Real-Time Imaging of Acrosomal Calcium Dynamics and Exocytosis in Live Mouse Sperm
Summary
The AcroSensE mouse model and live cell imaging methods described here provide a new approach to studying calcium dynamics in the subcellular compartment of the sperm acrosome and how they regulate intermediate steps leading to membrane fusion and acrosome exocytosis.
Abstract
Acrosome exocytosis (AE), in which the sperm's single exocytotic vesicle fuses with the plasma membrane, is a complex, calcium-dependent process essential for fertilization. However, our understanding of how calcium signaling regulates AE is still incomplete. In particular, the interplay between intra-acrosomal calcium dynamics and the intermediate steps leading to AE is not well-defined. Here, we describe a method that provides spatial and temporal insights into acrosomal calcium dynamics and their relationship to membrane fusion and subsequent exocytosis of the acrosome vesicle. The method utilizes a novel transgenic mouse expressing an Acrosome-targeted Sensor for Exocytosis (AcroSensE). The sensor combines a genetically encoded calcium indicator (GCaMP) fused with mCherry. This fusion protein was specifically designed to enable the concurrent observation of acrosomal calcium dynamics and membrane fusion events. Real-time monitoring of acrosomal calcium dynamics and AE in live AcroSensE sperm is achieved using a combination of high frame-rate imaging and a stimulant delivery system that can target single sperm. This protocol also provides several examples of basic methods to quantify and analyze the raw data. Because the AcroSensE model is genetically encoded, its scientific significance can be augmented by using readily available genetic tools, such as crossbreeding with other mouse genetic models or gene-editing (CRISPR) based methods. With this strategy, the roles of additional signaling pathways in sperm capacitation and fertilization can be resolved. In summary, the method described here provides a convenient and effective tool to study calcium dynamics in a specific subcellular compartment-the sperm acrosome-and how those dynamics regulate the intermediate steps leading to membrane fusion and acrosome exocytosis.
Introduction
Sperm acquire the ability to fertilize during a process called capacitation1. One endpoint of this process is that the sperm acquire the ability to undergo AE. Over two decades of data support the presence of a complex, multi-step model of AE in mammalian sperm (summarized in2,3). However, studying AE in live sperm is challenging, and currently available methods to monitor this process with adequate resolution are cumbersome and require multiple preparation steps4, are limited to the detection of the final step of AE (e.g., using PNA5), are limited to measurements of changes in cytosolic calcium (in contrast to acrosomal calcium dynamics), or are limited to measurements of either cytosolic calcium dynamics or AE6.
To overcome some of the key limitations of real-time AE studies under physiological conditions and to enable investigation of the interplay between calcium dynamics and AE, a unique mouse model was generated. In this mouse model, a fusion protein composed of the genetically encoded Ca2+-sensor (GCaMP3) and mCherry is expressed and targeted to the acrosome using an acrosin promoter and signaling peptide2. The targeted dual GCaMP3-mCherry sensor enables simultaneous real-time measurements of calcium concentrations and the status of the acrosomal contents in live sperm under physiological conditions using microscopy and a single-cell stimulant delivery system (Figure 1). As a component of the acrosomal matrix, membrane fusion, and AE would result in the loss of the photostable and pH-insensitive mCherry fluorescence from the sperm, as this protein diffuses out of the acrosome vesicle. In this regard, the model's ability to reflect the timing and occurrence of AE is akin to the benefits of the acrosome-targeted GFP mouse line7,8,9.
The GCaMP3 variant used in this transgenic mouse line has an approximate KD of 400 µM and a dynamic range for Ca2+ of 10-4-10-3 M10, which is suitable for this vesicle. We showed that this combination of features of GCaMP3 could reveal fusion pore formation between the plasma membrane and the outer acrosomal membrane (OAM)2. The fusion pore detection is a result of the pore size being too small to allow the AcroSensE protein to disperse out of the acrosome (via loss of acrosome content) while providing a membrane "channel" that enables the influx of Ca2+ ions into the acrosome lumen, leading to an increase in fluorescence intensity of the GCaMP3.
The bright, monomeric, non-calcium-sensitive fluorescent protein mCherry supports visualization of the acrosome while the GCaMP3 signal is faint (e.g., prior to Ca2+ binding, Figure 2), and importantly, it also allows for the identification of acrosome-intact sperm cells suitable for imaging.
