Dual Extracellular Recordings in the Mouse Hippocampus and Prefrontal Cortex
Summary
This protocol outlines the use of a custom-designed recording device and electrodes to record local field potentials and investigate information flow in the mouse hippocampus and prefrontal cortex.
Abstract
The technique of recording local field potentials (LFPs) is an electrophysiological method used to measure the electrical activity of localized neuronal populations. It serves as a crucial tool in cognitive research, particularly in brain regions like the hippocampus and prefrontal cortex. Dual LFP recordings between these areas are of particular interest as they allow the exploration of interregional signal communication. However, methods for performing these recordings are rarely described, and most commercial recording devices are either expensive or lack adaptability to accommodate specific experimental designs. This study presents a comprehensive protocol for performing dual-electrode LFP recordings in the mouse hippocampus and the prefrontal cortex to investigate the effects of antipsychotic drugs and potassium channel modulators on LFP properties in these areas. The technique enables the measurement of LFP properties, including power spectra within each brain region and coherence between the two. Additionally, a low-cost, custom-designed recording device has been developed for these experiments. In summary, this protocol provides a means to record signals with high signal-to-noise ratios in different brain regions, facilitating the investigation of interregional information communication within the brain.
Introduction
Local field potentials (LFPs) refer to the electrical activity recorded from the extracellular space, reflecting the collective activity of a localized group of neurons. They exhibit a diverse range of frequencies, spanning from slow waves at 1 Hz to fast oscillations at 100 Hz or 200 Hz. Specific frequency bands have been associated with cognitive functions such as learning, memory, and decision making1,2. Changes in LFP properties have been used as biomarkers for various neurological disorders, including dementia and schizophrenia3,4. Analyzing LFP recordings can offer valuable insights into the underlying pathological mechanisms associated with these conditions and potential therapeutic strategies.
Dual LFP recording is a technique used to measure localized electrical activity within and between two specific brain regions. This technique provides a valuable opportunity to investigate the intricate neural dynamics and signal communication occurring within and between distinct brain regions. Prior studies have revealed that detecting alterations in the neuronal properties of individual brain regions can be complex, but changes in interregional cortical communication can be observed5,6. Therefore, the utilization of dual LFP recording offers a potent means to address this issue.
Hippocampal-prefrontal connectivity plays a crucial role in modulating cognitive functions, and dysfunction has been linked to various neurological disorders7,8. Dual electrode recordings of these regions can provide information about these interactions. Unfortunately, there is limited information available on methods for performing dual electrode LFP recordings between these areas. Moreover, commercially available recording devices are generally expensive and lack adaptability to specific experimental designs. The conventional method for recording LFPs involves using a shielded cable to connect the recording device to electrodes implanted in an animal’s brain. However, this approach is susceptible to motion artifacts and environmental noise, impacting the quality and reliability of the recorded signals.
This protocol describes a comprehensive procedure for performing dual-electrode LFP recordings in the mouse hippocampus and prefrontal cortex, using a low-cost custom-designed headstage that can be placed on the animal’s head. These methods enable researchers to investigate region-specific oscillatory patterns within two discrete cerebral regions and explore interregional information exchange and connectivity between these areas.
Protocol
This study was approved by the Florey Animal Ethics Committee (The University of Melbourne, No. 22-025UM) in accordance with the Australian code for the care and use of animals for scientific purposes. C57BL/6 male mice (8 weeks), obtained from the Animal Resources Centre (Australia), were used for the present study.
1. Headstage design and fabrication
NOTE: The headstage PCB board is a compact 14 mm x 12 mm four-layer board designed to be placed directly on the animal's head. It utilizes a commercial amplifier chip (see Table of Materials), and all design and Gerber files are available online (GitHub link: https://github.com/dechuansun/Intan-headstage/tree/main/pcbway).
- Provide the following specifications to the manufacturer: Board thickness: 0.6 mm; Minimum tracking/spacing: 4 mils; Minimum hole size: 0.2 mm.
