Implantation Surgery for Abdominal Vagus Nerve Stimulation and Recording Studies in Awake Rats
Summary
The present protocol describes the surgical technique for implanting an electrode array onto the abdominal vagus nerve in rats, along with methods for chronic electrophysiology testing and stimulation using the implanted device.
Abstract
Abdominal vagus nerve stimulation (VNS) can be applied to the subdiaphragmatic branch of the vagus nerve of rats. Due to its anatomical location, it does not have any respiratory and cardiac off-target effects commonly associated with cervical VNS. The lack of respiratory and cardiac off-target effects means that the intensity of stimulation does not need to be lowered to reduce side effects commonly experienced during cervical VNS. Few recent studies demonstrate the anti-inflammatory effects of abdominal VNS in rat models of inflammatory bowel disease, rheumatoid arthritis, and glycemia reduction in a rat model of type 2 diabetes. Rat is a great model to explore the potential of this technology because of the well-established anatomy of the vagus nerve, the large size of the nerve that allows easy handling, and the availability of many disease models. Here, we describe the methods for cleaning and sterilizing the abdominal VNS electrode array and surgical protocol in rats. We also describe the technology required for confirmation of suprathreshold stimulation by recording evoked compound action potentials. Abdominal VNS has the potential to offer selective, effective treatment for a variety of conditions, including inflammatory diseases, and the application is expected to expand similarly to cervical VNS.
Introduction
Vagus nerve stimulation (VNS) delivered at the cervical site in the neck is The United States Food and Drug Administration (FDA)-approved treatment for refractory epilepsy, refractory depression, and post-ischemic stroke rehabilitation1, and European Commission-approved for heart failure in Europe2. Non-invasive cervical VNS is FDA-approved for migraine and headache1. Its application is expected to expand, with recent clinical trials showing efficacy of VNS in other indications such as Crohn's disease3, rheumatoid arthritis4,5 and impaired glucose tolerance and type 2 diabetes6,7. Although promising, cervical VNS can cause bradycardia and apnea due to off-target activation of the nerve fibers that innervate the lungs and the heart8,9,10. Side effects such as cough, pain, voice alteration, headache and increase in apnea-hypopnea index are commonly reported in patients receiving cervical VNS11,12. Reduction in stimulation strength is a common strategy for reducing these side effects, however reduced charge may limit efficacy of VNS therapy by failing to activate therapeutic fibers11. In support of this hypothesis, the responder rate of patients receiving high intensity stimulation for the treatment of epilepsy was higher than that of patients receiving low intensity stimulation13.
Abdominal VNS is applied on the subdiaphragmatic vagus nerve, above the hepatic and celiac branches14 (Figure 1). Our previous study demonstrated that in rats abdominal VNS does not cause cardiac or respiratory side effects associated with cervical VNS10. Earlier studies also demonstrate anti-inflammatory effects of abdominal VNS in a rat model of inflammatory bowel disease and rheumatoid arthritis10,15 as well as reduction in glycemia in a rat model of type 2 diabetes16. Recently, the abdominal VNS technology has been translated for a first-in-human clinical trial for the treatment of inflammatory bowel disease (NCT05469607).
The peripheral nerve electrode array used to deliver stimulation to the abdominal vagus nerve (WO201909502017) has been custom developed for use in rats, and comprises of two to three platinum electrode pairs placed 4.7 mm apart, supported by a medical-grade silicone elastomer cuff, a suturing tab to anchor the array to the esophagus, a lead wire and a percutaneous connector to be mounted on the lumbar region (Figure 2). The lead wire is tunneled under the skin on the left side of the animal. Multiple electrode pair design allows for electrical stimulation of the nerve as well as recording electrically evoked compound action potentials (ECAPs), which confirms correct placement of the implant onto the nerve and suprathreshold stimulation intensities. Abdominal VNS is well tolerated in freely moving rats for months10,15,16. This allows for assessment of its efficacy on disease models.
This manuscript describes the methods for the electrode array sterilization, abdominal vagus nerve implantation surgery, and chronic stimulation and recording of ECAPs in awake rats for studying the efficacy of abdominal VNS in a variety of disease models. These methods were originally developed for studying the efficacy of abdominal VNS in the rat model of inflammatory bowel disease10 and have also been successfully used for a rat model of rheumatoid arthritis15 and diabetes16.
Protocol
All procedures involving animals were approved by the Animal Ethics Committee of St. Vincent's Hospital (Melbourne) and complied with the Australian Code for the Care and Use of Animals for Scientific Purposes (National Health and Medical Research Council of Australia) and the Prevention of Cruelty to Animals (1986) Act. In total, 24 female Dark Agouti rats (8-9 weeks old) were used for this study. The experimental groups consisted of: a normal cohort (n = 8) that received no collagen injection or VNS implant; an unstimulated disease cohort (n = 8) that received an implant and a collagen injection (no electrophysiological tests conducted); and a stimulated disease cohort (n = 8) that received an implant, a collagen injection, electrophysiological testing and VNS therapy. Implantation surgery occurred 5 days prior to collagen injection, and habituation for VNS therapy started 4 days after collagen injection and occurred over 7 days. VNS therapy was applied from day 11 to 17 (inclusive) following collagen injection15. For the stimulated disease cohort, electrophysiological testing was performed immediately after the implantation surgery under anesthesia, on the day of collagen injection, 10 days after collagen injection, and the day of termination (17 days after collagen injection).
