Three-Dimensional Acoustic Assembly Device for Mass Manufacturing of Cell Spheroids
Summary
Cell spheroids have been considered one potential model in the field of biological applications. This article describes protocols for scalably generating cell spheroids using a 3D acoustic assembly device, which provides an efficient method for the robust and rapid fabrication of uniform cell spheroids.
Abstract
Cell spheroids are promising three-dimensional (3D) models that have gained wide applications in many biological fields. This protocol presents a method for manufacturing high-quality and high-throughput cell spheroids using a 3D acoustic assembly device through maneuverable procedures. The acoustic assembly device consists of three lead zirconate titanate (PZT) transducers, each arranged in the X/Y/Z plane of a square polymethyl methacrylate (PMMA) chamber. This configuration enables the generation of a 3D dot-array pattern of levitated acoustic nodes (LANs) when three signals are applied. As a result, cells in the gelatin methacryloyl (GelMA) solution can be driven to the LANs, forming uniform cell aggregates in three dimensions. The GelMA solution is then UV-photocured and crosslinked to serve as a scaffold that supports the growth of cell aggregates. Finally, masses of matured spheroids are obtained and retrieved by subsequently dissolving the GelMA scaffolds under mild conditions. The proposed new 3D acoustic cell assembly device will enable the scale-up fabrication of cell spheroids, and even organoids, offering great potential technology in the biological field.
Introduction
3D in vitro culture models, which provide more in vivo-like structural and morphological characteristics compared to conventional 2D culture models, have been recognized as promising systems in various biomedical applications such as tissue engineering, disease modeling, and drug screening1,2,3. As one type of 3D culture model, cell spheroids typically refer to cell aggregation, creating 3D spheroidal structures characterized by enhanced cell-cell and cell-matrix interactions4,5,6. Therefore, fabricating cell spheroids has become a powerful tool for enabling diverse biological studies.
Various techniques, including hanging drop7, non-adhesive plates8, or microwell devices9, have been developed to obtain spheroids. In principle, these methods commonly facilitate cell assembly by utilizing physical forces such as gravitational force while minimizing interactions between cells and the substrate. However, they often involve labor-intensive processes, have low productivity, and pose challenges for controlling spheroid size10,11. Importantly, the production of spheroids with the desired size and uniformity in sufficient quantity is of utmost importance to satisfy specific biological applications. In contrast to the above-mentioned methods, acoustic waves, as one type of external-force-driven technique12,13,14, have shown potential for mass manufacturing of cell spheroids with high quality and throughput, based on the principle of enhancing cell aggregation through external forces15,16,17,18. Unlike electromagnetic or magnetic forces, acoustic-based cell manipulation techniques are non-invasive and label-free, enabling spheroid formation with excellent biocompatibility19,20.
Commonly, standing surface acoustic waves (SAWs) and bulk acoustic waves (BAWs)-based devices have been developed to generate spheroids, utilizing the acoustic nodes (ANs) produced by corresponding standing acoustic fields21,22,23. Particularly, acoustic assembly devices based on BAWs, with the merits of convenient manufacture, easy operation, and excellent scalability, have gained attention for fabricating cell spheroids24,25. We have recently developed a facile BAWs-based acoustic assembly device with the ability to generate spheroids with high throughput26. The proposed device consists of a square polymethyl methacrylate (PMMA) chamber with three lead zirconate titanate (PZT) transducers arranged respectively in the X/Y/Z plane. This arrangement enables the creation of a 3D dot-array pattern of levitated acoustic nodes (LANs) for driving cell assembly. Compared to previously reported BAWs- or SAWs-based devices, which can only create a 1D or 2D array of ANs27,28,29, the present device enables a 3D dot-array of LANs for rapid cell aggregate formation within the gelatin methacryloyl (GelMA) solution. Subsequently, cell aggregates matured into spheroids with high viability within the photocured GelMA scaffolds after three days of cultivation. Finally, a large number of spheroids with uniform size were easily obtained from the GelMA scaffolds for downstream applications.
