Establishment and Characterization of Patient-derived Gastric Organoids from Biopsies of Benign Gastric Body and Antral Epithelium
Summary
Gastric patient-derived organoids find increasing use in research, yet formal protocols for generating human gastric organoids from single-cell digests with standardized seeding density are lacking. This protocol presents a detailed method for reliably creating gastric organoids from biopsy tissue obtained during upper endoscopy.
Abstract
Gastric patient-derived organoids (PDOs) offer a unique tool for studying gastric biology and pathology. Consequently, these PDOs find increasing use in a wide array of research applications. However, a shortage of published approaches exists for producing gastric PDOs from single-cell digests while maintaining a standardized initial cell seeding density. In this protocol, the emphasis is on the initiation of gastric organoids from isolated single cells and the provision of a method for passaging organoids through fragmentation. Importantly, the protocol demonstrates that a standardized approach to the initial cell seeding density consistently yields gastric organoids from benign biopsy tissue and allows for standardized quantification of organoid growth. Finally, evidence supports the novel observation that gastric PDOs display varying rates of formation and growth based on whether the organoids originate from biopsies of the body or antral regions of the stomach. Specifically, it is revealed that the use of antral biopsy tissue for organoid initiation results in a greater number of organoids formed and more rapid organoid growth over a 20-day period when compared to organoids generated from biopsies of the gastric body. The protocol described herein offers investigators a timely and reproducible method for successfully generating and working with gastric PDOs.
Introduction
Organoids are miniature three-dimensional (3D) cellular structures that resemble the architecture and functionality of the organs from which they were derived1,2. These lab-grown models are created by cultivating stem cells or tissue-specific cells in a controlled environment that allows these cells to self-organize and differentiate into various cell types1,2,3. One of the key advantages of organoids is their ability to recapitulate human biology more closely than traditional two-dimensional (2D) cell cultures1,2,3. In particular, human organoids have been shown to maintain the genetic diversity of their tissue of origin3,4,5. Organoids offer a unique opportunity to study human organ development, model diseases, and test potential therapeutics in a controlled laboratory setting. Furthermore, organoids can be derived from individual patient samples, enabling personalized medicine approaches and the potential development of individualized treatments3,6,7.
Researchers have used human gastric organoids to investigate various aspects of gastric biology and pathology. Prominent examples include the use of patient-derived organoids (PDOs) to predict gastric cancer chemotherapy responses8,9,10 and model the epithelial response to Helicobacter pylori infection11,12,13. Human gastric organoids consist of various cell types found in the stomach, including neck cells, pit cells, and other supporting cells11,14. Gastric organoids can either be generated from induced pluripotent stem cells (iPSCs) or stem cells directly isolated from gastric tissue obtained via biopsies or from gastric resection specimens11,14. The isolation of gastric stem cells from gastric tissue is commonly done by isolating and culturing gastric glands or enzymatically digesting tissue samples to liberate single cells9,13,15. Importantly, the differentiation of cells within gastric organoids generated using either of these techniques has been shown to be similar13. The protocol described herein focuses on a single-cell digest.
Organoids represent a scientific innovation that bridges the gap between traditional cell culture and whole organs. As research in the field continues to progress, organoids are poised to contribute to the development of more effective treatments and therapies for a wide range of applications. Given the rising utilization of gastric PDOs, there is a timely need for a standardized approach to their generation. Here, the protocol for generating human gastric PDOs from single cells isolated from benign gastric biopsy tissue acquired during upper endoscopy is described. Importantly and uniquely, a standardized number of single cells is determined for seeding to reliably generate gastric PDOs and allow subsequent characterization. Using this technique, reliable differences in the formation and growth of organoids generated from biopsies of either the gastric body or gastric antrum are demonstrated.
Protocol
All human tissue utilized in this protocol was collected from individuals who provided informed consent for tissue collection through a gastric tissue collection study approved by the University of Pennsylvania Institutional Review Board (IRB #842961). Participants in this study were required to undergo an upper endoscopy as part of their routine care, be at least 18 years old, and be able to provide informed consent. All research conducted adhered to the guidelines set forth by the University of Pennsylvania.
1. Experimental preparation
- Prepare conditioned L-WRN media as previously described16. Although not mandatory, the concentrations of Wnt-3A, R-spondin, and noggin contained in the conditioned media can be checked using a commercially available ELISA kits following the manufacturer's instructions (see Table of Materials).
- Prepare RPMI-Antibiotics (RPMI-ABX), PBS-Antibiotics (PBS-ABX), PBS-Dithiothreitol (PBS-DTT), digestion buffer, and gastric organoid media (see Supplementary Table 1 for detail composition). Gastric organoid media is stable for 1 week at 4 °C.