The following protocol describes the utilization of the unique AcroSensE mouse model and the methods for microscopy used experimentally to study AE and sperm calcium dynamics with high spatial and temporal resolution.
Protocol
All animal procedures were performed under the guidelines and approved by the Institutional Animal Care and Use Committee at Cornell University (#2002-0095). 8-10 weeks old AcroSensE mice2 were used for the present study. Requests for information on the availability of the AcroSensE mice can be submitted to the corresponding author.
1. Sperm collection and washing
- Collect cauda epididymal sperm (more details on this procedure are provided in11) from AcroSensE mice. Allow 15 min swim-out procedure in a 3.5 cm tissue-culture dish containing 0.5 mL of Modified Whitten's medium (MW) at 37 °C. Dish should be covered to reduce evaporation of the MW, and should be tilted so that the medium has enough depth to cover the cauda epididymides.
NOTE: Modified Whitten's medium (MW) includes 22 mM HEPES, 1.2 mM MgCl2, 100 mM NaCl, 4.7 mM KCl, 1 mM pyruvic acid, 4.8 mM lactic acid hemi-Ca2+ salt, pH 7.35. Glucose (5.5 mM), NaHCO3 (10 mM), and 2-hydroxypropyl–cyclodextrin (CD; 3 mM) are supplemented (see Table of Materials) as needed for induction of capacitation, and concentrations can be modified for specific experiments. In addition, alternative sterol acceptors could be used instead of CD (e.g., bovine serum albumin or high density lipoproteins). - Using fine forceps, remove any epididymal tissue from the swim-out collection dish. Transfer the medium containing the sperm into a 15 mL conical tube, and centrifuge the suspension at 100 x g for 1 min in a swinging bucket rotor at room temperature. Using a plastic pipette, remove the MW supernatant containing the sperm from any gross tissue debris that will have pelleted at this step.
- Bring the MW supernatant containing the sperm to a total volume of 3 mL by adding MW at 37 °C. Centrifuge at 400 x g for 8 min (at room temperature) in a round-bottomed tube. Slowly remove the supernatant of MW from the loosely-pelleted sperm.
- If viable, the sperm should appear as a "fluffy"-looking pellet. Gently resuspend the sperm via trituration with a large-bore transfer pipette or via gentle manual taps on the tube ("finger flicking"). Once resuspended evenly, transfer them into a new round-bottom tube. Use 10-20 µL of the sperm suspension to count and assess motility. Dilute sperm in MW for use.
NOTE: For most experiments involving mouse sperm capacitation, a final concentration of 5-10 million sperm/mL MW is utilized. The imaging protocol described here does not require the capacitation of sperm, although it is necessary for sperm to acquire the ability to undergo physiologically-relevant acrosome exocytosis. One could study pathological exocytosis or exocytosis induced through reagents such as a calcium ionophore without capacitating the sperm. Additionally, one could explore potential changes in acrosomal calcium in non-capacitated cells in response to various stimuli. - Use the sperm within 3-5 h from collection, keeping the time as short as possible and consistent among experimental trials.
- Perform all steps of collection and washing at 37 °C using MW medium, employing methods to minimize membrane damage. This includes the use of large-bore plastic transfer pipettes or large-orifice pipette tips, which are recommended for use during all stages of the procedure.
2. Poly-D-lysine-coating of coverslip dishes for imaging
NOTE: Poly-D-lysine (PDL) dishes should be prepared fresh before each experiment.
- Dispense a 0.5 μL droplet of PDL (0.5 mg/mL, see Table of Materials) at the center of a coverslip dish.
- Using a 10 μL graduated tip, smear the PDL droplet across the coverslip (crisscross pattern as shown in Figure 1B).
- Allow to dry at 37 ˚C for 10 min. Keep PDL-coated dishes covered and at 37 ˚C until use.
3. Capillary pulling, loading for puffing
- Pull borosilicate glass capillaries (O.D, 2 mm, I.D, 1.56 mm, see Table of Materials) using settings as follows- Heat: 330, Pull: 250, Velocity: 250, and Time: 70. This step should be optimized for the specific puller in use.
- Before each experiment, load a single capillary with the stimulating solution containing 3-5x the concentration of the stimulant (to account for the rapid diffusion of the solution upon puffing). Before filling, use fine tweezers to break open the capillary tip at approximately 1-2 mm from the end. This should produce a ∼5 µm opening diameter. Heat polishing is not required.