1. Sonication and sterilization of electrode array
- Set the ultrasonic cleaner to a frequency of 80 kHz and fill the ultrasonic tank with tap water. Submerge the electrode array in the cleaning solution in a clean plastic container and place it in the ultrasonic tank.
NOTE: The cleaning solution and sonication time to be used for each step are summarized in Table 1. Use a clean container for each step. - Place the sonicated electrode array into a sterilization bag using clean forceps sonicated with 0.5% liquid cleaning solution in distilled water and rinsed in distilled water. Autoclave the electrode array for 45 min with maximum temperature of 130 °C, and let it dry on a clean bench.
2. Implantation of electrode array on the abdominal vagus nerve
NOTE: In this study we have used female dark agouti rats (8-9 weeks of age)15. We have also successfully used this protocol to chronically implant adult male Sprague-Dawley rats (10-14 weeks of age)10,16. Surgery is conducted under aseptic conditions, and all instruments, electrode array and consumables such as gauze and cotton tips are sterilized by autoclaving.
- Anesthetize the rat in an induction chamber using 3% isoflurane and 1 L/min oxygen. Once there is no pedal reflex to toe pinching, move the rat to the heat mat with a thermostat on the surgical table and place an isoflurane mask over the nose.
- Monitor the respiratory rate and rectal temperature throughout the surgery and adjust the isoflurane level between 1.5% and 2.5% to maintain the respiratory rate between 40 to 62 breaths per min. Adjust the heat mat setting if required, to maintain the rectal temperature range between 35.9 – 37.5 °C.
- Administer analgesia pre-medication subcutaneously using 1 mL syringes with 25G needles (carprofen 5 mg/kg and buprenorphine 0.03 mg/kg subcutaneously) prior to the start of surgery.
- Shave generously around the incision site, including the area along the ventral midline from the xyphoid process to the end of the rib cage, the lumbar aspect of the back along the dorsal midline, and the left-hand side of the body between the forelimb and the hindlimb to allow subcutaneous tunneling of the array.
- Clean the surgical sites in a circular motion three times with alternating rounds of betadine and alcohol and place a surgical drape over the animal. Administer bupivacaine (1-2 mg/kg) subcutaneously using a 1 mL syringe with a 25G needle at the dorsal and ventral incision sites.
- Place the animal in ventral recumbency and make a 2 cm long incision on the back where the percutaneous pedestal will be anchored using a scalpel blade.
- Turn the rat to dorsal recumbency and make a 3 cm incision on the skin along the midline just below the xyphoid process using a scalpel blade. Hold up the skin near the incision site, and using dissecting scissors, blunt-dissect the skin layer from the muscle layer around the incision.
- To allow subcutaneous tunneling of the array from the pedestal to site of implantation, place the animal on its right side, insert a hemostat from the ventral incision and blunt dissect towards the dorsal incision site. Cut off the rim of a needle cap and insert the electrode array to protect it while in transit (Figure 1B). Using hands (wear sterile gloves), tunnel the electrode array under the skin towards the ventral incision.
- To access the esophagus and the vagus nerve, lay the animal in dorsal recumbency again. Make a 3 cm incision on the muscle layer along the midline below the xyphoid process, large enough to expose the whole length of the liver. Avoid damaging the liver during this step.
- Make a smaller incision (less than 1 cm) on the muscle layer laterally (the left side of the animal) to the main ventral incision. Tunnel the electrode array through this small incision using the needle cap used in step 2.8 to insert the array into the abdominal cavity.
NOTE: This step reduces tension applied to the main incision site and reduces the risk of sutures bursting. - Retract the skin and muscle layers to hold the abdominal cavity open. Ensure to keep tissues moist by using cotton tips and gauze soaked in sterile saline to handle the tissue.
- Gently retract the liver by cutting the connective tissue around it using Vannas scissors and placing a retractor over a small piece of gauze soaked in saline for protection. Gently retract the stomach, to allow straightening of the esophagus and overlying vagus nerve, by placing a retractor between the esophagus and the stomach.
NOTE: Retractors are made by rounding the pointy end of fishhooks. - Following exposure of the ventral surface of the esophagus, identify the abdominal vagus nerve and its sub-branches, including the hepatic nerve, the celiac nerve and two gastric branches (Figure 1D).
