Ex Vivo Placental Explant Flow Culture – Mimicking the Dynamic Conditions In Utero
Summary
Here is a protocol for culturing placental explants under constant flow conditions. This approach enhances traditional static villous culture systems by enabling the replication of dynamic physiological environments.
Abstract
The existing ex vivo placental explant culture models are primarily grounded in static culture systems using well plates. However, these models inadequately reflect the dynamic in utero setting, where the placenta encounters constant slight shear stress due to plasma or blood flow. To address this limitation, a flow culture system has been devised to bring ex vivo placental explant cultivation closer to the in utero flow conditions experienced within the maternal body. Within this approach, placental explants are cultivated in a sequence of five interconnected flow chambers. This setting maintains physiological oxygen concentrations and a consistent flow rate. The collected data reveals that under flow conditions, the preservation of tissue morphology exhibits notable enhancement compared to conventional static methods. This innovative technique introduces a straightforward means of ex vivo placental explant culture, offering a more faithful representation of the dynamic in vivo environment. Moreover, this study introduces new possibilities for investigating the functional dynamics of the feto-maternal interface. By embracing feasible dynamic methodologies, a deeper comprehension of placental biology is facilitated, underscoring its relevance for maternal-fetal health.
Introduction
Since the 1960s, placental explant cultivation on the bottom of a well-plate has been utilized for studying the feto-maternal interface1,2,3. This method is well-established and straightforward, enabling the utilization of human tissue for various studies, in addition to cultures of single cells2,3. Over time, experimental designs for placental explant cultures have been modified with regards to oxygen concentration4 and to prevent the tissue from settling at the well-plate's bottom2,5,6. However, this method has not been adapted to the in vivo conditions within the uterus, specifically the presence of a constant flow3.
The success of a pregnancy hinges on an adequate and consistent perfusion of the intervillous space with maternal blood, establishing a dynamic circuit with continuous inflow and outflow of blood and blood-borne substances7,8,9,10,11,12. The placenta features two distinct blood supply systems, one for maternal blood and one for fetal blood, resulting in dual perfusion by both fetal and maternal systems13. Maternal blood begins perfusing the placenta's intervillous space at the end of the first trimester, slowly flowing through widened uterine spiral arteries10,11,14. Consequently, the placental villous trees are bathed in maternal blood, delivering nutrients and oxygen to the fetus. This maternal blood flows through the intervillous space before returning to the maternal circulation via uteroplacental veins. During its passage through the intervillous space, diffusion and active uptake of oxygen and nutrients into fetal blood lead to lower oxygen and nutrient levels in maternal blood12,15. However, the intervillous space's blood is entirely replaced by fresh, oxygen-rich blood approximately two to three times per minute, ensuring a continuous supply of nutrients and gases13. Notably, the syncytiotrophoblast, the outermost part of the placental barrier, is the sole component of the placental villous tree directly exposed to maternal blood15,16,17. Consequently, the syncytiotrophoblast experiences a constant mild shear stress from the flowing maternal blood3,14.
Current scientific knowledge regarding the placental flow environment and modern technical advancements now permit an adapted and physiologically approximated cultivation of placental explants under flow conditions. Furthermore, evidence suggests that shear forces influence the biological functions of the syncytiotrophoblast18,19,20,21. A well-known approach that accounts for blood flow is the placental dual lobe perfusion system22. However, these experiments require significant expertise, are time-restricted (conducted for only a few hours), and are feasible only with third-trimester placental samples3,23. In contrast, we have developed a straightforward and non-intrusive technique for ex vivo placental villous explant culture under constant flow settings, accommodating both first and third-trimester placental tissues3. In this setup, placental explants are cultivated in five series-connected flow chambers. Villous explants are secured to the chamber's bottom using needle-shaped elevations on thin metal plates. The constructed flow circuit is subsequently transferred into a bioreactor, where both oxygen concentration and flow rate are regulated3. Flow culture results demonstrate that tissue integrity is better preserved compared to the typically used static method3. Furthermore, this dynamic approach enables novel and adapted experimental designs for tissue explant culturing, allowing in vitro experiments that more closely mimic the natural environment3.
Protocol
The ethics committee of the Medical University of Graz approved this study (31-019 ex 18/19 version 1.2 and 29-319 ex 16/17). Informed consent was obtained from all subjects involved in the study.
1. Preparation for the flow experiment
NOTE: The experiments are conducted in a bioreactor with integrated peristaltic pumps (see Table of Materials). The humidity, temperature and gas level inside the bioreactor can be adjusted.
- Turn on the bioreactor and make all prearrangements (e.g., calibration of the pumps, pre-warming, gas conditions and humidity) for the experiment according to the manual of the bioreactor. Before starting the experiment, the required settings (temperature, gas content, humidity) should be stabilized for a few hours or overnight. To do this, start the bioreactor and the software and then click on Change SetPoints under the menu item "Incubator".
- Pre-warm PBS and the required medium (Endothelial Cell Growth Medium supplemented with provided supplements hEGF-5, HC-500 as well as 5% exosome-depleted fetal bovine serum, 1% penicillin/streptomycin) (see Table of Materials) to 37 °C.