- Autoclave forceps, fine dissection scissors, and 1.5 mL tubes.
- Begin thawing the basement membrane matrix (Matrigel) on ice.
- Begin thawing the digestion buffer and Trypsin-EDTA in a 37 °C water bath.
- Set an incubator orbital shaker to 37 °C and 200 rpm.
2. Isolating single cells from the biopsy tissue
- Collect gastric biopsies during an upper endoscopy17 following the institutional clinical protocol, likely involving the use of jumbo forceps. Utilize a blunt-tipped needle to extract tissue samples from the forceps and place them in a 15 mL conical tube containing RPMI-ABX media. Keep the tube on ice for rapid transfer to the laboratory.
NOTE: At minimum, 2-4 biopsies should be collected. - Allow the biopsy tissue to settle at the bottom of the conical tube. Use a pipette to aspirate the media and wash the biopsies twice with 1 mL of PBS-ABX buffer. There is no need for centrifugation, as the tissue pieces settle naturally in the conical tube.
- Transfer a minimum of 20 mg of biopsy tissue to a 1.5 mL tube containing 1 mL of PBS-DTT.
- Use fine dissection scissors to cut the tissue into pieces that are 1-2 mm or smaller.
- Utilize a tabletop minicentrifuge for 15 s to aggregate tissue pieces at the tube's bottom and aspirate as much supernatant as possible. A small residual volume of PBS-DTT is acceptable.
- Add 5 mL of freshly warmed digestion buffer to a 50 mL conical tube. To transfer the small tissue pieces without losing tissue, take 500 µL of the digestion buffer and add it to the 1.5 mL tube containing the tissue. Next, modify the tip of a 1000 µL pipette tip with scissors to increase the tip's diameter, allowing for easy aspiration and transfer of tissue pieces to the 50 mL conical tube containing the digestion buffer.
- Incubate the digestion buffer and tissue mix at 37 °C for 30 min with orbital shaking at 200 rpm.
- Add 5 mL of warmed trypsin with 0.25% EDTA to the digestion buffer and incubate for an additional 10 min at 37 °C with orbital shaking at 200 rpm.
- Neutralize the digestion buffer and trypsin by adding an equal volume of Advanced (Adv.) DMEM/F12 media and pass the solution through a 70 µm cell strainer.
- Pellet the cells by centrifugation at 1400 x g for 4 min at 4 °C. Depending on the initial size of the biopsy tissue, a small cell pellet may or may not be visible.
- Resuspend the cell pellet in 1 mL of Adv. DMEM/F12 media and count the number of viable cells using Trypan Blue and a hemocytometer.
- Pellet the cells again through centrifugation at 1400 x g for 4 min at 4 °C and remove the supernatant.
NOTE: Figure 1 presents a schematic representation of the process for isolating single cells from the biopsy tissue.
3. Embedding single cells in a basement membrane matrix "dome"
- Based on the counted number of viable cells, calculate the volume of basement membrane matrix required to achieve a final concentration of 105 viable cells per 50 µL of basement membrane matrix.
- Perform the following swiftly to prevent the basement membrane matrix from polymerizing before plating. Remove the thawed basement membrane matrix from ice, add the previously calculated volume of basement membrane matrix to the cells, and gently mix by pipetting up and down for approximately 10 s.
NOTE: It is crucial to avoid creating bubbles during this step. Do not dilute the basement membrane matrix. - Rapidly pipette 50 µL aliquots of the basement membrane matrix/cell mixture into the center of individual wells in a 24-well tissue culture plate.
- Immediately cover the 24-well plate and, in a single smooth motion, flip the plate upside-down. Place the inverted plate in a 37 °C tissue culture incubator for 35 min to allow the basement membrane matrix to polymerize.
NOTE: Inverting the plate is essential to prevent the cells from sinking to the bottom of the plate and to enable the basement membrane matrix to polymerize into a 3D "dome" shape. - Add 500 µL of pre-warmed gastric organoid media to each well, ensuring that the top of each "dome" is fully submerged in the media. Dispense the media down the side of each well to avoid disturbing the "dome."
- Change the media every 2-3 days.
4. Routine passaging of organoids via fragmentation
- Once the organoids are ready for passaging, remove the media from each designated well.
NOTE: To determine the appropriate time for passaging, please refer to the Representative Results section. - Dispense 1 mL of ice-cold Adv. DMEM/F12 media directly onto a basement membrane matrix "dome." This should readily initiate the breakup of the "dome." Continue aspirating and dispensing until all fragments of the "dome" detach from the plate.