- Cut the connective tissue securing the abdominal vagus nerve to the esophagus using fine forceps and Vannas scissors and dissect the length of the nerve from just above the hepatic and celiac branches towards the diaphragm. Ensure to not tear, stretch, or pinch the nerve. Place an electrode array next to the nerve to confirm that enough length of the nerve is separated from the connective tissue to fit the array.
- Once connective tissue had been cleared around the nerve, pass the silk sutures (7-0) on the electrode-side of the array cuff under the nerve. Open the cuff of the array and place the nerve carefully into the array channel.
- Ensure that the entire length of the nerve sits inside the array channel. Tie the sutures around the cuff together to securely shut the cuff to ensure the nerve does not slip out of the channel. Trim the sutures.
- Using 7-0 silk suture, suture the tab of the array onto the esophagus to secure the array in place and prevent it from twisting. Avoid damaging the other branches of the vagus nerve or inserting needle too deep into the esophagus smooth muscle.
- Gently remove retractors and ensure all gauze has been removed from the abdominal cavity. Administer 1-2 mL of warm sterile saline using a 1 mL syringe in the abdominal cavity and reposition the liver to the correct position.
- Close the muscle layer with 3-0 silk suture using the simple running suture technique, making secure square knots with at least 3 throws at both ends. Space stitches closely together (approximately 3 mm apart) to prevent complications such as hernia/protrusion of the xyphoid process.
- Use suture to close the incision of the peritoneum together with the incision of the muscle layer, to reduce the chance of tissue adhesion.
- Using an absorbable suture material (Vicryl 4-0), close the skin incision. Use a buried suturing technique such as the running buried vertical mattress suture, or the running buried dermal suture to prevent the animal from removing the suture.
- Turn the animal to ventral recumbency, and using scissors, extend the dorsal incision to 4-5 cm, and blunt dissect between the muscle and the skin layer further so the connector base of the percutaneous connector can sit flat on the muscle layer.
- Using silk 3-0 suture, make 6 to 8 simple interrupted sutures around the connector base to secure it to the muscle layer underneath. Close the skin incision with silk 3-0 suture, using the horizontal mattress suture technique, ensuring secure square knots with at least 3 throws.
NOTE: In this step, braided silk sutures are preferred for their ease of handling and their capacity to create more secure knots compared to monofilament sutures. - At the completion of surgery, administer Hartmann's solution subcutaneously (1 mL/100 g/h). Turn off the isoflurane and let the animal recover on a heat mat while running oxygen (1.5 L/min). Once the rat is conscious and fully mobile, return the rat to its home cage, placed on a heat pad, until fully recovered from anesthetic.
- Closely observe the animal's recovery from isoflurane and ensure that the animal is able to access food and drink. In the next two days, provide subcutaneous administration of post-surgical analgesia (carprofen 5 mg/kg, daily) to alleviate pain. Monitor the animal at least 2x per day and check for evidence of defecation, quality of the coat, activity level and presence of any swelling or discharge from the surgical wounds.
- Record weight of the animal, and in the rare event the animal loses 10% or more, start intensive treatment. Intensive treatment includes subcutaneous administration of fluids (Hartmann's solution, 2x 10 mL) each day, providing additional food such as fresh vegetables and dietary gel supplement, and placing half the cage on a heat pad with a thermostat for extra warmth. Increase the frequency of monitoring until the animal recovers. Continue with administration of analgesia (carprofen 5 mg/kg, SQ, daily) if required based on a Grimace scale.
3. Electrophysiological testing
NOTE: Recording evoked compound action potentials (ECAPs) confirms appropriate placement of the electrode array on the vagus nerve. Additionally, recording of ECAPs using the electrode array described above provides likely confirmation of electrical activation of vagal C-fibers and suprathreshold VNS10,15.
- Measure the common ground impedance of electrodes to assess their integrity and detect any open or short circuits of wires prior to recording ECAPs. Functioning abdominal vagus nerve electrodes in vivo should have impedance values between 4 – 20 kΩ.
- Test animals while anaesthetized i.e., immediately after surgery, or awake and freely moving. Perform awake testing at least 2-3 days after the surgery to allow for the surgical skin wounds to heal and stabilize. Gather the equipment required for impedance and electrophysiological testing which include a custom-made stimulator, a data acquisition device, an isolated differential amplifier and a data acquisition and analysis software as listed in the Table of Materials.
- Wrap the animal in a towel if required, connect a cable to the back percutaneous connector, and connect the other end of the cable to a stimulator. To test the common ground impedance of electrodes, apply biphasic current pulses (100 µs per phase and current of 107 µA) between the electrode of interest and all other electrodes on the array.
- Measure the peak voltage at the end of the first phase of the voltage waveform (Vtotal) and calculate total impedance (Ztotal) using Ohm's law (Z = voltage/current).