2. Placental sample dissection
- Immediately after delivery, cut three times 2 cm3 placental samples from the mid-placental region as described in Kupper et al.3 Briefly, keep the samples in PBS. Discard the chorionic plate, the maternal decidua and areas of visible infarcts from the specimen.
- Dissect the remaining tissue specimen into villous explants with a cross-sectional diameter of about 0.5 cm (wet weight of about 7.5 mg). Transfer them into a Petri dish with fresh PBS.
- Wash the explants in PBS by gently shaking them in the liquid with tweezers to remove blood residues.
NOTE: Dissect the samples in a Petri dish with PBS to pre-wash and prevent them from drying out and use sterilized/autoclaved tools for processing the tissue.
3. Flow experiment
- Under a sterile hood, connect five chambers in series to the reservoir bottle using the luer locks according to the user manual of the flow chambers (see Table of Materials).
NOTE: Sterilize and/or autoclave all materials before use, according to the respective manual. Use an air filter on the reservoir bottle for sterile gas exchange. To open and close the chambers, gently squeeze in the lugs of the chambers. Step 3.1. may also be prepared earlier. - Turn the chambers upside down and open them by removing the bottom. Use forceps to transfer the metal plates centrally in the top part of the chambers with the pins pointing upwards.
- Fill the chambers with 1 mL of pre-warmed medium (37 °C). Then fill the reservoir with an additional 20 mL. The circuit requires a total of 25 mL, including the volume in each flow chamber, in the tubes, and reservoir bottle. Under the flow, the final volume of the medium in a filled chamber is 2 mL.
- Use forceps and transfer one villous explant after the other between the needles of the metal plate in the chamber. Let the needles slide between the placental villi to avoid puncture of the tissue. Transfer four explants into one chamber. Close the chambers by refitting the bottom carefully. A complete circuit contains a total of 20 explants. The chambers need to remain in an upside-down position.
NOTE: Grasp the explants gently with the forceps; try not to squeeze them. Ensure the chambers and the circuit are completely sealed to prevent leakage. The chambers are always used upside down. The number of explants per chamber and the number of chambers themselves are variable. The procedure for first-trimester tissue is similar to that for third-trimester tissue with a small addition: to fix the villi, bend the needles slightly over the explants after the tissue has been transferred to the metal plate (personal communication Brugger et al.). This fixes the fragile tissue on the metal plate and prevents the samples from slipping off. - Transfer the flow assembly into the bioreactor.
- Connect the flow circuit to the peristaltic pump within the bioreactor by connecting the pump tubing to the pump. Fix it on the 4th stage (one will hear it click four times).
- If a static control is required, then also place the well plate into the bioreactor.
NOTE: For static culture, five wells of a six-well plate are filled with 4 mL of medium per well and 4 villous explants per well. The filled well plate is also placed in the bioreactor and cultured in the same atmosphere as the flow culture explants. Further details are described in Kupper et al.3 - Set the pump mode to Manual under the menu item "Pumps". Then set the pump speed to 1 mL/min and start pumping the medium into the tubing by clicking on Run. While the circuit is being filled with medium, hold the chambers at an angle so that they are completely filled with medium.
NOTE: See Supplementary Table 1 for the experimental settings for placental villous flow and static culture. Specifications of the flow and static system are provided in Supplementary Table 2.
CAUTION: Carefully tilt the chamber during filling to prevent the specimens from slipping off the needles. - After the fill-up is completed, the chambers remain in their upside-down position. Ensure the chambers are standing securely and upright, and close both lids of the bioreactor.
NOTE: The final volume of the medium in a filled flow chamber is 2 mL. The experimental settings and the specifications of the flow chambers and well plates used in the experiments are described in Kupper et al.3 - Incubate the tissue for the desired time.
NOTE: The temperature, gas level and flow rate can be monitored on the computer without opening the lid of the bioreactor again. - Stop the pump after incubation of the tissue for the desired time by clicking on Abort under the menu item "Pumps". Open the two lids of the bioreactor and then one flow chamber at a time. Carefully remove the explants from the metal plate using forceps.
- Process the tissue and supernatant according to the selected downstream analysis. In this case, immunohistochemical staining and electron microscopy were performed3. See Supplementary Table 3 for the details of the antibodies used for immunohistochemistry and immunofluorescence.
NOTE: After removing the tissue, drain the medium from the circuit by counterclockwise rotation of the pump. - Disassemble and clean the flow circuit according to the manufacturer's instructions for the flow chambers and tubing.
Representative Results
Parts of this publication and its results have already been published (See references 3 and 23).