- During the PCB assembly process, follow this order:
- Solder the amplifier chip onto the board using a hot air gun set at 350 °C.
- Solder the passive components.
- Solder the SPI connector and the electrode connector (see Table of Materials).
- Inspect the soldering under a microscope for quality assurance. Secure the SPI connector in place using epoxy for added stability.
- Use third-party recording software and a control board (see Table of Materials) for signal acquisition. Refer to the software user guide for detailed instructions.
- The designed headstage supports 8 channels. In the software, enable channels 8, 9, 12, 13, 20, 21, 22, and 23 for recording.
2. Electrode fabrication
- Cut PFA-coated tungsten wires (see Table of Materials) to specific lengths for different electrode types: prefrontal cortex electrode (12 mm), hippocampus electrode (10 mm), and ground electrode (6 mm).
- Cut the brass tube (see Table of Materials) into 3 mm segments.
- Remove 2 mm of the coating at the end of each wire using a lighter, then securely solder the electrode wire to the brass tube. The brass tube has an inside diameter of 0.45 mm and an outside diameter of 0.60 mm.
- For the ground electrode, solder an M1.2 stainless steel screw (see Table of Materials) to the electrode. Apply phosphoric acid-based flux to the screw to enhance soldering. After soldering, clean the screw using alcohol.
NOTE: Wear gloves for protection during the soldering process.
3. Surgical procedure
- Anesthetize the mouse in an anesthesia chamber with 3% isoflurane and 1 L/min oxygen flow.
- Place the anesthetized mouse on a heating pad and secure it in a stereotaxic frame (see Table of Materials).
- Adjust the maintenance rate of isoflurane to 2.5-3% and reduce the oxygen flow to 500 mL/min. Using the toe pinch, verify if the animal is still under deep anesthesia.
- Subcutaneously inject carprofen at 0.5 mg/kg and apply eye ointment for eye protection.
- Shave and sterilize the mouse's head using povidone-iodine and 80% ethanol.
- Make an 8 mm incision along the midline of the scalp, removing connective tissue in the incision area.
- Apply hydrogen peroxide to clean the surface of the skull, being cautious not to touch the surrounding skin.
- Align the bregma and lambda landmarks to the same level for accurate electrode placement (bregma and lambda are where the sagittal suture intersects the coronal and lambdoid sutures).
- Drill holes for reference/ground electrode, anchor screws (0.9 mm drill burr), and active electrodes (0.3 mm drill burr) at specified coordinates.
- Attach the custom-made electrode (step 2) to the stereotaxic frame arm and ensure it is perpendicular to the brain.
- Implant the electrode in the hippocampal CA1 area (AP – 1.8 mm, ML – 1.3 mm, DV – 1.4 mm).
NOTE: AP, anteroposterior; ML, mediolateral; DV, dorsoventral. - Repeat electrode implantation in the prefrontal cortex (AP – 2.0 mm, ML – 0.3 mm, DV – 1.7 mm).
- Secure electrodes with a commercially available powerful adhesive and dental cement (see Table of Materials).
- Implant two 1.2 mm anchor screws (AP – 1.8 mm, ML -1.6 mm) to prevent movement.
- Position the reference/ground electrode in direct contact with the dura mater, 2 mm posterior and 2 mm unilateral to the lambda landmark.
- Connect the brass tube side of the electrodes to a multi-channel socket connector (see Table of Materials) with the ground electrode in the middle.
- Use 0.8 mm heat shrink tubing on the exterior of the middle pin for isolation.
- Secure the electrodes, anchor screws, and connector with adhesive and dental cement.
4. Postoperative care
- To alleviate postoperative pain, inject carprofen at a dose of 5-10 mg/kg subcutaneously every 12-24 hours based on an assessment of pain for a period of three days.
- Provide the animal with a one-week recovery period before starting any recording or experimental procedures.
5. Recording procedure
- Handle the animal for 15 min, twice daily, for three consecutive days.