- Using a thin plastic transfer pipette, slowly inject the stimulating solution into the capillary until three-fourths of its length is full.
- Remove any air bubbles formed during filling by gentle flicking of the capillary.
- Mount filled capillary on the micromanipulator (see Table of Materials).
4. Preparation of the delivery of stimulation for puffing
NOTE: To minimize the risk of user injury, safety goggles should be worn during the operation of the puffing procedure.
- Set up the single-cell delivery system settings to provide a 10 s pulse at 5 psi.
- Connect the borosilicate capillary to the single-cell delivery system pipette holder. Ensure seals are tight.
- While observing the capillary tip, apply a short puff of 2 s at 20 psi to ensure that the solution is indeed dispensed through the tip's end (a small drop should be obvious at the end of the tip).
5. Microscopy and image acquisition
NOTE: Multiple brands of microscopes are available and can be used; however, a minimum frame rate of 3 frames/s is desirable. Regarding temperature and environmental control, please note that the actual temperature of the medium in the dish should be confirmed, for example, using a non-contact infrared thermometer.
- Add 80 μL of diluted sperm to the center of a poly-D-lysine-coated 35 mm coverslip dish. For most experiments involving mouse sperm capacitation, a final concentration of 5-10 million sperm/mL is utilized.
- Slowly add to the sperm 3 mL of warm (37 ˚C) MW base media supplemented with 10 mM CaCl2.
NOTE: One could perform experiments at room temperature to slow cellular processes, but it's important to note that because capacitation is mediated by lipid exchanges and the fluidity of micro- and macro-domains, one must keep in mind that physiologically-relevant capacitation and acrosome exocytosis are temperature-dependent processes. - Mount the dish with the sperm on the microscope (pre-heated to 37 ˚C).
- Excite the GCaMP3 using a 488 nm laser line and view with a 505-550 nm bandpass (BP) filter. Excite the mCherry using a 555 nm laser line and view with a 575-615 nm BP filter.
- Using a micromanipulator (it is recommended to attach the manipulator to the air-table on which the microscope was set up.), position the tip of the capillary approximately 100 μm to the side and 5-10 μm above the plane of the cell of interest.
NOTE: The stimulating solutions are made at 3-5x the normal concentration to compensate for the rapid diffusion occurring in the volume between the capillary and the cell. - Start the imaging sequence on the microscope.
- Image sperm cells at a high frame rate, preferably >10 frames/s. Here, a resonant scanner was used that enables imaging at 33 ms/frame.
- Ten seconds after starting the image acquisition on the microscope, activate the single-cell delivery system to initiate the 10 s puff of stimulant.
- Image cells for a duration of 10-15 min.
- Using the single-cell delivery system, image multiple cells from a single dish according to the locations provided in Figure 1B.
NOTE: Untreated/non-stimulated sperm under the microscope should appear mostly red, with a low intensity of the green signal. Sperm heads should be attached to the coverslip, and live sperm can be identified by their moving tails. In sperm that undergo AE, the GCaMP3 green signal will initially increase, and both the mCherry red signal and GCaMP3 green signal will disappear if AE is complete, as illustrated in Figure 2. The reduction of mCherry fluorescence provides a more specific indicator of the time when AE begins because changes in Ca2+ concentrations will continually cause changes in the GCaMP3 fluorescence intensity.
6. Image and data analysis
NOTE: Offline image analysis is conducted using ImageJ (see Table of Materials). Previously, several intermediate steps were reported in the process leading to AE, including acrosomal calcium rise (ACR), and full membrane fusion. The latter leads to the loss of mCherry fluorescence and therefore represents full AE. In some cells, signals akin to pre-spike foot events (PSF) are observed when using amperometric approaches in studies of exocytosis (for more details, please see2). Several optional methods are available for analyzing the AcroSensE raw data to quantify ACR, AE, and its intermediate steps. The following describes some of the basic analytical methods.
- Depending on the specific microscope system file extension, use the split channel tool (Image > Colors > Split Channels) to generate individual windows for the GCaMP3 and mCherry channels.
- Synchronize the individual windows using the "Synchronize Window" tool (Analyze > Tools > Synchronize Windows).
- Select a region of interest (ROI) around the sperm head in the red channel to include the acrosome region (Figure 3A,B). The synchronize tool allows the same area selection to be applied automatically on the green channel, in which the sperm are still too dim to be visible.