Experimental setup
An experimental setup is illustrated in Figure 1. A composite flow cycle comprises five flow chambers that are interconnected in series (Figure 1A). Within each flow chamber, four explants, each with a cross-sectional diameter of approximately 0.5 cm, are cultivated (Figure 1 A,C). For the static control experiment, the explants are cultivated in individual wells of a six-well plate (Figure 1B). To prevent the explants from being flushed out, they are affixed onto metal plates featuring narrow needle-shaped protrusions (Figure 1 D,E). In order to subject the explants to a direct flow of the medium, the chambers are inverted, with the inlets and outlets positioned at the head section (Figure 1 F,G). Within the bioreactor, the flow cycle is linked to a peristaltic pump. For the purpose of comparing tissue integrity between flow-cultured tissue and conventionally static-cultured tissue, explants are placed in a six-well plate adjacent to the flow cycle. This ensures the verification of consistent culture conditions in terms of oxygen, temperature, and humidity (Figure 1A)3.
Morphological analysis
β-actin
Various immunohistochemical staining procedures were conducted to examine histological distinctions in tissue integrity associated with diverse cultivation conditions (Figure 2). Explants that were promptly embedded post-dissection served as the baseline reference. For the analysis of the actin cytoskeleton within villous explants, β-actin staining was executed (Figure 2A–E). Descriptive analysis unveiled a well-structured and organized visual presentation of the cytoskeleton in freshly obtained tissue (Figure 2A). Over time, as cultivation progressed, there was an observable aggregation of microfilaments, signifying a degradation of the cytoskeletal structure. This phenomenon was consistently observed in villous explants that underwent static cultivation3 (Figure 2C,E, indicated by asterisks).
H&E staining
H&E staining provided additional reinforcement to the observation that tissue integrity diminishes over the course of static culture, a trend that is ameliorated in the context of flow culture (Figure 2F–J). Fresh tissue exhibited a structured and characteristic histological presentation of the villous explants, characterized by a dense and tightly packed stroma (Figure 2F). Additionally, the syncytiotrophoblast was firmly adhered to the underlying stroma (Figure 2F). A comparable appearance was noted in villous explants cultured in a flow environment for 24 h (Figure 2G). However, after 48 h of cultivation under the flow, portions of the syncytiotrophoblast were observed to be partially detached (Figure 2I, indicated by arrow), accompanied by sporadic small lacunae within the stroma. Histological scrutiny of the tissue indicated that the integrity of the tissue following 24 h in a static culture condition was inadequately preserved (Figure 2H). Furthermore, this integrity further degraded markedly after 48 h in static culture (Figure 2J). The stroma exhibited a porous and pitted appearance, and significant detachment of the syncytiotrophoblast from the stroma was evident in larger regions (Figure 2J, arrows)3.
CD34II
CD34II staining was employed to visualize endothelial cells and, consequently, the feto-placental blood vessels within the villous explants (Figure 2K–O). Tissue that was directly embedded right after dissection displayed a distinctively organized arrangement of the endothelial cells (Figure 2K). The morphological integrity of the feto-placental blood vessels remained well maintained after 24 h of flow culture and frequently even after 48 h, although occasional instances of collapsed blood vessels were noted under flow conditions (Figure 2 L,N). However, following 24 h of static culture, the blood vessels exhibited partial collapse, as evidenced by their disrupted visual appearance (Figure 2M, indicated by arrowheads). This deterioration of blood vessels within the static culture setting appeared to exacerbate with prolonged cultivation time. In summary, the descriptive morphological assessment of the villous explants subsequent to both flow and static culture indicated that tissue integrity appears to be more effectively preserved within the flow system when contrasted with the static culture mode3.
Ultrastructural analysis of the cultivated tissue
Transmission electron microscopy
To conduct a more detailed examination of the morphology of the villous explants, additional ultrastructural analyses were performed using transmission electron microscopy (TEM) (Figure 3A–E). These findings corroborated the outcomes of the histological investigations. In tissue that was directly embedded immediately following preparation, the morphology was exceptionally well preserved (Figure 3A). Microvilli were clearly discernible on the surface of the syncytiotrophoblast. The syncytiotrophoblast presented its distinctive continuous layer without lateral cell boundaries, establishing direct contact with the basement membrane. The stroma of the fresh tissue exhibited dense packing without significant perforations or ruptures. Moreover, the ultrastructural appearance of the blood vessels and individualized intravascular erythrocytes also demonstrated excellent preservation (Figure 3A).
Even after 24 h of flow culture, the overall morphology of the tissue samples remained relatively well maintained (Figure 3D). While there were slightly fewer microvilli on the surface of the syncytiotrophoblast compared to fresh tissue, the syncytiotrophoblast remained primarily attached to the basal membrane. Nuclei and occasional small vacuoles were observable within the inner portion of the syncytiotrophoblast. The stroma within the placental villi appeared well-preserved and closely resembled fresh tissue (Figure 3D). Even following 48 h of flow culture, the stromal cells exhibited relatively good preservation, albeit with some perforations present (Figure 3E). Intriguingly, lipid droplets were detected within the tissue. While the syncytiotrophoblast displayed vacuoles and a reduction in the number of microvilli, it remained attached to the basal membrane in numerous regions, and syncytial and cell nuclei were clearly visible (Figure 3E).