Protocol
1. Fabrication of the 3D acoustic assembly device
- Begin by preparing four 1 mm thick PMMA sheets through laser cutting30, and then proceed to glue them together to form a square chamber with an inner width of 21 mm and a height of 10 mm.
- Next, attach another 1 mm thick PMMA sheet to the bottom of the chamber to serve as a holder for the bioink.
- Carefully affix three lead zirconate titanate (PZT) transducers (each measuring 20 mm in length, 10 mm in width, 0.7 mm in thickness, and with a primary resonant frequency of 3 MHz, see Table of Materials) to the exterior of the three orthogonal walls of the chamber, respectively. Ensure that the bottom PZT transducer is centered beneath the chamber.
- Solder wires to the two conductive areas of each PZT transducer.
- Finally, secure the device onto a hollow base to prevent the bottom PZT from coming into contact with other countertops.
2. Setting up the acoustic assembly system
- Begin by mounting the acoustic device onto a microscope stage, allowing for top-view observation of the chamber's interior.
- Position a digital microscope on the side of the device that is free from PZT transducers, enabling side-view observation of the chamber's interior.
- Independently connect the wires from the three PZT transducers in series to three power amplifiers and three output channels of function generators (see Table of Materials).
- Program the settings on the function generators for each PZT transducer, specifying parameters such as sinusoidal waveform, frequency, and amplitude.
- To ensure sterilization, fill the chamber of the acoustic device with 75% alcohol for 5 min, followed by a thorough cleaning with sterile PBS solution. Subsequently, irradiate the chamber with UV light in a clean bench for a minimum of 1 h.
3. Cell culture and harvest procedure
- Begin by culturing C3A cells, a human hepatocellular carcinoma cell line, in a T25 cell culture flask using Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (see Table of Materials).
- When the C3A cells reach approximately 80% confluence, perform cell passage as follows: firstly, wash the bottom of the culture flask twice with PBS. Then, add 2 mL of 0.05% trypsin-EDTA to the cell culture flask and incubate at 37 °C to facilitate cell detachment. Stop the trypsinization process by adding 2 mL of complete culture medium.
- Transfer the cell solution to a 15 mL tube, and centrifuge it at 200 × g for 5 min at room temperature to obtain a cell pellet.
4. Preparation of the bioink
- Prepare a 6% (w/v) GelMA solution containing 0.5% (w/v) lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) by dissolving 0.3 g of GelMA and 0.025 g of LAP (see Table of Materials) in 5 mL of phosphate buffer solution (PBS). Allow the mixture to sit in a 47 °C water bath for 1 h.
- Before mixing with cells, pass the GelMA solution through a 0.22 µm filter (see Table of Materials) for sterilization.
- Resuspend the cell pellet mentioned above (step 3.3) in cell culture medium. Stain a small volume of cells with a 0.4% trypan blue solution and then use a clean hemocytometer for cell counting.
- Mix an appropriate amount of C3A cells, calculated based on the cell density obtained above, with the sterilized GelMA solution to prepare the bioink with a cell density of 2 × 106 cells/mL.
- For visualization of the assembled cell spheroids, pre-stain C3A cells by incubating them with 2 µM cell tracker (DiO dye, see Table of Materials) at 37 °C for 20 min. Subsequently, wash the labeled cells with fresh cell culture medium three times before use.
5. Assembling the cell spheroids using the acoustic device
- Pipette more than 1 mL of the bioink into the sterilized chamber. Ensure that the face-to-face distance between the liquid surface and the chamber bottom is an integer multiple of half the acoustic wavelength.
NOTE: One can control this distance by adjusting the volume of bioink added. The acoustic wavelength (λ) can be estimated using the formula λ = c/f, where c represents the speed of sound in the medium, and f is the frequency of the PZT transducer. - Turn on the function generator and power amplifier to initiate the actuation of each PZT transducer.