- Choose "Z Profiler" plugin (Plugins > Stacks > Z Profiler) or "Time Series Analyzer" (Plugins > Stacks > Time Series Analyzer) to plot the change in fluorescence intensity as a function of frames/time for each of the two channels.
- Copy data to a spreadsheet software (e.g., Microsoft Excel) for plotting and further analysis.
NOTE: Once the data are copied to a spreadsheet, multiple parameters can be extracted for analysis, including the following, as illustrated in Figure 4.- Fusion Pore (FP) start time: measure the time from timepoint 0 to the timepoint where the GCaMP3 signal starts to rise. This parameter indicates the delay between the stimulation and the sperm ACR response.
- AE start time: measure the time from timepoint 0 to the timepoint where the mCherry signal starts to decay. This parameter indicates the delay between the stimulation and the sperm AE response. This parameter could also be used to calculate the delay between FP formation and AE response.
- Peak FP amplitude: measure the fluorescence signal from the base to the peak of the GCaMP3 signal rise. This parameter indicates the total increase in ACR response.
- Rate of loss of mCherry: determine this parameter by a linear or an exponential fit on the section of the mCherry signal loss (as indicated by "slope" in Figure 4B).
NOTE: Alternatively, determine the decay rate by the residual (R) signal method at various time points (e.g., R60: duration time in seconds to a residual signal of 60% from the baseline of the mCherry signal). This parameter can be used to quantify the rate at which the acrosomal content is dispersed upon AE. - Signal decrease for mCherry loss: determine the difference in amplitude between the baseline mCherry fluorescence intensity and the signal following AE. This parameter indicates the total amount of acrosomal content dispersed upon AE.
- Plot the changes in fluorescence (F) for both the red and green channels as raw data, or normalize them by the initial fluorescence (F0) and express them as ΔF/F0. The latter provides better visualization of the changes in both red and green on a single plot (e.g., see Figure 3D and Figure 3H).
NOTE: Finally, the different measured parameters can be compared between different conditions to determine the relationship between changes in acrosomal calcium concentration and AE. For examples of various experimental condition comparisons, see reference2.
Representative Results
Figure 2 provides a simplified illustration showing the sequence of fluorescence changes expected following the successful stimulation of sperm. The top panel of Figure 2 illustrates the changes in GCaMP3 fluorescence intensity, where the signal is initially dim (baseline acrosomal calcium concentrations are lower than GCaMP3 KD), and upon the entry of calcium ions via fusion pores, the fluorescence increases in brightness. Finally, upon AE, there is a loss of signal due to diffusion and the loss of the sensor to the extracellular space. The bottom panel of Figure 2 illustrates the changes in mCherry fluorescence intensity, where the initial signal is bright, and only upon AE and diffusion of the sensor along with other contents of the acrosomal matrix, there is a dimming of the red signal.
Figure 3 provides actual measured data for the stages illustrated above in two live sperm stimulated with 125 µM of the ganglioside GM1 (for more details on how GM1 regulates AE in sperm12), captured in a single field of view. Figure 3A,B, and Figure 3E,F show the red and green signals measured in two sperm at time point 0 (before stimulation), where initially, the mCherry signal is bright, and the GCaMP3 is dim. Figure 3C, Figure 3G, and Figure 3D, Figure 3H provide the Z Profiler analysis for the full duration of the experiment (raw data or F/F0, respectively). Insert panels provide a zoomed-in view of the time window where ACR and AE occur.
Because sperm are both highly heterogeneous and very sensitive to membrane damage due to various handling procedures, Figure 5 provides examples of two types of negative results. Figure 5A,C show several sperm captured within one field of view. The yellow outline highlights two sperm cells that demonstrate a high GCaMP3 signal at time point 0 (before stimulation). In the present experiments, such cells were avoided, as they indicate that the acrosomal vesicle is already going through some membrane fusion process or that they have experienced some membrane damage that allows calcium to leak. The sperm in Figure 5A indicated by the arrow (circle ROI in Figure 5A,C) demonstrates an initial mCherry signal but no response in the red or green channels (Figure 5B,D, respectively, showing the Z-Profiler analysis window as provided by ImageJ) following stimulation with 125 µM GM1. Although a subpopulation of sperm, such as the one provided in this example, shows no response to stimulation, these are included in the final analysis and are calculated in the percent response data analysis2 (Figure 3).