In stark contrast to the tissue from the flow culture, the morphology of villous tissue subjected to static culture exhibited deterioration as early as 24 h (Figure 3B). The syncytiotrophoblast became dissociated from the basal membrane at multiple sites and displayed relatively substantial perforations. Additionally, lipid droplets were frequently evident in both the syncytiotrophoblast and the stroma (Figure 3B). Following 48 h of static culture, a progressive decline in ultrastructure was apparent (Figure 3C). The syncytiotrophoblast presented numerous perforations and detachment from the basal membrane to a considerable extent. Identifying cells within the stroma, as well as endothelial cells comprising the blood vessels, became challenging. Furthermore, there was a notable accumulation of lipid droplets within the villous explants after 48 h of static culture (Figure 3C). In summary, the ultrastructure of tissue in static culture exhibited successive deterioration over the duration of the cultivation period, a trend that was mitigated by cultivation under flow conditions3.
Scanning electron microscopy
Utilizing scanning electron microscopy (SEM), a detailed examination of the surface of the villous explants was facilitated (Figure 4A–J). Tissue that had been freshly embedded exhibited a densely populated array of microvilli across its surface (Figure 4 A,B). Some regions exhibited vesicle-like structures. In contrast, tissue from the static culture manifested a substantial reduction in microvilli after 24 h (Figure 4C,D), a reduction that persisted after 48 h (Figure 4E,F). While certain areas showed aggregation of vesicle-like structures that had not been released, other regions appeared bare and eroded (Figure 4D,F). In tissue subjected to flow culture, microvilli were still present on the surface after 24 h (Figure 4G,H), as well as after 48 h (Figure 4I,J), although to a lesser extent than in fresh tissue. In comparison to the static culture, the prevalence of vesicle-like structures on the surface was diminished. Intriguingly, these vesicle-like structures were notably concentrated in specific recesses where flow could be reduced or absent (Figure 4H,J), suggesting that they might have been dislodged from the flow-exposed tissue surface due to the flow of the medium3.
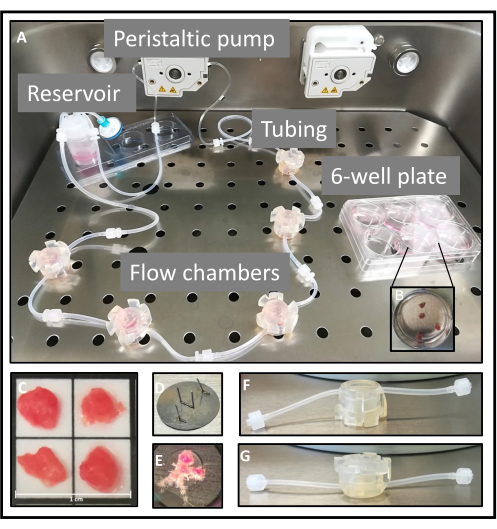
Figure 1: Set up of the flow system. (A) The assembled flow system, consisting of the reservoir and five flow chambers, is connected to one of the peristaltic pumps. On the right side is a six-well plate in which the explants are statically cultured. (B,C) For both cultivation methods, the placental samples are dissected into villous explants of approximately 0.5 cm2, of which four explants are then used per well or chamber. In an experimental approach, five chambers or wells are used. (D,E) For flow culture, a metal plate with narrow needle-shaped elevations is used to secure the explants. (F,G) The openings of the tubes are located at the head of the chambers, and are thus used upside down to guarantee that the tissue is exposed to direct flow. This figure is reproduced from Kupper et al.3. Please click here to view a larger version of this figure.
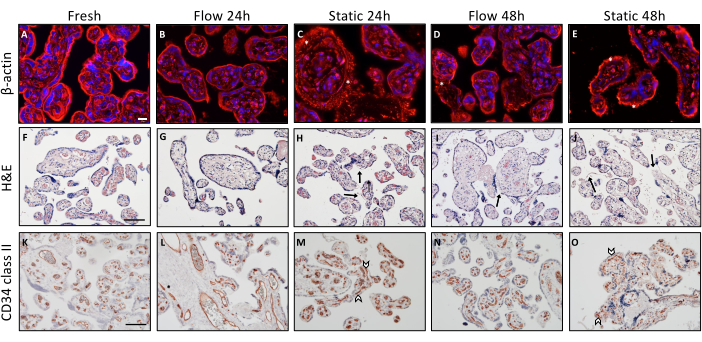
Figure 2: Morphological analysis of placental villous explants upon flow and static culture. (A–E) Immunofluorescence staining for β-actin to visualize the cytoskeleton of explants upon culture. For the analysis, six randomly selected spots were used per slide. Representative images are shown. (A) Visualization of the cytoskeleton of tissue embedded directly after preparation. Scale bar: 20 µm. (B–E) Representative depiction of both time-dependent and cultivation mode-dependent degeneration of the actin cytoskeleton in cultured explants of flow and static culture. (C–E) Asterisks signify increased actin microfilament accumulation, which is an indication of actin cytoskeleton degradation. (F–J) Hematoxylin-eosin staining of villous explants. Scale bar: 100 µm. (F,G) Freshly embedded tissue (F) and flow culture explants for 24 h (G) show a well-preserved morphology of a villous explant. (I) Flow-cultured explants for 48 h show intermittently detached areas of the syncytiotrophoblast (arrow). (H,J) Time-dependent deterioration of structural integrity after static explant culture, indicated by dislodgment of the syncytiotrophoblast (arrow) and perforated stroma. (K–O) CD34 II was used to stain villous endothelial cells. Scale bar: 100 µm. (K,L) Fresh tissue (K) and explants cultured for 24 h under flow conditions (L) exhibit a characteristic structurally aligned endothelial cell pattern. (N) After 48 h in flow culture, the vascular integrity decreases to some extent. (M,O) In static culture, collapsed blood vessels are already visible after 24 h (M), which was observed to increase with longer static cultivation time (O). This figure is reproduced from Kupper et al.3. Please click here to view a larger version of this figure.