- Pick up the mice by gently closing the hand around them without applying excessive pressure.
- Place the headstage board on the animal's head for 30 min once a day for three consecutive days.
- On the recording day, acclimate the animal to the recording room for 30 min.
- Place the animal in a small recording chamber within a Faraday cage to reduce external electrical interference. Attach the custom headstage for recording.
- Open the recording software and select a 2.00 kHz sampling rate. Disable all channels except for 13 and 20 by selecting each channel and pressing the space bar.
- In the hardware bandwidth window, set the lower bandwidth to 2 Hz and the upper bandwidth to 100 Hz.
- In the software filtering window, adjust the low-pass filter to 100 Hz and the high-pass filter to 2 Hz.
- Choose the storage path by clicking on Select File Name, and then click on Record.
- Start each recording session with a 10 min habituation period followed by a 15 min baseline EEG recording.
- After the baseline recording, administer the drug via intraperitoneal injection and continue recording for an additional 30 min without delay.
NOTE: See the Results section for the details on the drugs used.
Representative Results
The results shown here demonstrate the effects of several drugs on local field potentials (LFPs) properties tested in four cohorts of C57BL/6 male mice (n = 8 for each cohort; age: 8 weeks; weight: 24.0 ± 0.42 g). The drugs tested included the antipsychotic drug clozapine, the potassium channel modulators 4-Aminopyridine (4-AP), and retigabine, as well as the control vehicle saline.
As shown in Figure 1, the mouse was placed in a small recording chamber and LFPs were collected from the hippocampus (HIP) and the pre-frontal cortex (PFC) using a custom-designed headstage. As this study primarily focused on the examination of the theta and gamma frequency bands, the recorded signal first underwent initial bandpass filtering within a frequency range of 2-100 Hz, followed by sampling at 2000 Hz. The signal was then sectioned into several 2 s epochs. Any epochs displaying pronounced motion artifacts were identified and subsequently excluded from subsequent analysis processes. Power spectra of LFPs in both the HIP and PFC as well as the HIP-PFC coherence were measured using a multi-taper-based analysis method9. The analysis used five Slepian tapers, and the time-bandwidth was set to three to achieve the optimal spectral concentration. A sliding window of 1 s and a step size of 100 ms were used to generate the time-frequency spectrograms and the time-course of the HIP-PFC coherence.
As shown in Figure 2 and Figure 3, the administration of saline did not produce any discernible effects on the power spectra of LFPs in HIP and PFC, nor HIP-PFC coherence. Both retigabine and clozapine demonstrated clear reductions in the gamma band (30-100 Hz) power in HIP and PFC, as well as the gamma band HIP-PFC coherence. In contrast, 4-AP exhibited the opposite effects, characterized by enhanced gamma band power in HIP and PFC, along with an increased coherence within the gamma band between HIP and PFC.
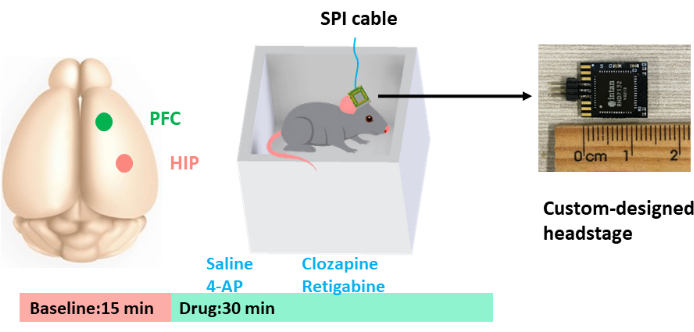
Figure 1: Schematic of the experimental setup. Electrodes were implanted in the hippocampus (HIP) and the prefrontal cortex (PFC) for recording local field potentials. The animal was placed in a small recording chamber, and a custom-designed headstage was attached to the electrode connector. Each recording session commenced with a 10 min baseline session, which was followed by a 30 min drug session. The effects of saline, clozapine, 4-AP, and retigabine were tested. Please click here to view a larger version of this figure.
