Gut Isolation from Zebrafish Larvae for Single-cell RNA Sequencing
Summary
Here, we describe a method for gut isolation from zebrafish larvae at 5 days post fertilization, for single-cell RNA sequencing analysis.
Abstract
The gastrointestinal (GI) tract performs a range of functions essential for life. Congenital defects affecting its development can lead to enteric neuromuscular disorders, highlighting the importance to understand the molecular mechanisms underlying GI development and dysfunction. In this study, we present a method for gut isolation from zebrafish larvae at 5 days post fertilization to obtain live, viable cells which can be used for single-cell RNA sequencing (scRNA-seq) analysis. This protocol is based on the manual dissection of the zebrafish intestine, followed by enzymatic dissociation with papain. Subsequently, cells are submitted to fluorescence-activated cell sorting, and viable cells are collected for scRNA-seq. With this method, we were able to successfully identify different intestinal cell types, including epithelial, stromal, blood, muscle, and immune cells, as well as enteric neurons and glia. Therefore, we consider it to be a valuable resource for studying the composition of the GI tract in health and disease, using the zebrafish.
Introduction
The gastrointestinal (GI) tract is a complex system that plays a vital role in overall health and well-being. It is responsible for the digestion and absorption of nutrients, as well as the elimination of waste products1,2. The GI tract is composed of multiple cell types, including epithelial cells, smooth muscle cells, immune cells, and the enteric nervous system (ENS), which communicate closely together to regulate and maintain proper intestinal function3,4,5. Defects in the development of the GI tract can have far-reaching effects on various aspects such as nutrient absorption, microbiota composition, the gut-brain axis, and the ENS, leading to several enteric neuromuscular disorders, such as Hirschsprung disease and Chronic intestinal pseudo-obstruction6,7. These disorders are characterized by severe gut dysmotility caused by alterations in various key cells, such as the interstitial cells of Cajal, smooth muscle cells, and the ENS6,8,9. However, the molecular mechanisms underlying GI development and dysfunction are still poorly understood.
The zebrafish is a valuable model organism for studying GI development and dysfunction due to its rapid embryonic development, transparency during embryonic and larval stages, and genetic tractability10,11,12,13,14. Numerous transgenic zebrafish lines expressing fluorescent proteins are available. An example of such a line is the tg(phox2bb:GFP) zebrafish, commonly used to study the ENS, as all phox2bb+ cells, including enteric neurons, are labeled15,16. Here, using the tg(phox2bb:GFP) zebrafish line, we present a method for intestinal isolation of 5 days post fertilization (dpf) larvae for single-cell RNA sequencing (scRNA-seq) analysis (Figure 1).
Protocol
All zebrafish husbandry and experiments were conducted according to the institutional guidelines of the Erasmus MC and Animal welfare legislation. The use of zebrafish larvae at 5 days post fertilization falls under the category of experiments that do not require formal ethical approval, as outlined by the Dutch regulations.
1. Obtaining 5 days post fertilization (dpf) wildtype and tg(phox2bb:GFP) larvae
- Set up breeding of wildtype zebrafish and collect 50 eggs in HEPES-buffered E3 medium (hereafter referred to as E3) in a 15 cm Petri dish. Use these fish as a negative control for fluorescence-activated cell sorting (FACs).
- Set up breeding of tg(phox2bb:GFP) zebrafish and collect approximately 300 eggs in E3 in a 15 cm Petri dish.
- Keep fertilized eggs in E3 (maximum 50 eggs/dish) on a 14 h/10 h light/dark cycle, in an incubator at 28.5 °C.
- At 1 dpf, remove unfertilized eggs and let the fertilized ones develop to 5 dpf.
- Select tg(phox2bb:GFP) larvae at 1 dpf.
2. Wildtype whole larvae dissociation
- Anesthetize 5 dpf larvae with 0.016% Tricaine.
- Chop 30 zebrafish into small pieces using a razor blade in a Petri dish containing 1 mL of 10x trypsin-EDTA solution.