NOTE: It is recommended to individually actuate each PZT transducer first to achieve the optimal parallel-line cellular pattern. This can be achieved by conducting a frequency sweep with a step size of 0.001 MHz near the primary resonant frequency for each PZT transducer. Afterward, simultaneously apply these optimal signals to all three PZT transducers to obtain the expected 3D-dot cellular pattern. Before applying each input signal, ensure that the cells are evenly dispersed in the bioink by gently agitating. - Crosslink the bioink using blue light (405 nm, 60 mW/cm2, 30 s) to create a 3D hydrogel scaffold that encapsulates the cell aggregates assembled acoustically. Afterward, turn off the function generator and power amplifier.
- Carefully transfer the 3D hydrogel scaffold from the chamber into a Petri dish and cut it into small pieces (e.g., 2 mm × 2 mm × 2 mm) using a clean razor.
- Add cell culture medium for cultivation.
NOTE: Remember to change the culture medium every day.
6. Retrieving cell spheroids
- Begin by observing spheroid formation at different layers of the scaffolds using an inverted microscope.
- After 3 days of culture, remove the culture medium and thoroughly wash the hydrogel scaffolds twice with PBS.
- Incubate the scaffolds with more than 2 mL of GelMA lysis buffer at a 1: 200 dilution with cell culture medium for 30 min in a cell incubator. This step aims to dissolve the hydrogel scaffolds.
- Transfer the solution from the previous step into a tube and then centrifuge it at 200 × g for 5 min at room temperature to obtain the cell spheroid pellet.
- Discard the supernatant and resuspend the pellet in fresh culture medium for downstream applications.
7. Spheroid viability analysis
- Assess the viability of cell spheroids either within the hydrogel scaffolds or in the retrieved spheroids using a live/dead staining kit (see Table of Materials).
- Incubate the samples at different culture time points with 1 mL of PBS solution containing 1 µL of Calcein-AM and 2 µL of Propidium Iodide (PI) for 15 min at 37 °C.
- For samples within the hydrogel scaffolds, wash them directly with PBS twice. For retrieved spheroids, centrifuge at 200 × g for 5 min at roomtemperature to obtain a spheroid pellet, and wash it twice before observation.
- Observe the samples using a fluorescence microscope and capture the fluorescence images.
- Calculate the ratio of the area stained with Calcein-AM to the total area stained with both Calcein-AM and PI using ImageJ to determine spheroid viability.
Representative Results
This study designed a 3D acoustic assembly device for mass manufacturing of cell spheroids. The acoustic device comprised a square chamber with two PZT transducers attached to the X-plane and Y-plane on the outer surface of the chamber and one PZT transducer on the chamber's bottom (Figure 1A,B). Three output channels from two function generators were connected to three power amplifiers to generate three independently sinusoidal signals to actuate the PZT transducers (Figure 1C).
The optimal resonant frequencies used to actuate the three PZT transducers attached to the X/Y/Z planes of the chamber were 3.209 MHz, 3.283 MHz, and 3.215 MHz, respectively. The optimal amplitude for all three PZT transducers was 10 peak-to-peak output voltage (Vpp), measured by an oscilloscope. Figure 2A illustrates the working mechanism of cell aggregates generated using the 3D acoustic assembly device. When the signal is applied, cells are driven to the acoustic nodes under the influence of acoustic radiation force (ARF). To visualize cell spheroids, cells were pre-stained with 2 µM DiO (green fluorescence). After acoustic cell assembly, a confocal microscope was used to observe the 3D acoustically assembled cell aggregates. These cell aggregates were observed to be arranged in a regular 3D-dot array pattern with uniform green fluorescent signals (Figure 2B). Different top views of bright-field images also demonstrated that the formed aggregates in each layer were arranged in a 2D-dot array pattern (Figure 2C).