- Connect a pair of electrodes to the stimulator and a pair of electrodes to the recording equipment and apply bipolar stimulation to generate ECAPs using the reference electrode of the VNS implant placed under the skin as a reference for the differential recording of ECAPs. Make two sets of recordings averaged from a total of 50 repetitions using the data acquisition and analysis software.
- Use the following settings for measurements.
Currents: 0 to 2 mA in 0.1 mA increments;
Pulse width: 25 – 200 µs;
Interphase gap: 8 – 50 µs;
Stimulation rate: 10 – 30 pulses/s;
Sampling rate: 100 kHz;
Filter: High pass 200 Hz, low pass 2000 Hz, voltage gain 1 x 102. - Using the data analysis software, analyze the ECAP response by measuring the peak-to-peak voltage of the waveforms within the analysis window (4 – 10 ms post-stimulus, indicated by shading in Figure 3A,B). ECAP threshold is defined as the minimum stimulus current intensity producing a response amplitude of at least 0.1 µVpeak-peak in both sets of average electrophysiology recordings. A valid response will be repeated for at least two current levels above the threshold, and not present for at least two current levels below the threshold10,15.
4. Chronic abdominal VNS in awake rats
NOTE: Abdominal VNS can be applied to awake animals once the surgical wound around the percutaneous connector has healed and stabilized. To reduce any stress response and allow for better data collection, animals are habituated to the testers' handling and stimulation environment, one hours a day over seven days prior to the implantation surgery and commencement of VNS therapy.
- Measure the impedance of each electrode as described in step 3.4, prior to applying any VNS. Ensure that the impedance of stimulating electrodes is below 20 kΩ.
- Connect a cable to the back percutaneous connector and connect the other end of the cable to a stimulator programmed to apply appropriate stimulation (e.g., 27 Hz, 1.6 mA, 200 µs pulse-width with 50 µs interphase gap, 30 s ON, 2.5 min off15), and turn on the stimulator.
NOTE: Although animals are often observed falling asleep during stimulation if appropriately habituated, use a cable with protective outer material such as steel coils where possible to prevent it from being chewed. - Observe the animal at the start of each VNS therapy session to ensure there is no adverse reaction such as excessive grooming or sudden increase/decrease in activity level in sync with the timing of stimulation.
- Monitor every 30 min to check for twisting or disconnection of the cable. To apply VNS chronically (e.g., 3 h a day over 7 days15), repeat steps 4.1-4.3 at the start of each session.
NOTE: The use of a commutator may reduce the chance of cables becoming twisted and could require less frequent monitoring.
Representative Results
Recording evoked compound action potentials (ECAPs, Figure 3A,B) immediately after surgery is a technique that can be used to help confirm correct placement of the nerve within the array channel, and that stimulation is efficacious in activating the vagus nerve.
In Figure 3, female dark agouti rats (8-9 weeks of age) were implanted with the VNS electrode array. In rats randomly selected to receive therapeutic stimulation, ECAPs were recorded immediately after surgery (day 0, Figure 3A) and at the end of the VNS therapy session (day 23, Figure 3B). The presence of ECAPs (Figure 3B) indicated that stimulation intensity was above neural threshold and that the nerve was successfully activated. Animals in the VNS treatment group were excluded if ECAPs were not recorded as there was no guarantee that stimulation was successfully delivered15. The latency of the neural response (Figure 3A,B, indicated by green arrow) can be used to assess which class of fibers were activated.
In previous studies, we have observed that most neural responses typically occur between 4 ms and 10 ms10,15. Given the distance between stimulating and recording pairs is 4.7 mm, the approximate conduction velocity of this response window is 0.47 – 1.2 m/s, which is consistent with the conduction velocity of C-fibers18.
There is an increase in neural threshold between day 0 (377 µA, Figure 3A) and day 23 (1335 µA, Figure 3B), which occurs over time likely due to minor benign fibrosis that forms around the tissue-electrode interface10,15.
Figure 3C indicated the experimental testing set up and the positioning of the back connector, which remained stable for the duration of the 3 week testing period15.