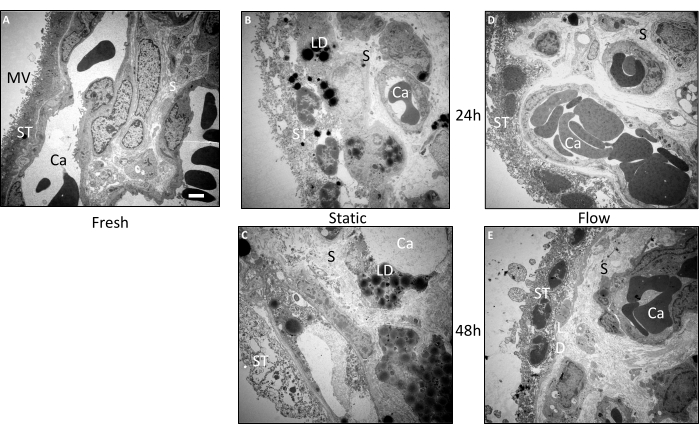
Figure 3: Ultrastructural pre- and post-cultivation examination of villous explants using transmission electron microscopy. Tissue from three independent experiments was used to analyze the images. (A) A representative image of freshly embedded tissue shows a large amount of microvilli (MV) on the surface of the syncytiotrophoblast (ST). Structurally intact capillaries (Ca) are visible in the well-preserved stroma (S). (B) In the tissue that has been statically cultured for 24 h, there is a deterioration in the structural integrity of the syncytiotrophoblast, which appears to be disconnected from the basal membrane in some areas. There is also a noticeable accumulation of lipid droplets (LD). (C) After 48 h in static culture, severe ultrastructural deterioration is observed. The stroma as well as the syncytiotrophoblast are perforated and a massive accumulation of lipid droplets is evident. Blood vessels could scarcely be traced. (D,E) The ultrastructure of the tissue from the flow culture was relatively well preserved after 24 h (D) as well as after 48 h (E). Scale bar: 2 µm. MV: Microvilli, ST: Syncytiotrophoblast, S: Stroma, Ca: Capillary, LD: Lipid droplets. This figure is reproduced from Kupper et al.3. Please click here to view a larger version of this figure.
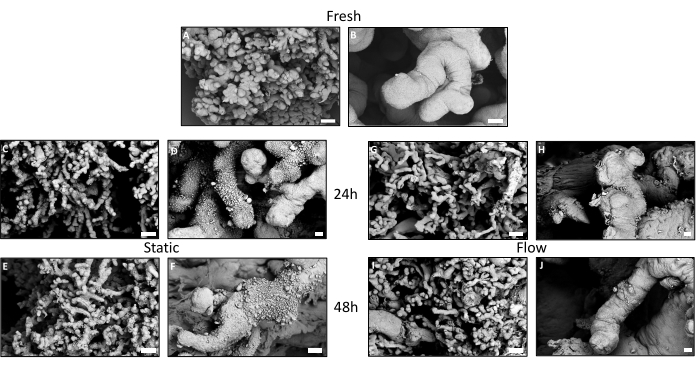
Figure 4: Ultrastructural pre- and post-cultivation examination of villous explants using scanning electron microscopy. (A,C,E,G,I) Overview images of the surface of the placental villous trees with respective detailed images (B,D,F,H,J). (A,B) Freshly embedded tissue exhibits a dense seam of microvilli. (B) Vesicular-like structures can be noticed in some locations. (C–F) After 24 h and 48 h in static culture, a decrease in microvilli on the surface of the syncytiotrophoblast is visible. Striking is the extensive accumulation of vesicular-like particles on the surface of the explant. (F) The particles appear to wither after 48 h in static culture. (G–J) The surface of the tissue from the flow culture seems to be better preserved after 24 h (G,H) as well as after 48 h (I,J) compared to the static culture. Microvilli are visible on the surface (H,J), although not in the same high density as in the fresh tissue. (B) Vesicular particles can be seen scattered in the niches with reduced flow. This figure is reproduced from Kupper et al.3. Please click here to view a larger version of this figure.