The growth of cell aggregates within the hydrogel at different time points was observed. Results showed that the assembled aggregates gradually integrated and formed tight spheroids by day 3, accompanied by an increase in spheroid diameter (Figure 3A,B). A live/dead staining was conducted to evaluate the viability of the cell spheroids. Good cell viabilities (>90%) were achieved before day 3, while viability decreased slightly after a week of culture (Figure 3C,D).
For the retrieval of spheroids, a GelMA lysis buffer was used to dissociate the hydrogel scaffolds, releasing the encapsulated cell spheroids (Figure 4A). Consequently, after three days of cultivation, the small pieces of hydrogel scaffolds were treated with GelMA lysis buffer at 37 °C for 30 min. The released spheroids maintained a good spherical morphology with a narrow size distribution, along with the expression of albumin and desirable viability (Figure 4B–D).
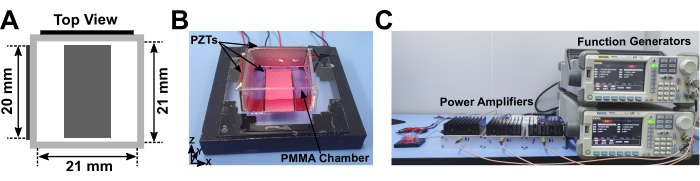
Figure 1: 3D acoustic assembly device. (A) Schematic diagram depicting the top view of the 3D acoustic assembly device, consisting of a PMMA chamber attached with three PZT transducers. (B) Photograph displaying the actual 3D acoustic assembly device. (C) Photograph showing the 3D acoustic assembly device connected with two function generators and three power amplifiers. Please click here to view a larger version of this figure.
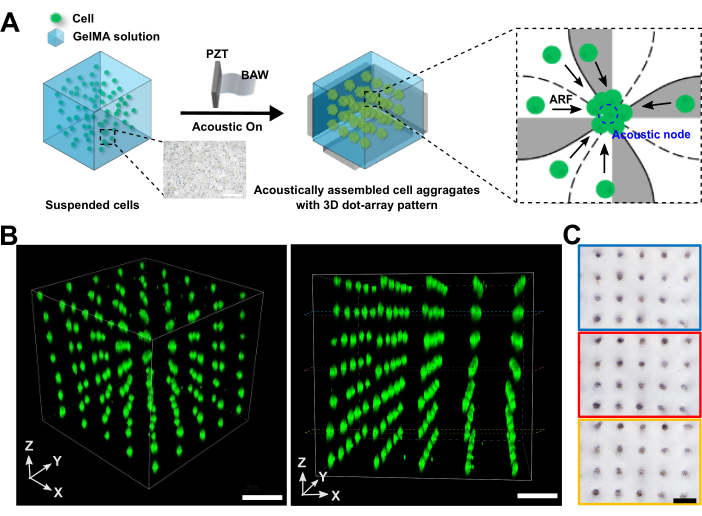
Figure 2: Acoustically assembled cell aggregates. (A) Schematic illustrating the working mechanism of cell aggregates generated by the 3D acoustic assembly device, where cells are driven to the acoustic nodes by acoustic radiation force. (B) Confocal images showcasing the 3D acoustically assembled cell aggregates from different perspectives. (C) Bright-field images displaying the formed cell aggregates at various layers within the hydrogel scaffold. The scale bar represents 250 µm. Please click here to view a larger version of this figure.
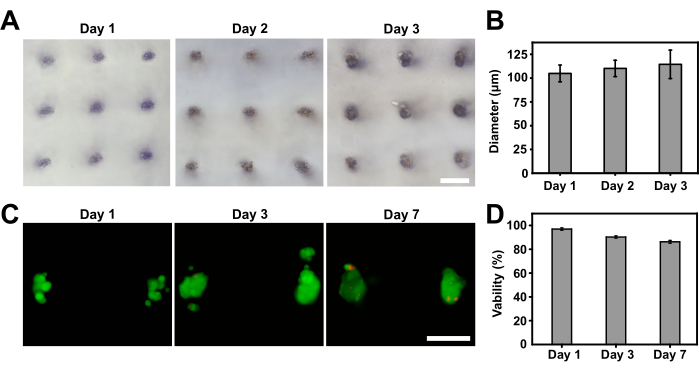
Figure 3: Growth of cell aggregates into spheroids within the GelMA scaffold. (A) Bright-field images showing the formation of compact cell spheroids after a 3-day culture period. (B) Quantification of spheroid sizes. (C) Live/dead staining of spheroids within the hydrogel scaffold after one week of culture. (D) Quantification of cell spheroid viability. The scale bar represents 250 µm. Please click here to view a larger version of this figure.