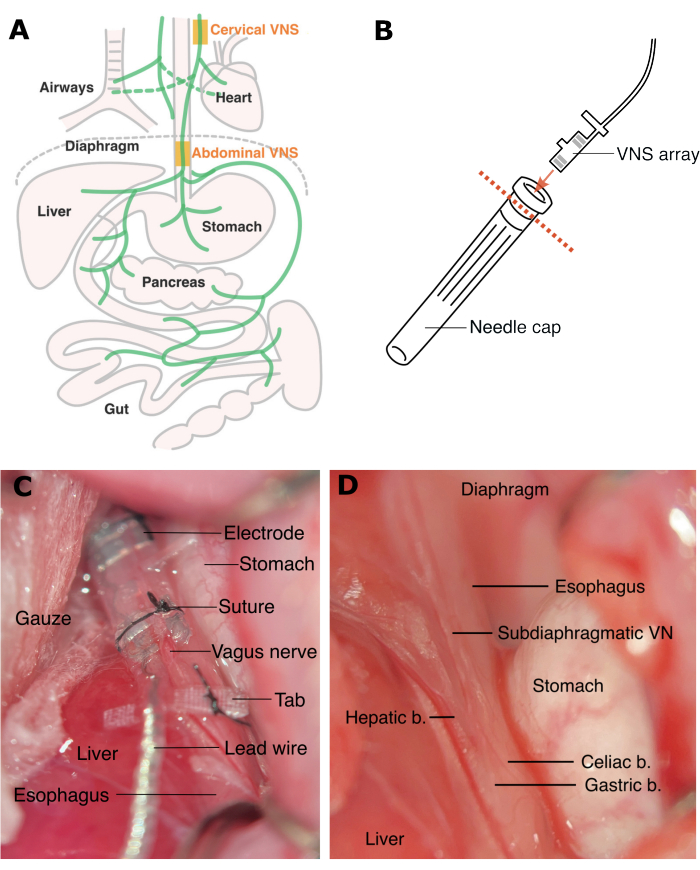
Figure 1: Sites for cervical VNS and abdominal VNS. (A) Cervical VNS is applied above the branches to the heart and the airways, and abdominal VNS is applied below these branches. (B) The electrode array is inserted to a needle cap (with the rim removed) to protect it during tunneling under the skin. (C) Rat abdominal VNS array is implanted and secured with sutures (D) above the celiac and hepatic branches below the diaphragm. Abbreviations: VN = vagus nerve, b. = branch. Please click here to view a larger version of this figure.
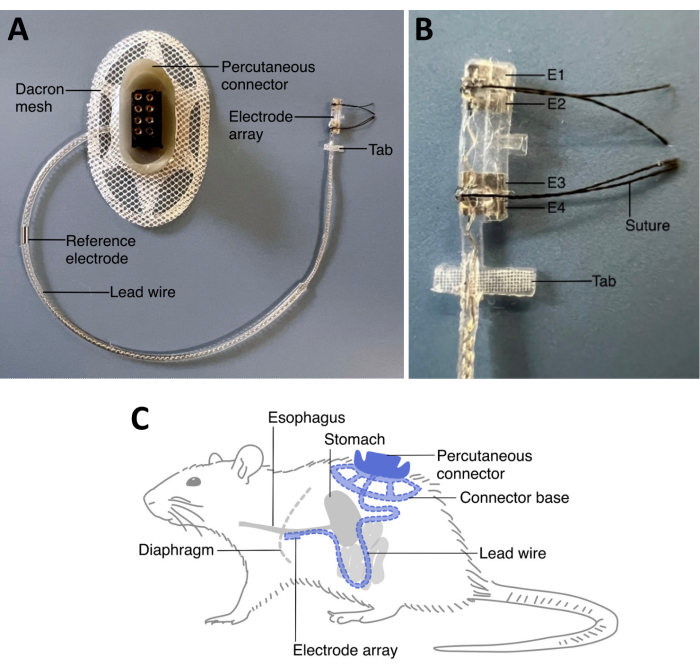
Figure 2: Rat VNS array. (A) A rat VNS array comprises of a percutaneous connector and an electrode array connected by lead wire. (B) A tab near the electrode array can be sutured to the esophagus to help stabilize the position of the array. The standard rat abdominal vagus nerve array comes with two electrode pairs (E1 and E2, and E3 and E4). Both electrode pairs can be used for either stimulation or recording. The percutaneous connector is mounted on the lumber region of the animal, and the lead wire is tunneled under the skin on the left side of the animal. The reference electrode along the lead wire is placed under the skin on the left side of the animal once the electrode array is implanted. The electrode array is implanted on the vagus nerve along the esophagus above the stomach and just below the diaphragm. (C) The extra length of the lead wire on the left side of the animal under the skin provides strain relief. Abbreviations: E = electrode. Please click here to view a larger version of this figure.
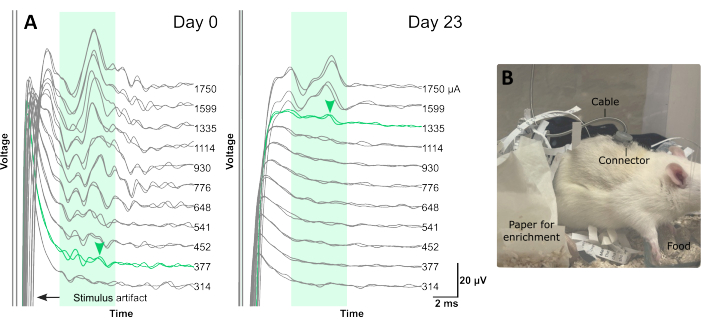
Figure 3: Typical electrophysiology traces recorded from abdominal vagus nerve during chronic implantation and a rat receiving VNS therapy. (A) Each electrophysiology trace is an average of 25 repetitions. The green shaded boxes indicate the typical latency of C-fiber response in the rat abdominal vagus nerve, between 4 ms and 10 ms (using an electrode array with distance of 4.7 mm between stimulating and recording electrode pairs, center to center). ECAPs are labelled with green arrowheads. (B) A rat receiving VNS therapy via the percutaneous connector on its back in the home cage. Please click here to view a larger version of this figure.