- Transport both the media and the fragmented basement membrane matrix to the next well, repeating this process for all wells slated for passaging. After disassembling the final basement membrane matrix "dome," dispense the media and the mixture of organoids and basement membrane matrix into a 1.5 mL tube.
- Attach a 1000 µL tip to a P1000 pipette, and then insert this tip into a 200 µL tip. This will create a pipette tip with a small enough diameter to fragment the organoids while still allowing the aspiration of a larger volume.
- Vigorously pipette the mixture of organoids and basement membrane matrix up and down approximately 25 times to fragment the organoids into small pieces.
- Centrifuge the fragmented organoid mixture using a tabletop centrifuge at 4 °C for 30 s at 2000 x g. This will result in a pellet of fragmented organoids separated from the basement membrane matrix and media. Use a pipette to aspirate the media and basement membrane matrix supernatant. Do not use a vacuum to aspirate the supernatant, as the pellet is loose.
- Calculate the volume of freshly thawed basement membrane matrix required so that the number of wells/domes is split at a 1:2 ratio (50 µL/dome). Add the fresh basement membrane matrix and gently pipette up and down to mix, taking care to avoid creating bubbles.
- Swiftly aliquot 50 µL of the mixture of organoid fragments and basement membrane matrix into individual wells of a 24-well plate.
- Cover the plate, flip it upside down, and place it in a 37 °C tissue culture incubator for 35 min to allow the basement membrane matrix to polymerize.
- Add 500 µL of pre-warmed gastric organoid media to each well.
NOTE: Figure 2 presents a schematic overview of the passaging of gastric patient-derived organoids through fragmentation.
Representative Results
The subsequent representative results are derived from biopsies taken from the benign epithelium of both the gastric body and gastric antrum regions of the stomachs of five different patients undergoing upper endoscopy. Two to four "domes"/wells were plated and analyzed per patient for both gastric body and antrum biopsies. Organoids were successfully generated from the gastric body and gastric antrum biopsy tissue from all five patients. On average, 41 organoids were analyzed per "dome"/well. All images are z-projections acquired using a confocal microscope, and the quantification of organoid size and sphericity was performed using commercially available image analysis software (see Table of Materials).
Organoids are generally identifiable within 10 days post-seeding of single cells (Figure 3A). By day 20, the organoids are large and typically need to be passaged. While the number of organoids that form post single-cell seeding can be somewhat variable, expected results for the number of organoids formed from body and antral gastric biopsies are shown in Figure 3B. The number of body and antral organoids peaks at day 10 post-seeding. While not significant, the total number of organoids begins to decrease from day 10 to day 15 and day 15 to day 20. There appears to be a subpopulation of small organoids that form by day 10 and then cease growth and die off over the following 10 days, which would explain this trend. Importantly, it is shown that the number of organoids formed from antral biopsy tissue is significantly higher than biopsy tissue from the body at days 10, 15, and 20. The number of antral organoids formed was on average 2-fold higher than the number of organoids formed from the body.
In Figure 3C, representative results for organoid growth after single-cell seeding between day 10 and day 20 are shown. While organoids from both antral and body biopsies saw steady growth from day 10 to 20, organoids generated from antral biopsy tissue displayed a greater growth rate compared to organoids generated from body biopsy tissue. In particular, antral organoids had nearly a 4-fold greater area than body organoids at day 20.
Across different patients, a diversity of organoid morphology is typically observed in any single "dome"/well (Figure 4A). Some organoids are more round or spherical while others displayed a more irregular morphology. However, on average, sphericity, a measurement of how spherical an organoid is (where a score of 1 = a perfect sphere)18, showed little variation within and between organoids generated from body or antral biopsy tissue (Figure 4B). Therefore, although there are differing growth rates, there are typically no significant morphological differences between organoids generated from biopsy tissue of the gastric body or antrum.
By day 20 post-initiation, gastric organoids are typically ready to be passaged. A large organoid size (≥1500 µm in diameter) or a darkened interior (suggestive of extensive cellular turnover) of the organoid are key signs that organoids need to be passaged (Figure 5A). Organoids left to go beyond this point may begin to break down into 2D monolayers (Figure 5B) that do not reliably re-form organoids after passaging, perhaps indicating a loss of viability or stemness. After passaging and reseeding gastric organoids using the fragmentation protocol described herein, the "domes" will contain many organoid fragments (Figure 5C) that will reorganize themselves into many more organoids and grow much more quickly compared to the initial seeding of single cells (Figure 5D). If organoid growth still needs characterization and/or standardization at the time of passaging, gastric organoids can instead be digested to single cells, as previously described19.