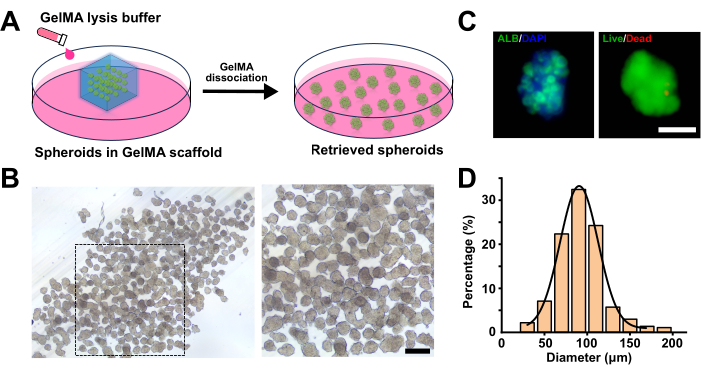
Figure 4: Retrieval of acoustically fabricated cell spheroids. (A) Illustration depicting the steps for retrieving acoustically fabricated cell spheroids. (B) Bright-field images displaying the retrieved spheroids at different magnifications. The scale bar represents 250 µm. (C) Analysis of viability and functionality of the retrieved spheroids. The scale bar represents 100 µm. (D) Size distribution of the spheroids after retrieval. Please click here to view a larger version of this figure.
Discussion
Efficient and stable fabrication of cell spheroids with high throughput using technologies like the 3D acoustic assembly device holds great promise for advancing biomedical engineering and drug screening1,2,3. This approach simplifies the mass production of cell spheroids through straightforward procedures.
However, there are critical factors to consider when using this acoustic device. The creation of standing waves is essential for constructing a 3D-dot array acoustic field. In this device, only one PZT transducer is present in each dimension, serving as a sound excitation source. Therefore, for each dimension, it's crucial that the face-to-face distance between the opposite walls of the PMMA chamber or PMMA-water surface is an integer multiple of half the wavelength. The primary frequency of the PZT transducers we used is 1 MHz, which results in a wavelength in the water or bioink of about 500 µm. Consequently, for the horizontal dimension, the face-to-face distance between the two opposite walls of the PMMA chamber in the acoustic device was set to 21 mm, accounting for the size of the PZT transducers. For the vertical dimension, the face-to-face distance between the bottom PMMA wall and the water surface can be adjusted by varying the bioink volume to meet the conditions for standing wave formation. During experimental operation, slight frequency adjustments may be necessary to compensate for distance deviations. Additionally, it's important to keep the input voltage at a moderate level to prevent PZT transducer overheating, which could heat up the bioink and potentially damage the cells. In the experiments, a peak-to-peak voltage of 10 Vpp was used, completing cell aggregate assembly within 30 s while maintaining the bioink temperature below 30 °C.
One limitation of this approach is the reliance on the GelMA scaffold26, which plays a crucial role in maintaining the structural integrity of the acoustically assembled cell aggregates as they mature into cell spheroids when the acoustic field is removed. However, the step involving the dissolution of the hydrogel scaffolds for spheroid retrieval may lead to reduced cell viability and potential spheroid loss. Additionally, the volume of the GelMA scaffold needs to be within the range of several millimeters to allow for nutrient penetration and exchange for the encapsulated cells. The limited scaffold size in the millimeter range can constrain the production yield of cell spheroids, even if larger scaffolds are initially produced and then cut into smaller pieces. Future efforts may focus on maintaining acoustically assembled cell aggregates in situ, suspended in the culture medium, to enable them to grow into spheroids without the need for an external scaffold.