| Cleaning solution | Sonication time |
| 1. 0.5% pyroneg in ultrapure water | 15 min |
| 2. Ultrapure water | 5 min |
| 3. Ultrapure water | 5 min |
| 4. 96% ethanol | 10 min |
| 5. Ultrapure water | 5 min |
| 6. Ultrapure water | 5 min |
Table 1: Sonication steps. The table provides details of the sonication performed here.
Discussion
This method of abdominal VNS implant surgery and chronic stimulation of the vagus nerve and recording of ECAPs have been successfully used and well-tolerated for 5 weeks in rats following implantation10,15,16. Retraction of the stomach, liver, and gut to gain a good view of the esophagus and the vagus nerve is one of the key steps in the surgery. Once these organs are retracted, the vagus nerve becomes accessible. Retraction of the stomach risks compromising breathing, in which case the retractor is loosened. Additionally, when cutting the connective tissue surrounding the esophagus to access the vagus nerve, care should be taken to avoid damage to the diaphragm, which can lead to disruption in the intra-thoracic pressure, severe respiratory suppression, and death of the animal during surgery. It is recommended that the connective tissue surrounding the esophagus is cleared just enough to fit the electrode array above the hepatic and celiac branches.
Possible post-surgical complications include tissue adhesion around the esophagus, hernia, and post-operative ileus. Tissue adhesion can be minimized by liberal application of sterile saline in the abdominal cavity during surgery and prior to closing the abdominal cavity. Suture closing the peritoneum layer together with the muscle layer should also help protect the internal organs and reduce tissue adhesion. As the liver is easily bruised and manipulation of the intestine can cause post-operative ileus, minimal and gentle handling of these organs is important. Finally, particularly in heavier animals, it is critical that running suture stitches are placed close together or interrupted sutures are used instead when closing the abdominal cavity to prevent hernia and xyphoid process protrusion.
As standard post-operative care, animals should be checked regularly following surgery for evidence of defecation, weight change, coat, activity level and presence of any swelling or discharge from the surgical wounds. In rare occasions, excessive discharge, redness and swelling around surgical wounds may occur, suggesting infection. On such occasions, antibiotics such as Baytril (0.2 mg/mL, in drinking water for 3-5 days) are administered until the infection has resolved. While normal rats generally recover well from the surgery, rats with compromised health conditions (i.e., disease models) may require more time before testing and stimulation can begin post-surgery. Appropriate post-operative care (as summarized in step 2.26) for such animals is essential for the welfare of the animals.
One of the limitations of this protocol is that while the design of the rat VNS electrode array is excellent for recording slower C-fiber response, the spacing between the electrode pairs (4.7 mm) may not be suitable for capturing activity of some of the faster fiber types. Although the total length of the array is limited by the available length of the subdiaphragmatic vagus nerve above the hepatic and celiac branches, these VNS arrays can be purchased with additional electrode pairs. Such arrays may be used to explore the application of blocking stimulation that can manipulate the direction of VNS16,19, expanding the possible use of this model.
Recording ECAPs can be used to assess the placement of the array around the nerve, the quality of the electrode interface and the ability of the device to activate vagal fibers. The vagus is an autonomic nerve, consisting of 97%-99% C-fibers18,20, with the remaining 1%-3% of fibers being myelinated fibers (function unknown), as confirmed by transmission electron microscopy studies20. The responses in Figure 3 are likely from electrically-evoked activity of the vagus nerve, rather than myogenic activity, as they fit the shape and form of the compound action potential for a peripheral nerve21,22. Furthermore, the typical conduction velocity of the rat abdominal vagus nerve ECAPs is 0.47 – 1.2 m/s, which is consistent with the conduction velocity of C-fibers18. In initial device development pilot studies, the recordings were validated in anesthetized rat studies by cutting the vagus nerve between the stimulating and recording electrode, resulting in the elimination of any evoked responses (data not shown). The electrode array has been designed so that the vagus nerve sits within a platinum-silicone channel, effectively electrically isolating it from surrounding structure structures (e.g., the oesophagus). Stimulation and recording are also both performed using bipolar configurations utilizing adjacent electrodes, further minimizing opportunities for spread of stimulation and contamination of recording. We have consistently reported similar waveforms following electrical stimulation of the abdominal vagus nerve in anesthetized and awake preparations10,15,16,19,23, including studies in which physiological effects of stimulation confirmed vagal nerve activation10,15,16. Although it is impossible to exclude contamination of the recording by myogenic activity, myogenic responses are usually distinguishable from neural response due to their rapid and large growth amplitude profile24 in contrast to the graded, smaller growth profiles observed in our studies10,15 and in Figure 3A,B: Day 0: current level 377 µA latency: 7.24 ms > current level 1750 µA: 6.74 ms.