Supplementary Table 1: Experimental settings for placental villous flow and static culture. Please click here to download this File.
Supplementary Table 2: Specifications of the flow and static system. Please click here to download this File.
Supplementary Table 3: Antibodies for immunohistochemistry and immunofluorescence used for this study. Please click here to download this File.
Discussion
This study introduces a unique perspective on a flow culture technique for placental explants designed to replicate the dynamic in utero environment3,23. The findings reveal that the morphology of tissue cultured under flow conditions is better preserved compared to the traditional static cultivation method3. Notably, even though neither static nor flow culture conditions facilitate perfusion of placental vessels, the destruction of feto-placental blood vessels inside the villous stroma was predominantly observed in static culture, whereas the integrity of blood vessels appeared to be better maintained over a longer duration in flow culture3.
One possible explanation for this observation could be linked to the crucial protective and endocrine role of the syncytiotrophoblast, a function well-documented in the literature12,24,25,26. Given this, it is conceivable that the overall integrity of the outer layer of the villi significantly contributes to the maintenance of the underlying stroma, including the blood vessels. Consequently, the sustained cellular integrity of the blood vessels under flow conditions might be attributed to the continuous flow of the medium. This movement aids in the passive motion of the explants, facilitating the exchange of gases, nutrients, and nanoparticles (like extracellular vesicles) across the placental barrier. This, in turn, could positively impact the preservation of blood vessel morphology. Moreover, the phenomenon of mechanosensation plays a role in tissue morphogenesis across various tissues27,28. Studies have demonstrated that mechanosensitivity influences cellular processes at multiple levels, triggering a range of biochemical responses that ultimately influence tissue and organ functionality29. Notably, mechanosensitive proteins are expressed by the syncytiotrophoblast throughout gestation28. Furthermore, the study suggests that microvilli on the tissue surface may be implicated in this context28.
An additional perspective worth considering is the potential role of mitochondria in the cellular response to flow. For instance, in endothelial cells, mitochondria serve as signal transducers for cellular responses to environmental stimuli30. Increased accumulation of lipid droplets, observed in static cultured tissue through TEM3, has been associated with apoptosis induction due to mitochondrial dysfunction31. Further investigations are necessary to unveil the underlying mechanisms and key factors, linking them to downstream signaling pathways. This exploration could enhance our understanding of how the tissue perceives and reacts to shear stress, translating into improved viability and integrity of villous explants in culture23.
Several critical protocol steps must be reiterated and executed with care. After placental delivery, tissue should be cultured as swiftly as possible. During explant preparation, avoiding areas with visible infarcts is crucial. Gently handling explants with forceps to prevent squeezing is important. Keeping the tissue covered with liquid throughout the procedure and conducting it rapidly is recommended.
It's important to acknowledge that this study is unable to specify the exact shear stress within the presented flow system, which should be considered as a limitation in future investigations3,23. Nevertheless, it's important to recognize that precise flow velocity and shear stress for a specific placental villus in vivo are influenced by numerous parameters, such as the geometrical characteristics of the intervillous space, the villus's location within this space, and its proximity and angle to maternal spiral arteries and uterine veins3,19,23,32. The complexity of the placenta's geometric structure, which varies among individuals, must be taken into account as well23,32. Mathematical models estimating blood flow within the intervillous space32 and calculations on wall shear stress on the syncytiotrophoblast19,28already exist. Interestingly, one study predicted that shear stress on the syncytiotrophoblast is lower in the third trimester compared to the first trimester28, while another demonstrated spatially heterogeneous wall shear stress on the syncytiotrophoblast19. Determining the precise flow velocity and shear stress for a specific placental villus remains a challenge3,19,23,32. Such calculations offer an approximation of the shear stress range for future investigations, but they may require ongoing anatomical adjustments and optimization23. Moreover, future studies may develop new and refined flow-through culture techniques that account for the intricate geometry of the intervillous space and strategies to increase the number of specimens per experiment3. Ongoing progress and development of the flow system are anticipated, potentially employing alternative flow chambers (Brugger et al., unpublished data, 2023).
In conclusion, this study lays a robust foundation by demonstrating an easily implementable ex vivo flow culture technique that upholds the structural integrity of cultured villous explants. It underscores the significance of dynamic techniques in placental functional biology studies, paving the way for further advancements in flow culture systems and the generation of new ideas and hypotheses3,23.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors gratefully appreciate the excellent technical support of Bettina Amtmann and Petra Winkler for tissue sampling. This research was funded by the Austrian Science Fund FWF (DOC 31-B26) and the Medical University of Graz, Austria, through the PhD Program Inflammatory Disorders in Pregnancy (DP-iDP).