- Transfer the chopped zebrafish into a microcentrifuge tube with a total volume of 2 mL of 10x trypsin-EDTA solution and leave on ice for 3 h. Pipette up and down with a P1000 pipette every hour to stimulate dissociation.
3. Gut isolation
- Place 6-10 5 dpf larvae anesthetized with 0.016% Tricaine in a row, on a 1.8% agarose plate (0.45 g of agarose in 25 mL of E3), under a dissection microscope
- Put one insect pin in the head of the zebrafish.
- Remove all remaining E3 using a tissue.
- Isolate the intestine using another insect pin, without disturbing any other organs. Make sure that the yolk is removed (Supplementary Figure S1A).
- Once isolated, inspect the intestine and remove all non-intestinal material (e.g., skin, fat, liver) if needed.
- Collect the intestine with tweezers and place it in a microcentrifuge tube, containing phosphate-buffered saline (PBS) with 10% fetal calf serum (FCS), on ice.
NOTE: A minimum of 244 intestines should be isolated, keeping the total dissection time at 3 h. This is feasible with two people working in parallel.
4. Gut dissociation
- Immediately after the dissection of all intestines, centrifuge the microcentrifuge tube at full speed (13,800 × g) for 30 s.
- Remove the PBS/10% FCS but leave a small amount (~100 µL) to prevent the intestines from drying out. Add 500 µL of 2.17 mg/mL papain in HBSS containing CaCl2 and MgCl2, to dissociate cells.
- Activate papain with 2.5 µL of cysteine (1 M).
- Incubate the guts in a water bath at 37 °C for 10 min. Pipette up and down halfway (after 5 min) to stimulate enzymatic tissue digestion.
- Transfer cells into a FACS tube using a 35 µm cell strainer, prewetted with 0.5 mL of PBS/10% FCS.
- Wash the strainer by adding a total of 2 mL of PBS/10% FCS in 0.5 mL steps.
- Centrifuge at 700 × g for 5 min at 4 °C.
- Remove the supernatant and resuspend the pellet in 300 µL of PBS/10% FCS.
- Add 1 µg/mL of 4',6-diamidino-2-phenylindole (DAPI) to label dead cells (1:1,000) and incubate for 5 min to allow their exclusion during FACS.
5. FACS enrichment
- Use the wildtype sample to set the gates of the sorted cell population by recording 3,000 cells on the flow cytometer (Supplementary Figure S1B-E).
- Use a 100 µm nozzle, set sort precision on purity, and put the collection tube containing 200 µL of PBS/5% FCS, in the FACS machine.
- Load the cell suspension of the guts and sort live (DAPI-negative), single cells into the collection tube (Figure 2C), by excluding doublets (Figure 2B) and dead cells (Figure 2C).
NOTE: For the tg(phox2bb) zebrafish, the proportion of GFP+ cells is only checked for reference, as all single live cells are sorted. - Keep the collection tube on ice after cell sorting.
- Count the cell suspension with a hemocytometer, including a viability check with trypan blue. If the viability is at least 80%, continue with the next step.
- Cells are now ready to process for scRNAseq (i.e., 10x Genomics Chromium platform). Use approximately 20,000 cells per sample.
Representative Results
With this protocol, we achieved successful isolation and dissociation of entire intestines from 5 dpf larvae. Using papain as the dissociation enzyme, we significantly enhanced cell viability, enabling the capture of 46,139 events involving single, viable cells (6.4% of all cells) out of 244 isolated guts (Figure 2A). Wildtype whole larvae were used as a control to ensure that the sorting process was optimized, enabling effective cell identification and sorting. Whole larvae could be used as we only sort for live, single cells (Supplementary Figure S1B-E). All sorted intestinal cells were subsequently submitted to the scRNA seq platform. In total, 9,858 cells were sequenced with mean reads per cell of 21,106. For scRNA-seq analysis, we used Seurat V317. In total, 48 clusters were identified representing 12 different cell types, including epithelial, stromal, blood, muscle, and immune cells, as well as enteric neurons and glia18.