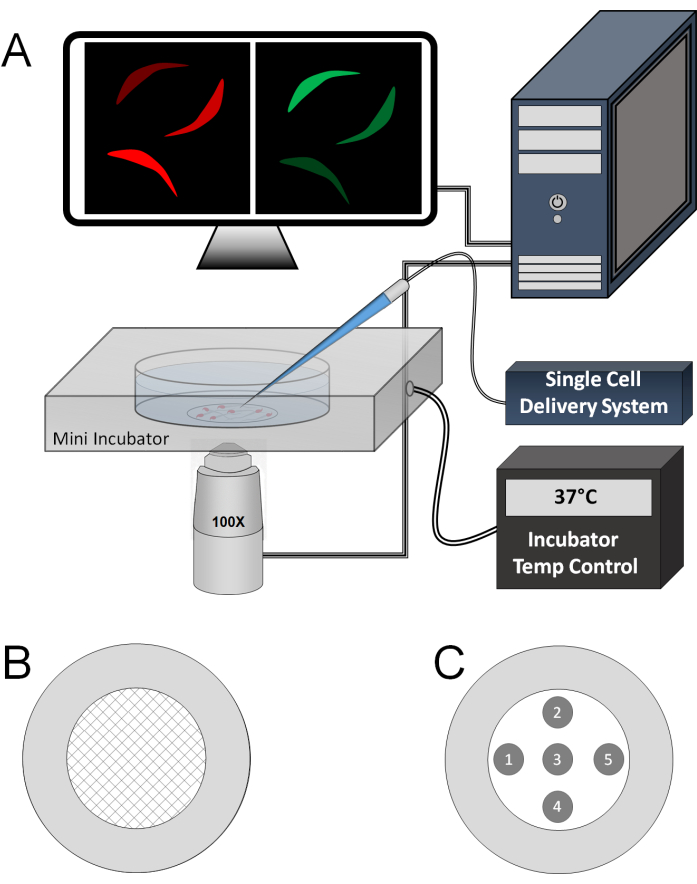
Figure 1: Instrumentation and experimental setup. (A) Live sperm cells on a PDL-coated coverslip dish placed in an incubation chamber at 37 °C. A borosilicate capillary, pulled and attached to a single-cell delivery system, is positioned near the sperm cell in the center of the imaging area for direct stimulant delivery. Both green (GCaMP3) and red (mCherry) channels are continuously monitored and recorded at a high frame rate (e.g., 10 frames/s) during stimulation. (B) Schematic representation of the PDL deposition pattern on the cover-slide dish. (C) Schematic representation of the specific areas on the dish where sperm can be stimulated using the capillary to minimize non-specific stimulation from prior puffs in the same dish. Please click here to view a larger version of this figure.
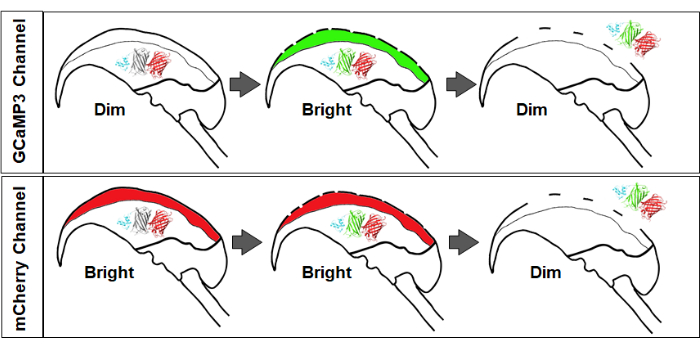
Figure 2: Changes in fluorescence signal and intensity. Top panel: An increase in GCaMP3 signal signifies calcium influx into the acrosome, followed by signal loss due to diffusion of the AcroSensE fusion protein. Bottom panel: mCherry signal remains stable during initial membrane fusion, with subsequent dimming upon AcroSensE diffusion and acrosomal exocytosis (AE). Please click here to view a larger version of this figure.