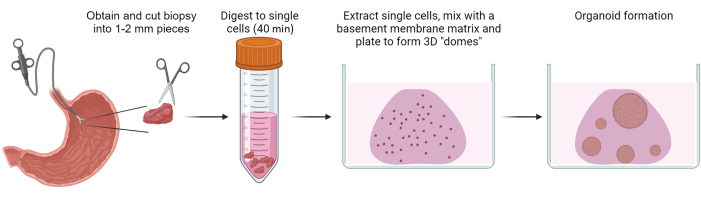
Figure 1: Generation of gastric patient-derived organoids. Schematic overview depicting the process of generating gastric patient-derived organoids from biopsies of benign gastric epithelium. Please click here to view a larger version of this figure.
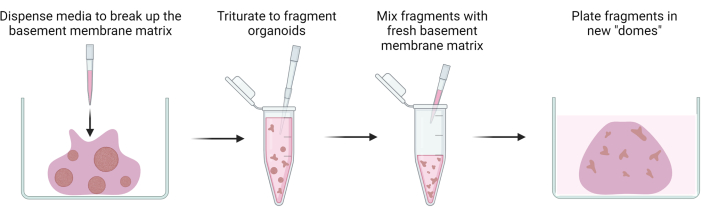
Figure 2: Passaging of gastric patient-derived organoids. Schematic overview illustrating the passaging of gastric patient-derived organoids through fragmentation. Please click here to view a larger version of this figure.
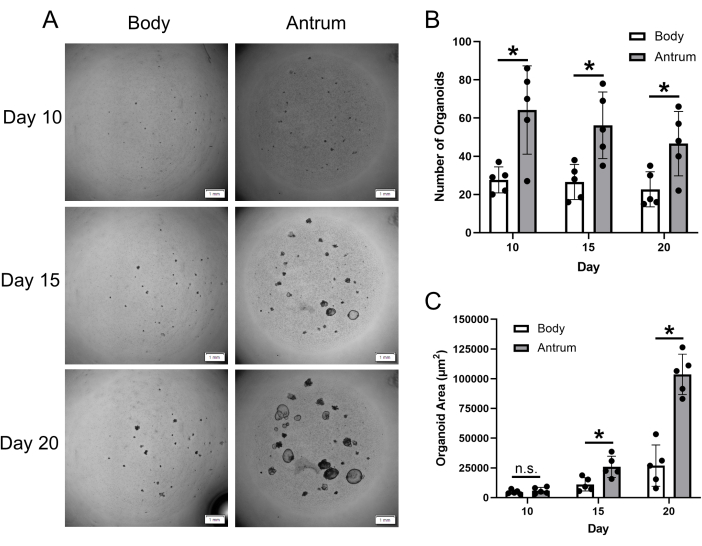
Figure 3: Gastric patient-derived organoid formation and growth. (A) Representative z-projection images displaying the growth of gastric organoids at day 10, 15, and 20 post-single-cell seeding. Images are of organoids generated from gastric body and antrum biopsy tissue from the same patient. Scale bar = 1 mm. (B) Mean (±SD) number of gastric body and antrum organoids at indicated timepoints post-single-cell seeding. (C) Mean (±SD) area (µm2) of gastric body and antrum organoids at indicated timepoints post-single-cell seeding. n = 5 patients per group and timepoint. * = statistically significant difference (p ≤ 0.05) at the indicated timepoint. n.s. = no statistically significant difference at the indicated timepoint. Statistical comparisons conducted via 2-way ANOVA. Please click here to view a larger version of this figure.
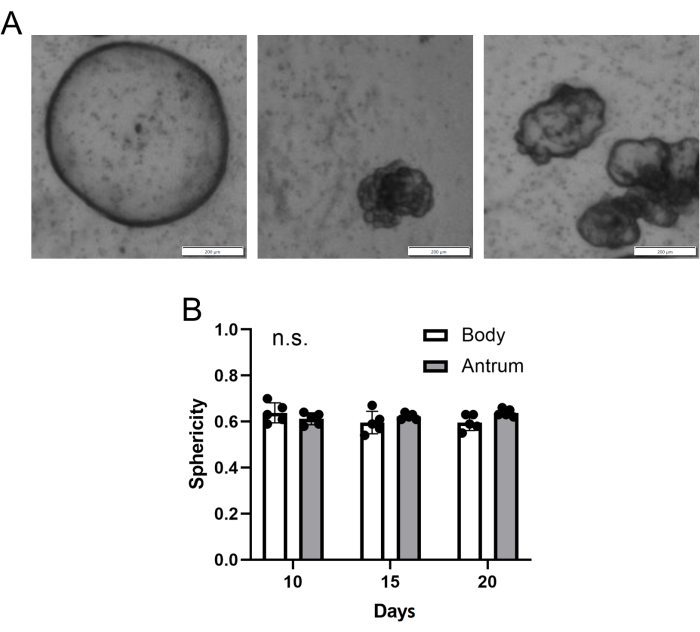
Figure 4: Gastric patient-derived organoid morphology. (A) Representative z-projection images of different gastric organoid morphologies from an individual "dome"/well of gastric body-derived organoids at day 15 post-single-cell seeding. Scale bar = 200 µm. (B) Mean (±SD) gastric body and antrum organoid sphericity (where a value of 1 = a perfect sphere). n = 5 patients per group and timepoint. n.s. = no statistically significant difference at any timepoint. Statistical comparisons conducted via 2-way ANOVA. Please click here to view a larger version of this figure.