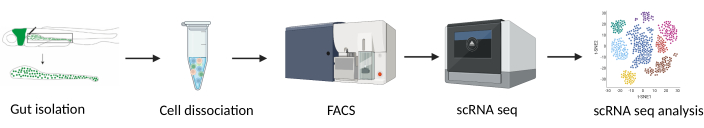
Figure 1: Experimental design for single-cell RNA sequencing of isolated zebrafish guts. Abbreviations: FACS = fluorescence-activated cell sorting; scRNA-seq = single-cell RNA-sequencing. Please click here to view a larger version of this figure.
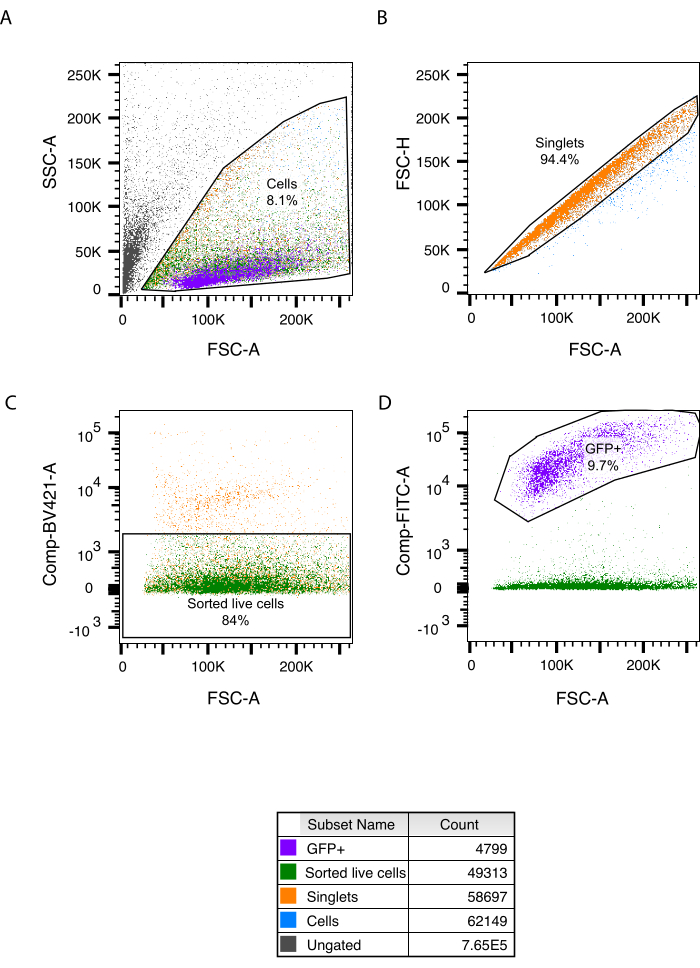
Figure 2: Gating strategy to sort live, single cells from the zebrafish gut. (A) Post sort analysis with FCS/SSC size gating. (B) Doublet discrimination. (C) Live/dead cell gating and sorted population. (D) Phox2bb:GFP+ cells present in the sorted live population. In each panel, the percentage of gated cells is shown. Abbreviations: FCS-A = forward scatter-peak area; SSC-A = side scatter-peak area; FSC-H = forward scatter-peak height; GFP = green fluorescent protein; FITC = fluorescein isothiocyanate. Please click here to view a larger version of this figure.
Supplemental Figure S1: Gut isolation and gating strategy to sort live, single cells from whole wildtype zebrafish larvae. (A) Brightfield image of gut isolation from 5 days post fertilization zebrafish larvae. The red line indicates the dissection line. (B) Post-sort analysis, showing all cells with FCS/SSC size gating. (C) Singlet selection. (D) DAPI gating. (E) Phox2bb:GFP+ gating. The percentage of gated cells is shown in each panel. Abbreviations: FCS-A = forward scatter-peak area; SSC-A = side scatter-peak area; FSC-H = forward scatter-peak height; GFP = green fluorescent protein; FITC = fluorescein isothiocyanate. Please click here to download this File.