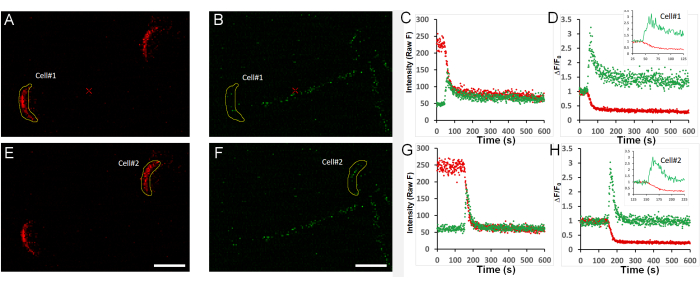
Figure 3: Representative data and fluorescence traces. A field containing two sperm cells was imaged. Green/red channel fluorescence was quantified offline using ImageJ and plotted in Excel (as described in step 6). (A–D) Quantification of red (A) and green (B) channel fluorescence intensity for cell #1 over time, calculated for selected ROIs, and then presented as raw data (C) or normalized to the initial signal (D, F/F0). The insert in (D) provides a zoomed-in view of the time frame from 25-125 s. (E–H) Quantification of red (E) and green (F) channel fluorescence intensity for cell #2 over time, calculated for selected ROIs, and then presented as raw data (G) or normalized to the initial signal (H, F/F0). The insert in (H) provides a zoomed-in view of the time frame from 25-125 s. Scale bar = 5 µm. All x-axes represent time in seconds (s). Please click here to view a larger version of this figure.
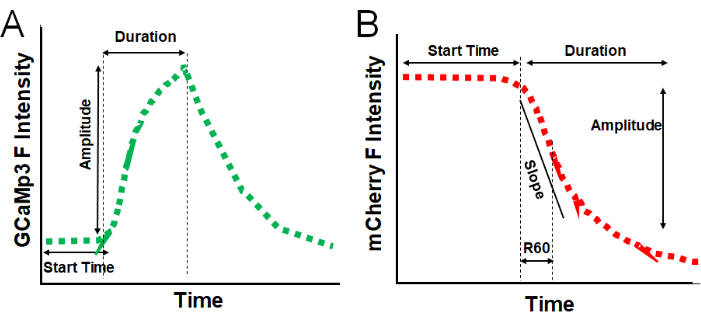
Figure 4: Data analysis and quantification parameters. (A) Illustration of a typical GCaMP3 fluorescence intensity trace, displaying quantifiable parameters, including Start Time (seconds), Amplitude (fluorescence intensity), and Duration (seconds). (B) Illustration of a typical mCherry fluorescence intensity trace, displaying quantifiable parameters, including Start Time (seconds), Duration (seconds), Slope (F/s), R60 (fluorescence intensity), and Amplitude (fluorescence intensity). Please click here to view a larger version of this figure.
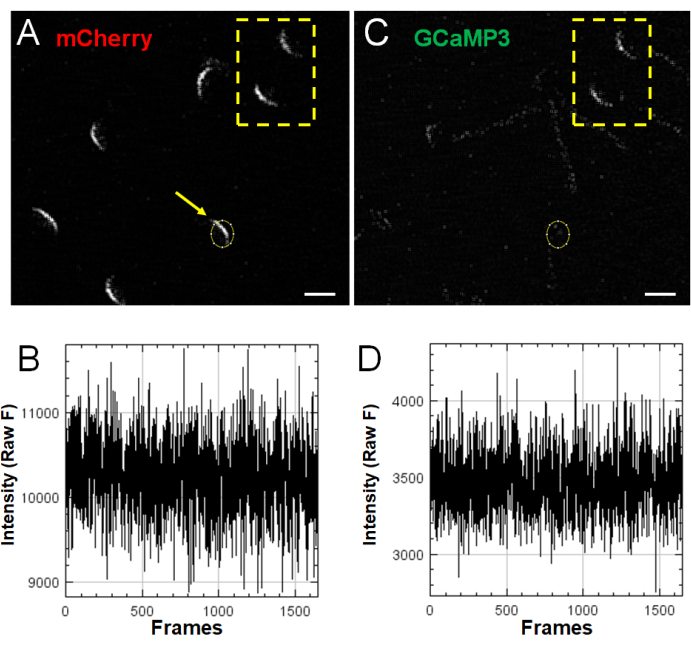
Figure 5: Negative results. (A,C) mCherry (red) and GCaMP3 (green) channels showing multiple sperm cells captured in a single field of view. The arrow in (A) indicates a sperm cell initially exhibiting mCherry fluorescence but no subsequent fluorescence intensity changes in response to 125 µM GM1 stimulation, as observed in the Z-Profiler generated traces provided in (B) (mCherry) and (D) (GCaMP3). Cells within the yellow rectangle exhibit elevated GCaMP3 fluorescence intensity at time point 0, before stimulation. Scale bar = 5 µm. Please click here to view a larger version of this figure.