While the activation of the subdiaphragmatic vagus nerve which consists almost entirely of C-fibers20,25 has been shown to be effective for treatment of pre-clinical models of inflammatory bowel disease10, rheumatoid arthritis15 and diabetes16, the optimal abdominal VNS parameters to maximize its therapeutic effect have not been fully explored14. Further research on this topic would be of great benefit, as application of abdominal VNS for treatment of inflammatory bowel disease is currently being investigated in a first-in-human clinical trial. As the anti-inflammatory effect of abdominal VNS is considered to be systemic26, there is a great potential that this therapy is also effective for other inflammatory conditions such as systemic lupus erythematosus27 and chronic kidney disease28.
Our rat studies show abdominal VNS has a unique advantage over cervical VNS in that it does not cause cardiac or respiratory off-target effects10. Higher intensity stimulation can be applied for longer periods of time without compromising respiration or the heart rate of the animal. Paired with the ability to monitor the evoked neural response confirming suprathreshold stimulation intensities, this method provides a great model to study the efficacy of abdominal VNS for treating a variety of diseases. As the application of VNS is continuing to expand, the application of this method of VNS is expected to expand as well.
Divulgations
The authors have nothing to disclose.
Acknowledgements
Development of the rat abdominal VNS implant was funded by the Defense Advanced Research Projects Agency (DARPA) BTO, under the auspices of Dr. Doug Weber and Dr. Eric Van Gieson through the Space and Naval Warfare Systems Center (Contract No. N66001-15-2-4060). Research reported in this publication was supported by the Bionics Institute Incubation Fund. The Bionics Institute acknowledges the support they receive from the Victorian Government through its Operational Infrastructural Support Program. We would like to thank Mr. Owen Burns for mechanical design, Prof. John B Furness for anatomical expertise, Prof. Robert K Shepherd for peripheral interface, neuromodulation and recording expertise, Ms. Philippa Kammerer and Ms. Amy Morley for animal husbandry and testing, Ms. Fenella Muntz and Dr. Peta Grigsby for their advice on post-operative animal care, and Ms. Jenny Zhou and the electrode fabrication team from NeoBionica for production of the VNS arrays.
Materials
| 0.9% saline | Briemarpak | SC3050 | |
| Baytril | Bayer | ||
| Betadine | Sanofi-Aventis Healthcare | ||
| Buprelieve (Buprenorphine) | Jurox | ||
| Data acquisition device | National Instruments | USB-6210 | |
| DietGel Boost (dietary gel supplement) | ClearH2O | ||
| Dumont tweezer, style 5 | ProSciTech | T05-822 | |
| Dumont tweezer, style N7, self-closing | ProSciTech | EMS72864-D | |
| Elmasonic P sonicator | Elma | ||
| Hartmann's solution | Baxter | AHB2323 | |
| Hemostat | ProSciTech | TS1322-140 | |
| HPMC/PAA Moisturising Eye Gel | Alcon | ||
| Igor Pro-8 software | Wavemetrics, Inc | ||
| Isoflo (Isoflurane) | Zoetis | ||
| Isolated differential amplifier | World Precision Instruments | ISO-80 | |
| Liquid pyroneg | Diversey | HH12291 | cleaning solution |
| Marcaine (Bupivacaine) | Aspen | ||
| Plastic drape | Multigate | 22-203 | |
| Rat vagus nerve implant | Neo-Bionica | ||
| Rimadyl (Carprofen) | Zoetis | ||
| Silk suture 3-0 | Ethicon | ||
| Silk suture 7-0 | Ethicon | ||
| SteriClave autoclave | Cominox | 24S | |
| Sterile disposable surgical gown | Zebravet | DSG-S | |
| Suicide Nickel hooks | Jarvis Walker | ||
| Ultrapure water | Merck Millipre | Milli-Q Direct | |
| Underpads | Zebravet | UP10SM | |
| Vannas scissors | ProSciTech | EMS72933-01 | |
| Vicryl suture 4-0 | Ethicon |
References
- Fang, Y. T., et al. Neuroimmunomodulation of vagus nerve stimulation and the therapeutic implications. Front Aging Neurosci. 15, 1173987 (2023).
- Fudim, M., et al. Device therapy in chronic heart failure: JACC state-of-the-art review. J Am Coll Cardiol. 78 (9), 931-956 (2021).
- Sinniger, V., et al. A 12-month pilot study outcomes of vagus nerve stimulation in Crohn’s disease. Neurogastroenterol Motil. 32 (10), 13911 (2020).
- Koopman, F. A., et al. Vagus nerve stimulation in patients with rheumatoid arthritis: 24 month safety and efficacy. Arthritis Rheumatol. 70, (2018).