Discussion
Here, we present a method for isolation and dissociation of the gut of 5 dpf zebrafish larvae using FACS. With this method, different intestinal cell types were successfully collected and analyzed by scRNA-seq, using the 10x Genomics Chromium platform. We selected the tg(phox2bb:GFP) zebrafish line, as we wanted an indication that viable ENS cells would be also isolated (Figure 2D). However, it is important to note that this method can be readily extended to other zebrafish lines of interest, as we only used the phox2bb gating to assess neuronal viability, and not to select for a specific cell population. Since we decided to use an unbiased approach to collect all intestine cells present at 5 dpf, the sorting criterion primarily hinges on identifying live, individual cells. This is also the reason behind the use of whole larvae to establish a reliable and consistent gating strategy. Considering the labor involved in isolating individual guts, it becomes impractical to isolate more intestines to serve as a negative control, within the given time frame of this experiment. Therefore, while we acknowledge that cell types from the whole larvae differ from those found in the intestine, and even distinct dissociation methods were used, it is notable that the gating strategy applied to sort intestinal cells can be initially validated using whole larvae, as we were only interested in assessing cell viability.
To perform scRNA-seq, a critical step in this protocol would be to isolate a sufficient number of viable cells (~20,000 cells). At the same time, the time spent for gut isolation should be kept as short as possible to avoid a detrimental effect on cell viability. Previous reports focusing on zebrafish larvae intestines employed accutase or trypsin for cell dissociation19,20,21. However, while both dissociation enzymes were able to isolate enough viable cells for scRNA-seq, accutase did not allow the capture of enteric neuronal cells19,20. Trypsin, however, predominantly captured epithelial cells, yielding only a low number of enteric neuronal cells21. Papain is commonly recognized for its gentleness, which can be particularly beneficial for preserving enteric neuronal integrity22. Our results showed that papain is indeed effective in maintaining the viability of neuronal cells, confirming previous applications in which this enzyme was used to digest zebrafish adult retina and brain23,24. However, it is noteworthy to mention that even with papain, cell viability post gut isolation and dissociation was only 6.4%. This suggests that the subsequent FACS step is, thus, crucial to select for viable cells. Such results can be considered a limitation particularly when dealing with scarce and challenging-to-capture cells of interest.
Future studies should focus on new dissociation methods to improve cell viability during the isolation protocol. Once increased, this method becomes feasible to sort out reporter-positive cells and perform scRNA-seq on specific intestinal cell types. Nevertheless, the method presented here allows the capture of enough viable cells for subsequent identification of different cell types within the gut, such as epithelial, stromal, blood, muscle, immune cells, and even enteric neurons and glia, which have not been captured before at this cell stage using similar approaches. Since the ability to isolate and analyze these different cell types is essential to get more insights into GI development and dysfunction, we consider this method to be particularly valuable, as it provides a reliable approach to study intestinal composition using the zebrafish, as a model organism.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was funded by the friends of Sophia Foundation (SSWO WAR-63).
Materials
| 10x Trypsin (0.5%)-EDTA (0.2%) | Sigma | 59418C | |
| 5 mL round bottom tube with cell-strainer cap | Falcon | 352235 | |
| Agarose | Sigma-Aldrich | A9539 | |
| BD Falcon Round-Bottom Tube 5 mL (FACS tubes) snap cap | BD Biosciences | 352054 | |
| Cell Ranger v3.0.2 | 10X Genomics | N/A | |
| DAPI | Sigma-Aldrich | Cat#D-9542 | |
| Dissection microscope | Olympus SZX16 | ||
| FACSAria III sorter machine | BD Biosciences | N/A | |
| HBSS with CaCl2 and MgCl2 | Gibco | 14025050 | |
| Insect pins | Fine Science Tools | 26000-25 | |
| L-Cysteine | Sigma | C7352 | |
| MS-222, Tricaine | Supelco | A5040-250G | |
| Papain | Sigma | P4762 | |
| Seurat v3 | Stuart et al. (2019) | N/A | |
| Trypan blue | Sigma | Cat#T8154 |
References
- Saldana-Morales, F. B., Kim, D. V., Tsai, M. T., Diehl, G. E. Healthy intestinal function relies on coordinated enteric nervous system, immune system, and epithelium eesponses. Gut Microbes. 13 (1), 1-14 (2021).