Discussion
Here, a microscopy-based method is described to utilize the newly generated AcroSensE mouse model for real-time, single-cell monitoring and analysis of the interplay between acrosomal calcium dynamics and intermediate steps leading to AE. Together with readily available genetic approaches, such as crossbreeding with other mouse genetic models or gene editing, this model and method provide a powerful system to study the role of various components and pathways that take part in sperm signaling pathways related to capacitation, AE, and fertilization.
Critical steps
It is important in all sperm handling steps to use large-bore plastic transfer pipettes or large-orifice pipette tips and to perform all steps of collection and washing at 37 ˚C to minimize membrane damage. Similarly, a swinging-bucket rotor is preferable to a fixed-angle rotor to avoid membrane damage due to cells interacting with the side wall of the micro-centrifuge tube. If Z-position drift correction is available, applying z-position correction hardware/software is highly recommended to avoid the drift of the imaged sperm out of the focal plane during the imaging interval.
Modifications and troubleshooting
The protocol described here was optimized for studies of lipid-mediated signaling2,3 and can be applied to both capacitated and non-capacitated sperm. Different methods for sperm capacitation can be used; for example, either albumin or cyclodextrin can serve as sterol acceptors. In addition, various energy substrates or bicarbonate can be added or omitted. However, note that bicarbonate will form in aqueous media from exposure to air, so testing of truly 'bicarbonate-free' conditions would require careful use of a nitrogen environment in all steps of making/preparing/handling the medium and performing the experiment. The protocol above includes using 10 mM calcium added to the experimental solution; however, calcium ions can be omitted, chelated (e.g., EGTA), or added in lower concentrations for calcium-sensitive experimental applications. Please note that lowering or omitting calcium concentrations will diminish the expected GCaMP3 signal upon AE2. In addition, one can use other media for this protocol (versus the MW used here) that better fit their experimental design and objectives. The provided protocol describes the use of single-cell imaging and single-cell delivery of stimulants; however, a simplified variation is attainable by bulk stimulation, such as by adding the stimulating reagent to the whole dish and not via puffing. In addition, whole-population experiments are also possible using the AcroSensE model via the use of fluorimeters or plate readers with fluorescence capability.
Limitations
The method provided here requires the availability, maintenance, and use of an AcroSensE mouse colony. The method also depends on the availability of an advanced imaging system, which is costly and requires trained personnel. For some experimental applications, a possible limitation concerns the inability to concurrently measure cytosolic calcium in AcroSensE sperm, as many available synthetic calcium indicators are of similar wavelength to GCaMP3. Although beyond the scope of this protocol, potential limitations of population-level experiments might include the reading of the mCherry signal after AE and dispersal in the medium or GCaMP3 signal coming from dead/permeabilized cells. To optimize such methods, one might explore employing z-focusing to minimize background noise from secreted mCherry, and/or utilize sham-treated controls to ensure baseline GCaMP quantification from dead or permeabilized sperm.
The significance of the method with respect to existing/alternative methods
Over the last two decades, there have been reports of several methods for real-time studies of AE dynamics in live sperm, including acrosome-targeted GFP transgenic mice7, calcium dyes loaded into the acrosome (e.g., Fluo-412), or using exocytosis-specific indicators such as the synthetic dye FM-6413. However, in comparison to these methods, the AcroSensE model provides the convenience of using minimally treated sperm that do not require any dye loading or other potentially membrane-perturbing procedures. Importantly, there is a great advantage to having sperm that express a dual indicator that can monitor acrosomal calcium dynamics, membrane fusion, and pore formation, as well as AE and the consequent release of the acrosomal content.
Importance and potential applications of the method in specific research areas
Note that the experimental application examples below could be performed as single-cell or population assays, with the latter having significant advantages when high-throughput analysis is desired. Studies of AE dynamics in various mouse models can be conducted by means of crossbreeding with the AcroSensE mouse model, including (1) null or mutated forms of various calcium channels or calcium channel subunits (e.g., crossing with α1E- and CatSper-null models); (2) null or mutated forms of proteins that mediate membrane fusion (e.g., SNAREs). Additionally, studies can be conducted to investigate how various pharmacological agents related to different signaling pathways modulate acrosomal calcium and AE dynamics. Furthermore, research can be conducted on how environmental changes affect AE, including temperature, pH, and the presence or concentration of various ions or small molecules.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by National Institutes of Health grants R01-HD093827 and R03-HD090304 (A.J.T).