Materials
| 6-well plates | NUNC, ThermoFisher Scientific, Waltham, MA, USA | 140675 | |
| Alexa Fluor 555 goat-anti-mouse | ThermoFisher Scientific, Waltham, MA, USA | A21422 | Diluted in PBS, 1:200 |
| antibody diluent | Dako, Santa Clara, CA, USA | S3022 | |
| anti-β-actin (AC-15) | Abcam, Cambridge, UK | ab6276 | Stock concentration: 2.1 mg/mL, diluted in antibody diluent, 1:10,000 |
| Bioreactor TEB500 | TEB500, EBERS Medical Technology SL, Zaragoza, Spain | Serial Number: TEB505 / 1000EW/ 117 | |
| CD34 Class II (QBEnd-10) | Dako, Santa Clara, CA, USA | M7165 | Stock concentration: 12 mg/l, diluted in antibody diluent, 1:500 |
| CPD 030 critically point dryer | Bal-Tec, Balzers, Liechtenstein) | Critically point dryer | |
| DAPI | ThermoFisher Scientific, Waltham, MA, USA | D21490 | Diluted in PBS, 1:1000 |
| Ebers TEB505 Series Software | TEB500, EBERS Medical Technology SL, Zaragoza, Spain | Series Software 1.4 | |
| Endothelial Cell Growth Medium MV | PromoCell PC-C-22120, Heidelberg, Germany; | C-22120 | Used without EGCS/h and FCS, any other medium suitable for the tissue can be used |
| Excelsior AS Tissue Processor | ThermoFisher Scientific, Waltham, MA, USA | ||
| Exosome-depleted fetal bovine serum | Gibco by Life Technologies, ThermoFisher Scientific, Waltham, MA, USA | A2720803 | |
| Histolab Clear | Histolab, Askim, Sweden | 14250-TY | |
| Hydrogen Peroxide Block | ThermoFisher Scientific, Waltham, MA, USA | TA125H202Q | |
| Kaiser’s Glycerin Gelatine | Merck, Darmstadt, Germany | 1092420100 | |
| Leica DM 6000 B microscope | Leica, Wetzlar, Germany | Equipped with an Olympus DP 72 Camera | |
| Leica UC7 ultramicrotome | Leica Microsystems, Vienna, Austria) | ||
| Metal plate with needles | In-house construction | ||
| Microtome | Microtome Microm HM 355 S, ThermoFisher Scientific, Waltham, MA, USA | ||
| Microwave oven | Miele, Guetersloh, Germany | ||
| Olympus microscope (BX63) | Olympus, Hamburg, Germany | Serial Number: 1A52421 | |
| PBS | ThermoFisher Scientific, Waltham, MA, USA | 10010015 | |
| Penicillin/Streptomycin | Gibco by Life Technologies, ThermoFisher Scientific, Waltham, MA, USA | 2585627 | |
| Primary antibody enhancer | ThermoFisher Scientific, Waltham, MA, USA | TL-125-PB | |
| ProLong Gold Antifade Reagent | ThermoFisher Scientific, Waltham, MA, USA | P36934 | |
| Pumping tube | Tygon, Bartelt, Graz, Austria | 6.078 175 | 1.02 mm diameter |
| QV500 Flow chambers | Kirkstall Ltd., Quasi Vivo, North Yorkshire, UK | QV500 | Other chambers would work as well |
| SCD 500, sputter coater | Bal-Tec, Balzers, Liechtenstein | Sputter coater | |
| Substrate amino-ethyl carbazole, AEC substrate kit | Abcam, Cambridge, UK | ab64252 | |
| Superfrost Plus slides | Menzel-Glaeser, Braunschweig, Germany | J1800AMNZ | |
| Syringe Filter | Corning Incorporated, NY, USA | 431219 | 0.2 µm Pore SFCA Membrane, air filter for the reservoir bottle |
| TAAB epoxy resin | Agar Scientific, Stansted, Essex, UK | T001 | |
| UltraVision LP-Detection System HRP-Polymer | ThermoFisher Scientific, Waltham, MA, USA | TL-125-HL | |
| UltraVision Protein Block | ThermoFisher Scientific, Waltham, MA, USA | TA125BPQ | |
| Zeiss EM 900 transmission electron microscope | Zeiss, Oberkochen, Germany | ||
| Zeiss Sigma 500 field emission scanning electron microscope | Zeiss, Cambridge, UK | Used with a back-scattered electron detector at 5 kV acceleration voltage |
References
- Villee, C. A. The metabolism of human placenta in vitro. Journal of Biological Chemistry. 205 (1), 113-123 (1953).
- Miller, R. K., et al. Human placental explants in culture: Approaches and assessments. Placenta. 26 (6), 439-448 (2005).
- Kupper, N., Pritz, E., Siwetz, M., Guettler, J., Huppertz, B. Placental villous explant culture 2.0: flow culture allows studies closer to the in vivo situation. International Journal of Molecular Sciences. 22 (14), 7464 (2021).
- Reti, N. G., et al. Effect of high oxygen on placental function in short-term explant cultures. Cell and Tissue Research. 328 (3), 607-616 (2007).
- Simán, C. M., Sibley, C. P., Jones, C. J. P., Turner, M. A., Greenwood, S. L. The functional regeneration of syncytiotrophoblast in cultured explants of term placenta. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 280 (4), R1116-R1122 (2001).