In summary, the acoustic assembly device described in this protocol can be easily assembled and operated with relatively simple steps, offering the advantage of efficiently fabricating cell spheroids. Moreover, this acoustic assembly device is compatible with various cell types, including the fabrication of organoids, showcasing its significant potential for a wide array of biomedical applications.
Divulgations
The authors have nothing to disclose.
Acknowledgements
Tis work was supported by the National Key Research and Development Program of China (2022YFA1104600), and the Zhejiang Provincial Natural Science Foundation of China (LQ23H160011).
Materials
| 0.22-μm filter | Merck | SLGSM33SS | Used for GelMA solution sterilization |
| 35 mm-cell culture dish | Corning | 430165 | Used for culturing cells |
| Confocal microscope | Nikon | A1RHD25 | Fluorescent cell observation |
| DiO dye | Beyotime | C1038 | Dye used to stain cells |
| DMEM | Gibco | 12430054 | Cell culture media |
| FBS | Gibco | 10099141C | Cell culture media supplement |
| Function generator | Rigol | DG5352 | For RF signal generation |
| GelMA | Regenovo | none | Used to prepare bioink |
| GelMA lysis buffer | EFL | EFL-GM-LS-001 | Used to dissolve GelMA scaffolds |
| Inverted microscope | Nikon | Ti-U | Cell observation |
| LAP | Sigma-Aldrich | 900889 | Used as photoinitiator |
| Live-Dead kit | Beyotime | C2015M | Cell vability analysis |
| PBS | Gibco | 10010002 | Used as buffer |
| Penicillin-streptomycin | Gibco | 15070063 | Prevent cell culture contamination |
| Power amplifer | Minicircuit | LCY-22+ | Increase the voltage amplitude of the RF signal |
| PZT transducers | Yantai Xingzhiwen Trading Co.,Ltd. | PZT-41 | Functional units for acoustic assembly device |
| T25 cell culture flask | Corning | 430639 | Used for culturing cells |
| Trypan blue | Gibco | 15250061 | Cell counting |
| Trypsin-EDTA | Gibco | 25200056 | Cell dissociation enzyme |
References
- Eiraku, M., et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 472 (7341), 51-56 (2011).
- Lancaster, M. A., Knoblich, J. A. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 345 (6194), 1247125 (2014).
- Habanjar, O., Diab-Assaf, M., Caldefie-Chezet, F., Delort, L. 3D cell culture systems: tumor application, advantages, and disadvantages. International Journal of Molecular Sciences. 22 (22), 12200 (2021).
- Decarli, M. C., et al. Cell spheroids as a versatile research platform: formation mechanisms, high throughput production, characterization and applications. Biofabrication. 13 (3), 032002 (2021).
- Lee, Y. B., et al. Engineering spheroids potentiating cell-cell and cell-ECM interactions by self-assembly of stem cell microlayer. Biomaterials. 165, 105-120 (2018).
- Zhuang, P., Chiang, Y. H., Fernanda, M. S., He, M. Using spheroids as building blocks towards 3d bioprinting of tumor microenvironment. International Journal of Bioprinting. 7 (4), 444 (2021).
- Foty, R. A simple hanging drop cell culture protocol for generation of 3D spheroids. Journal of Visualized Experiments. 51, e2720 (2011).
- Laschke, M. W., Menger, M. D. Life is 3D: boosting spheroid function for tissue engineering. Trends in Biotechnology. 35 (2), 133-144 (2017).
- Fu, W., et al. Combinatorial drug screening based on massive 3d tumor cultures using micropatterned array chips. Analytical Chemistry. 95 (4), 2504-2512 (2023).
- Kang, S. M., Kim, D., Lee, J. H., Takayama, S., Park, J. Y. Engineered microsystems for spheroid and organoid studies. Advanced Healthcare Materials. 10 (2), 2001284 (2021).