Materials
| 100x oil objective | Olympus Japan | UPlanApo, | |
| 2-hydroxypropyl-b-cyclodextrin | Sigma | C0926 | |
| 35 mm coverslip dish, 1.5 thickness | MatTek Corp. | P35G-1.5-20-C | |
| 5 mL round-bottomed tube | Falcon | 352054 | |
| Borosilicate glass capilarries | Sutter Instrument Co. CA USA | B200-156-10 | |
| CaCl2 | Sigma | C4901 | |
| Confocal microscope | Olympus Japan | Olympus FluoView | |
| Glucose | Sigma | G7528 | |
| Graduated tip | TipOne, USA Scientific | ||
| HEPES | Sigma | H7006 | |
| ImageJ | National Institutes of Health | https://imagej.nih.gov/ij/plugins/index.html | |
| KCl | Sigma | P9541 | |
| Lactic acid | Sigma | G5889 | |
| Live-Cell Microscope Incubation Systems | TOKAI HIT Shizuoka, Japan | Model STX | |
| MgCl2 | Sigma | M8266 | |
| Micropipette Puller | Sutter Instrument Co. CA USA | Model P-97 | |
| NaCl | Sigma | S3014 | |
| NaHCO3 | Sigma | S6297 | |
| Plastic transfer pipette | FisherBrand | 13-711-6M | |
| Poly-D-lysine | Sigma | P7280 | |
| Pyruvic acid | Sigma | 107360 | |
| Single cell delivery system | Parker, Hauppauge, NY | Picospritzer III |
References
- Austin, C. R. Observations on the penetration of the sperm in the mammalian egg. Aust J Sci Res B. 4 (4), 581-596 (1951).
- Cohen, R., et al. A genetically targeted sensor reveals spatial and temporal dynamics of acrosomal calcium and sperm acrosome exocytosis. J Biol Chem. 298 (5), 101868 (2022).
- Cohen, R., Mukai, C., Travis, A. J. Lipid regulation of acrosome exocytosis. Adv Anat Embryol Cell Biol. 220, 107-127 (2016).
- Harper, C. V., Cummerson, J. A., White, M. R., Publicover, S. J., Johnson, P. M. Dynamic resolution of acrosomal exocytosis in human sperm. J Cell Sci. 121, 2130-2135 (2008).
- Sosa, C. M., et al. Kinetics of human sperm acrosomal exocytosis). Mol Hum Reprod. 21 (3), 244-254 (2015).
- Balestrini, P. A., et al. Seeing is believing: Current methods to observe sperm acrosomal exocytosis in real time. Mol Reprod Dev. 87 (12), 1188-1198 (2020).
- Nakanishi, T., et al. Real-time observation of acrosomal dispersal from mouse sperm using GFP as a marker protein. FEBS Lett. 449 (2-3), 277-283 (1999).
- Kim, K. S., Gerton, G. L. Differential release of soluble and matrix components: evidence for intermediate states of secretion during spontaneous acrosomal exocytosis in mouse sperm. Dev Biol. 264 (1), 141-152 (2003).
- Hasuwa, H., et al. Transgenic mouse sperm that have green acrosome and red mitochondria allow visualization of sperm and their acrosome reaction in vivo. Experimental animals / Japanese Association for Laboratory Animal Science. 59 (1), 105-107 (2010).
- Henderson, M. J., et al. A Low-Affinity GCaMP3 Variant (GCaMPer) for imaging the endoplasmic reticulum calcium store. PloS one. 10 (10), 0139273 (2015).
- Travis, A. J., et al. Functional relationships between capacitation-dependent cell signaling and compartmentalized metabolic pathways in murine spermatozoa. J Biol Chem. 276 (10), 7630-7636 (2001).
- Cohen, R., et al. Lipid modulation of calcium flux through CaV2.3 regulates acrosome exocytosis and fertilization. Dev Cell. 28 (3), 310-321 (2014).
- Sanchez-Cardenas, C., et al. Acrosome reaction and Ca(2)(+) imaging in single human spermatozoa: new regulatory roles of [Ca(2)(+)]i. Biol Reprod. 91 (2), 67 (2014).