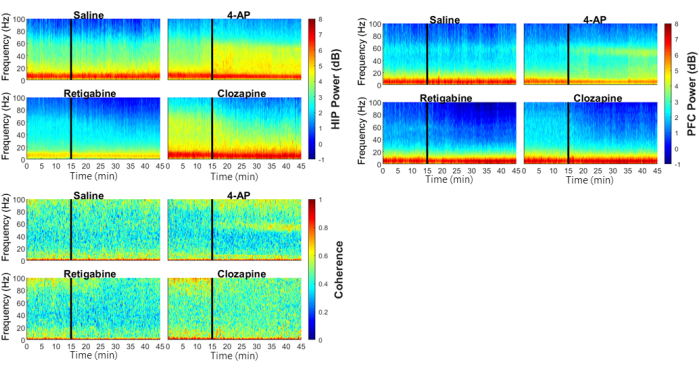
Figure 2: The impact of antipsychotic drugs and potassium channel modulators on the ongoing electrophysiological activity in the hippocampus (HIP) and prefrontal cortex (PFC). The normalized spectrogram of the local field potentials in the HIP and PFC, along with the HIP-PFC coherence, exhibited time-dependent effects for all the drugs studied.The drugs were administered via intraperitoneal injection at time t = 15 min. Please click here to view a larger version of this figure.
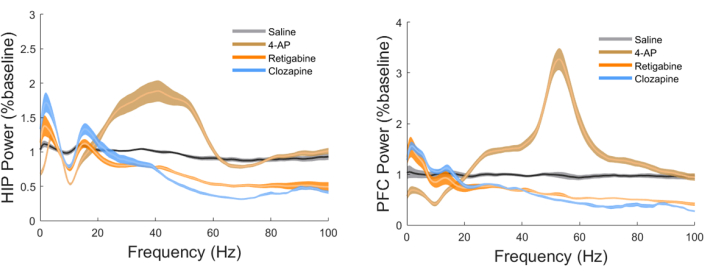
Figure 3: The impact of antipsychotic drugs and potassium channel modulators on the power spectra density in HIP and PFC. 4-AP significantly enhanced gamma band power in both brain regions, while clozapine and retigabine suppressed gamma band power in both regions. Please click here to view a larger version of this figure.
Discussion
The protocol presented here outlines the procedure for constructing a customized headstage specifically designed for the simultaneous recording of dual local field potentials (LFPs) in the hippocampus (HIP) and the prefrontal cortex (PFC). The detailed steps provided in this protocol offer sufficient information for researchers to thoroughly examine signal communication both within each region and between the HIP and PFC.
The custom-designed headstage utilizes a commercial amplifier chip. While the current configuration supports the recording of 8 channels, the headstage can be easily adapted to accommodate the full 32-channel capacity, thereby providing potential for expanded channel capacity suitable for electrode array recording. Considering the low-cost of the headstage, one option is to permanently affix the board to the animal’s head. This approach offers the advantage of minimizing motion artifacts and reducing the overall level of disturbance caused by movement.
The custom-made electrode demonstrates stable long-term recording of LFPs, maintaining good signal quality over a span of 3-5 months. Another viable approach involves employing polyimide-based flexible circuit boards as electrode arrays10,11. These flexible circuit boards can be integrated with the recording headstage to enable multi-channel recording. This method provides the advantage of simplifying electrode preparation and surgical procedures. The weight of the implant with and without the headstage is very light, at 0.198 g and 0.812 g, respectively, making it suitable for very young mice.
One limitation of the current recording technique is the potential interference caused by the hanging cable, which may interfere the natural behavior of the animal during experiments. To address this issue, alternative solutions such as utilizing an SD card for data storage or implementing a wireless signal transmitter module can be considered.