- Toro, A. R., et al. Leptin is an anti-apoptotic effector in placental cells involving p53 downregulation. PLoS ONE. 9 (6), e99187 (2014).
- Morley, L. C., Debant, M., Walker, J. J., Beech, D. J., Simpson, N. A. B. Placental blood flow sensing and regulation in fetal growth restriction. Placenta. 113, 23-28 (2021).
- Wang, Y. Z. S., Wang, Y., Zhao, S. Placental blood circulation. Vascular biology of the placenta. Chapter 2, (2010).
- Huppertz, B. The anatomy of the normal placenta. Journal of Clinical Pathology. 61 (12), 1296-1302 (2008).
- Weiss, G., Sundl, M., Glasner, A., Huppertz, B., Moser, G. The trophoblast plug during early pregnancy: a deeper insight. Histochemistry and Cell Biology. 146 (6), 749-756 (2016).
- Burton, G. J., Woods, A. W., Jauniaux, E., Kingdom, J. C. P. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 30 (6), 473-482 (2009).
- Gude, N. M., Roberts, C. T., Kalionis, B., King, R. G. Growth and function of the normal human placenta. Thrombosis Research. 114 (5-6), 397-407 (2004).
- Wang, Y. Vascular biology of the placenta. Colloquium Series on Integrated Systems Physiology: From Molecule to Function. 2 (1), 1-98 (2010).
- Moser, G., Windsperger, K., Pollheimer, J., de Sousa Lopes, S. C., Huppertz, B. Human trophoblast invasion: new and unexpected routes and functions. Histochemistry and Cell Biology. 150 (4), 361-370 (2018).
- Kupper, N., Huppertz, B. The endogenous exposome of the pregnant mother: Placental extracellular vesicles and their effect on the maternal system. Molecular Aspects of Medicine. 87 (October 2020), 100955 (2022).
- Huppertz, B. IFPA award in placentology lecture: biology of the placental syncytiotrophoblast – myths and facts. Placenta. 31 (SUPPL), S75-S81 (2010).
- Gauster, M., Moser, G., Wernitznig, S., Kupper, N., Huppertz, B. Early human trophoblast development: from morphology to function. Cellular and Molecular Life Sciences. 79 (6), 345 (2022).
- Lecarpentier, E., et al. Fluid shear stress promotes placental growth factor upregulation in human syncytiotrophoblast through the cAMP-pKA signaling pathway. Hypertension. 68 (6), 1438-1446 (2016).
- Lecarpentier, E., et al. Computational fluid dynamic simulations of maternal circulation: wall shear stress in the human placenta and its biological implications. PLOS ONE. 11 (1), e0147262 (2016).
- Miura, S., Sato, K., Kato-Negishi, M., Teshima, T., Takeuchi, S. Fluid shear triggers microvilli formation via mechanosensitive activation of TRPV6. Nature Communications. 6 (1), 8871 (2015).
- Jauniaux, E., et al. Onset of maternal arterial blood flow and placental oxidative stress. The American Journal of Pathology. 157 (6), 2111-2122 (2000).
- Sodha, R. J., Proegler, M., Schneider, H. Transfer and metabolism of norepinephrine studied from maternal-to-fetal and fetal-to-maternal sides in the in vitro perfused human placental lobe. American Journal of Obstetrics and Gynecology. 148 (4), 474-481 (1984).
- Kupper, N. . Extracellular vesicles from advanced placental explant flow culture and their role in preeclampsia [Dissertation]. , (2022).
- Burton, G. J., Fowden, A. L. The placenta: a multifaceted, transient organ. Philosophical Transactions of the Royal Society B: Biological Sciences. 370 (1663), 20140066 (2015).
- Arora, N., Sadovsky, Y., Dermody, T. S., Coyne, C. B. Microbial vertical transmission during human pregnancy. Cell Host & Microbe. 21 (5), 561-567 (2017).
- Cheong, M. L., et al. A Positive feedback loop between glial cells missing 1 and human chorionic gonadotropin (hCG) regulates placental hCGβ expression and cell differentiation. Molecular and Cellular Biology. 36 (1), 197-209 (2016).
- Heisenberg, C. P., Bellaïche, Y. Forces in tissue morphogenesis and patterning. Cell. 153 (5), 948-962 (2013).
- Lee, T. C., Moulvi, A., James, J. L., Clark, A. R. Multi-scale modelling of shear stress on the syncytiotrophoblast: could maternal blood flow impact placental function across gestation. Annals of Biomedical Engineering. 51 (6), 1256-1269 (2023).
- Kluge, M. A., Fetterman, J. L., Vita, J. A. Mitochondria and endothelial function. Circulation Research. 112 (8), 1171-1188 (2013).
- Boren, J., Brindle, K. M. Apoptosis-induced mitochondrial dysfunction causes cytoplasmic lipid droplet formation. Cell Death & Differentiation. 19 (9), 1561-1570 (2012).
- Chernyavsky, I. L., Jensen, O. E., Leach, L. A Mathematical model of intervillous blood flow in the human placentone. Placenta. 31 (1), 44-52 (2010).