- Genovese, M. C., et al. Safety and efficacy of neurostimulation with a miniaturised vagus nerve stimulation device in patients with multidrug-refractory rheumatoid arthritis: a two-stage multicentre, randomised pilot study. Lancet Rheumatol. 2 (9), e527-e538 (2020).
- Lu, J. Y., et al. A randomized trial on the effect of transcutaneous electrical nerve stimulator on glycemic control in patients with type 2 diabetes. Sci Rep. 13 (1), 2662 (2023).
- Huang, F., et al. Effect of transcutaneous auricular vagus nerve stimulation on impaired glucose tolerance: a pilot randomized study. BMC Complement Altern Med. 14, 203 (2014).
- Chang, R. B., Strochlic, D. E., Williams, E. K., Umans, B. D., Liberles, S. D. Vagal sensory neuron subtypes that differentially control breathing. Cell. 161 (3), 622-633 (2015).
- McAllen, R. M., Shafton, A. D., Bratton, B. O., Trevaks, D., Furness, J. B. Calibration of thresholds for functional engagement of vagal A, B and C fiber groups in vivo. Bioelectron Med (Lond). 1 (1), 21-27 (2018).
- Payne, S. C., et al. Anti-inflammatory effects of abdominal vagus nerve stimulation on experimental intestinal inflammation). Front Neurosci. 13, 418 (2019).
- Ben-Menachem, E., Revesz, D., Simon, B. J., Silberstein, S. Surgically implanted and non-invasive vagus nerve stimulation: a review of efficacy, safety and tolerability. Eur J Neurol. 22 (9), 1260-1268 (2015).
- Parhizgar, F., Nugent, K., Raj, R. Obstructive sleep apnea and respiratory complications associated with vagus nerve stimulators. J Clin Sleep Med. 7 (4), 401-407 (2011).
- Mao, H., Chen, Y., Ge, Q., Ye, L., Cheng, H. S. h. o. r. t. -. and long-term response of vagus nerve stimulation therapy in drug-resistant epilepsy: A systematic review and meta-analysis. Neuromodulation. 25 (3), 327-342 (2022).
- Payne, S. C., Furness, J. B., Stebbing, M. J. Bioelectric neuromodulation for gastrointestinal disorders: effectiveness and mechanisms. Nat Rev Gastroenterol Hepatol. 16 (2), 89-105 (2019).
- Payne, S. C., Romas, E., Hyakumura, T., Muntz, F., Fallon, J. B. Abdominal vagus nerve stimulation alleviates collagen-induced arthritis in rats. Front Neurosci. 16, 1012133 (2022).
- Payne, S. C., et al. Blood glucose modulation and safety of efferent vagus nerve stimulation in a type 2 diabetic rat model. Physiol Rep. 10 (8), 15257 (2022).
- Shepherd, R. K., Fallon, J. B., Payne, S. C., Burns, O., Furness, J. B. Peripheral nerve electrode array. US patent. , (2019).
- Castoro, M. A., et al. Excitation properties of the right cervical vagus nerve in adult dogs. Exp Neurol. 227 (1), 62-68 (2011).
- Payne, S. C., et al. Differential effects of vagus nerve stimulation strategies on glycemia and pancreatic secretions. Physiol Rep. 8 (11), 14479 (2020).
- Prechtl, J. C., Powley, T. L. The fiber composition of the abdominal vagus of the rat. Anat Embryol (Berl). 181 (2), 101-115 (1990).
- Gasser, H. S., Erlanger, J. The role played by the sizes of the constituent fibers of a nerve trunk in determining the form of its action potential wave. Am J Physiol-Legacy Content. 80 (3), 522-547 (1927).
- Parker, J. L., Shariati, N. H., Karantonis, D. M. Electrically evoked compound action potential recording in peripheral nerves. Bioelectron Med. 1 (1), 71-83 (2018).
- Villalobos, J., et al. Stimulation parameters for directional vagus nerve stimulation. Bioelectron Med. 9 (1), 16 (2023).
- Verma, N., et al. Characterization and applications of evoked responses during epidural electrical stimulation. Bioelectron Med. 9 (1), 5 (2023).
- Hoffman, H. H., Schnitzlein, H. N. The numbers of nerve fibers in the vagus nerve of man. Anat Rec. 139, 429-435 (1961).
- Bassi, G. S., et al. Anatomical and clinical implications of vagal modulation of the spleen. Neurosci Biobehav Rev. 112, 363-373 (2020).
- Courties, A., Berenbaum, F., Sellam, J. Vagus nerve stimulation in musculoskeletal diseases. Joint Bone Spine. 88 (3), 105149 (2021).
- Hilderman, M., Bruchfeld, A. The cholinergic anti-inflammatory pathway in chronic kidney disease-review and vagus nerve stimulation clinical pilot study. Nephrol Dial Transplant. 35 (11), 1840-1852 (2020).