An essential and critical step of the protocol involves the accurate positioning of the electrode. It is crucial to ensure precise and consistent electrode placement to enable comparability across experiments. To verify the electrode location, histology must be conducted12. A useful technique to enhance proper electrode positioning in the HPC is to record while the electrode is vertically inserted, as strong theta rhythms and neuronal firing will indicate correct placement. It is advisable to utilize adult mice older than 8 weeks, as the signal quality may decline over time or result in incorrect placement as the mice age. Taking these considerations into account will help maintain the reliability and validity of the experimental results.
In conclusion, the protocol presented in this article provides a framework to study the signal communication between distinct brain regions. It enables researchers to explore the neuronal dynamics and interactions within and between these regions.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Royal Melbourne Hospital Neuroscience Foundation (A2087).
Materials
| Brass tube | Albion Alloys, USA | Inside diameter of 0.45 mm |
| Carprofen | Rimadyl, Pfizer Animal Health | |
| Commercial amplifier chip | Intantech | RHD 2132 |
| Control board | Intantech | RHD recording system |
| Dental cement | Paladur | |
| Heat shrinks | Panduit | 0.8 mm diameter |
| M1.2 stainless steel screw | Watch tools | Clock and watch screw |
| Multichannel socket connector | Harwin, AU | 1.27 mm pitch, PCB socket |
| PFA-coated tungsten wires | A-M SYSTEMS, USA | Inside diameter of 150 µm |
| Phosphoric acid-based flux | Chip Quik | CQ4LF-0.5 |
| Recording software | Intantech | RHX recording software |
| Stereotactic Frame | World Precision Instruments | Mouse stereotactic instrument |
| Super glue | UHU | Ultra fast |
References
- Einevoll, G. T., Kayser, C., Logothetis, N. K., Panzeri, S. Modelling and analysis of local field potentials for studying the function of cortical circuits. Nat Rev Neurosci. 14 (11), 770-785 (2013).
- Buzsaki, G., Anastassiou, C. A., Koch, C. The origin of extracellular fields and currents-EEG, ECOG, LFP and spikes. Nat Rev Neurosci. 13 (6), 407-420 (2012).
- Sigurdsson, T., Stark, K. L., Karayiorgou, M., Gogos, J. A., Gordon, J. A. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 464 (7289), 763-767 (2010).
- Witton, J., et al. Disrupted hippocampal sharp-wave ripple-associated spike dynamics in a transgenic mouse model of dementia. J Physiol. 594 (16), 4615-4630 (2016).
- Englot, D. J., Konrad, P. E., Morgan, V. L. Regional and global connectivity disturbances in focal epilepsy, related neurocognitive sequelae, and potential mechanistic underpinnings. Epilepsia. 57 (10), 1546-1557 (2016).
- Pievani, M., De Haan, W., Wu, T., Seeley, W. W., Frisoni, G. B. Functional network disruption in the degenerative dementias. Lancet Neurol. 10 (9), 829-843 (2011).
- Sigurdsson, T., Duvarci, S. Hippocampal-prefrontal interactions in cognition, behavior and psychiatric disease. Front Syst Neurosci. 9, 190 (2015).
- Sun, D., et al. Effects of antipsychotic drugs and potassium channel modulators on spectral properties of local field potentials in mouse hippocampus and pre-frontal cortex. Neuropharmacology. 191, 108572 (2021).
- Bokil, H., Andrews, P., Kulkarni, J. E., Mehta, S., Mitra, P. P. Chronux: A platform for analyzing neural signals. J Neurosci Methods. 192 (1), 146-151 (2010).
- Bozkurt, A., Lal, A. Low-cost flexible printed circuit technology based microelectrode array for extracellular stimulation of the invertebrate locomotory system. Sens Actuator A Phys. 169 (1), 89-97 (2011).
- Du, P., et al. High-resolution mapping of in vivo gastrointestinal slow wave activity using flexible printed circuit board electrodes: Methodology and validation. Ann Biomed Eng. 37, 839-846 (2009).
- JoVE Science Education Database. Neuroscience. Histological Staining of Neural Tissue. JoVE. , (2023).