- Kim, S. J., Kim, E. M., Yamamoto, M., Park, H., Shin, H. Engineering multi-cellular spheroids for tissue engineering and regenerative medicine. Advanced Healthcare Materials. 9 (23), 2000608 (2020).
- Yang, Y., et al. 3D acoustic manipulation of living cells and organisms based On 2D array. IEEE Transactions on Biomedical Engineering. 69 (7), 2342-2352 (2022).
- Armstrong, J. P. K., et al. Engineering anisotropic muscle tissue using acoustic cell patterning. Advanced Materials. 30 (43), 1802649 (2018).
- Drinkwater, B. W. A perspective on acoustical tweezers-devices, forces, and biomedical applications. Applied Physics Letters. 117 (18), 180501 (2020).
- Bouyer, C., et al. A Bio-Acoustic Levitational (BAL) assembly method for engineering of multilayered, 3d brain-like constructs, using human embryonic stem cell derived neuro-progenitors. Advanced Materials. 28 (1), 161-167 (2016).
- Chansoria, P., Narayanan, L. K., Schuchard, K., Shirwaiker, R. Ultrasound-assisted biofabrication and bioprinting of preferentially aligned three-dimensional cellular constructs. Biofabrication. 11 (3), 035015 (2019).
- Wu, Y., et al. Acoustic assembly of cell spheroids in disposable capillaries. Nanotechnology. 29 (50), 504006 (2018).
- Hu, X., et al. On-chip hydrogel arrays individually encapsulating acoustic formed multicellular aggregates for high throughput drug testing. Lab on a Chip. 20 (12), 2228-2236 (2020).
- Wu, Z., et al. The acoustofluidic focusing and separation of rare tumor cells using transparent lithium niobate transducers. Lab on a Chip. 19 (23), 3922-3930 (2019).
- Chen, B., et al. High-throughput acoustofluidic fabrication of tumor spheroids. Lab on a Chip. 19 (10), 1755-1763 (2019).
- Sriphutkiat, Y., Kasetsirikul, S., Zhou, Y. Formation of cell spheroids using Standing Surface Acoustic Wave (SSAW). International Journal of Bioprinting. 4 (1), 130 (2018).
- Guex, A. G., Di Marzio, N., Eglin, D., Alini, M., Serra, T. The waves that make the pattern: a review on acoustic manipulation in biomedical research. Materials Today Bio. 10, 100110 (2021).
- Harley, W. S., et al. Advances in biofabrication techniques towards functional bioprinted heterogeneous engineered tissues: A comprehensive review. Bioprinting. 23, 00147 (2021).
- Yang, Y., Dejous, C., Hallil, H. Trends and applications of surface and bulk acoustic wave devices: a review. Micromachines (Basel). 14 (1), 43 (2022).
- Ma, Z., et al. Acoustic holographic cell patterning in a biocompatible hydrogel). Advanced Materials. 32 (4), 1904181 (2020).
- Miao, T. K., et al. High-throughput fabrication of cell spheroids with 3D acoustic assembly devices. International Journal of Bioprinting. 9 (4), 733 (2023).
- Jeger-Madiot, N., et al. Self-organization and culture of Mesenchymal Stem Cell spheroids in acoustic levitation. Scientific Reports. 11 (1), 8355 (2021).
- Cai, H., et al. Acoustofluidic assembly of 3D neurospheroids to model Alzheimer’s disease. Analyst. 145 (19), 6243-6253 (2020).
- Mei, J., Zhang, N., Friend, J. Fabrication of surface acoustic wave devices on lithium niobate. Jove-Journal of Visualized Experiments. (160), e61013 (2020).
- Niculescu, A. G., Chircov, C., Bîrcă, A. C., Grumezescu, A. M. Fabrication and applications of microfluidic devices: a review. International Journal of Molecular Sciences. 22 (4), 2011 (2011).