- Sitrin, M. . The Gastrointestinal System. , (2014).
- Furness, J. B. The organisation of the autonomic nervous system: peripheral connections. Autonomic Neuroscience: Basic and Clinical. 130 (1-2), 1-5 (2006).
- Furness, J. B. The enteric nervous system and neurogastroenterology. Nature Reviews. Gastroenterology & Hepatology. 9 (5), 286-294 (2012).
- Obata, Y., Pachnis, V. The effect of microbiota and the immune system on the development and organization of the enteric nervous system. Gastroenterology. 151 (5), 836-844 (2016).
- Heuckeroth, R. O. Hirschsprung disease – integrating basic science and clinical medicine to improve outcomes. Nature Reviews. Gastroenterology & Hepatology. 15 (3), 152-167 (2018).
- Antonucci, A., et al. Chronic intestinal pseudo-obstruction. World Journal of Gastroenterology. 14 (19), 2953-2961 (2008).
- De Giorgio, R., Sarnelli, G., Corinaldesi, R., Stanghellini, V. Advances in our understanding of the pathology of chronic intestinal pseudo-obstruction. Gut. 53 (11), 1549-1552 (2004).
- Bianco, F., et al. Enteric neuromyopathies: highlights on genetic mechanisms underlying chronic intestinal pseudo-obstruction. Biomolecules. 12 (12), 1849 (2022).
- Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B., Schilling, T. F. Stages of embryonic development of the zebrafish. Dev Dyn. 203 (3), 253-310 (1995).
- Lieschke, G. J., Currie, P. D. Animal models of human disease: zebrafish swim into view. Nature Reviews. Genetics. 8 (5), 353-367 (2007).
- Howe, K., et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 496 (7446), 498-503 (2013).
- Wallace, K. N., Akhter, S., Smith, E. M., Lorent, K., Pack, M. Intestinal growth and differentiation in zebrafish. Mechanisms of Development. 122 (2), 157-173 (2005).
- Wallace, K. N., Pack, M. Unique and conserved aspects of gut development in zebrafish. Biologie du développement. 255 (1), 12-29 (2003).
- Harrison, C., Wabbersen, T., Shepherd, I. T. In vivo visualization of the development of the enteric nervous system using a Tg(-8.3bphox2b:Kaede) transgenic zebrafish. Genesis. 52 (12), 985-990 (2014).
- Kuil, L. E., Chauhan, R. K., Cheng, W. W., Hofstra, R. M. W., Alves, M. M. Zebrafish: a model organism for studying enteric nervous system development and disease. Frontiers in Cell and Developmental Biology. 8, 629073 (2020).
- Stuart, T., et al. Comprehensive Integration of Single-Cell Data. Cell. 177 (7), 1888-1902 (2019).
- Kuil, L. E., et al. Unbiased characterization of the larval zebrafish enteric nervous system at a single cell transcriptomic level. iScience. 26 (7), 107070 (2023).
- Gao, Y., et al. Unraveling differential transcriptomes and cell types in zebrafish larvae intestine and liver. Cells. 11 (20), 3290 (2022).
- Jin, Q., et al. Cdx1b protects intestinal cell fate by repressing signaling networks for liver specification. Journal of Genetics and Genomics. 49 (12), 1101-1113 (2022).
- Willms, R. J., Jones, L. O., Hocking, J. C., Foley, E. A cell atlas of microbe-responsive processes in the zebrafish intestine. Cell Reports. 38 (5), 110311 (2022).
- Kline, M. . Fishing for answers: Isolating enteric neurons and identifying putative ENS mutants. , (2016).
- Allan, K., DiCicco, R., Ramos, M., Asosingh, K., Yuan, A. Preparing a single cell suspension from zebrafish retinal tissue for flow cytometric cell sorting of Muller glia. Cytometry A. 97 (6), 638-646 (2020).
- Lopez-Ramirez, M. A., Calvo, C. F., Ristori, E., Thomas, J. L., Nicoli, S. Isolation and culture of adult zebrafish brain-derived neurospheres. Journal of Visualized Experiments. 53617 (108), 53617 (2016).