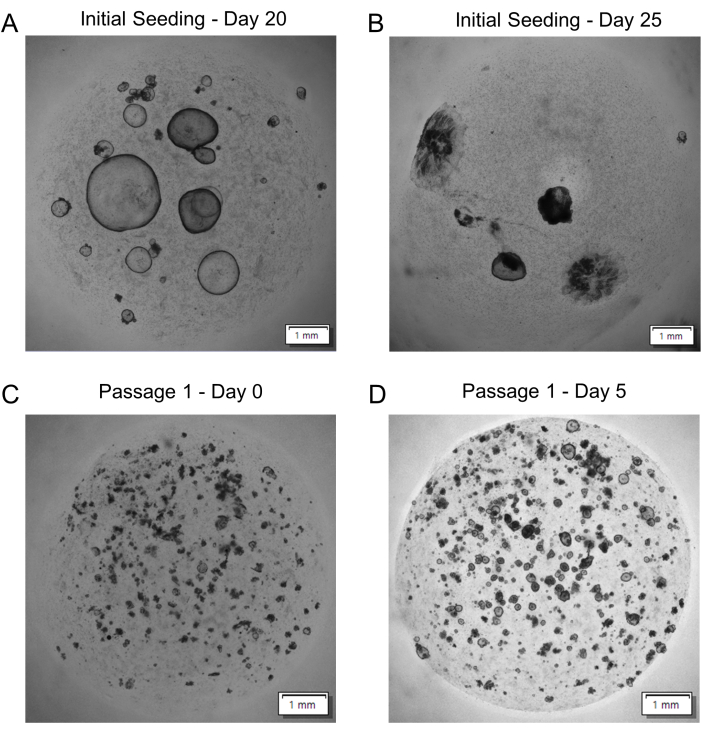
Figure 5: Gastric patient-derived organoid passaging. (A) Representative image of organoids ready to be passaged at day 20 post-single-cell seeding. (B) Representative image of organoids overdue for passaging at day 25 post-single-cell seeding. (C) Representative image of fragmented organoids after reseeding (Passage 1 – Day 0). (D) Representative image of organoid growth over 5 days after reseeding (Passage 1 – Day 5). All images are z-projections. Scale bars = 1 mm. Please click here to view a larger version of this figure.
Supplementary Table 1: Solutions and media recipes. Please click here to download this File.
Discussion
Herein, a detailed protocol for reliably generating human gastric organoids from single cells isolated from biopsies of benign epithelium from the gastric body and antrum is outlined. Critical steps in the protocol revolve around timing as well as handling the basement membrane matrix. To preserve viability, it is essential to initiate the protocol as soon as possible after acquiring the biopsy tissue. The aim is to start digesting the biopsy tissue within 30 min of the biopsy being performed. Handling the basement membrane matrix can also be challenging. When thawed on ice, it remains a liquid; however, at temperatures above 4 °C, it polymerizes. Therefore, swiftly transferring the basement membrane matrix from ice to mix with single cells or organoid fragments for plating "domes" must be done promptly, as it begins to polymerize within one minute. Once it has polymerized in a tube, it cannot be aspirated into a pipette tip. If this occurs, the tube can be placed on ice until the Matrigel depolymerizes back into a liquid. When gently pipetting up and down to mix single cells or organoid fragments with the basement membrane matrix, it is also crucial to avoid creating bubbles. While bubbles in a basement membrane matrix "dome" do not seem to hinder organoid formation and growth, they can obstruct visualization. Additionally, after aliquoting the basement membrane matrix/cell mixture into the well(s) of a cell culture plate, the plate must be inverted and placed into an incubator. This step is critical to allow the basement membrane matrix to polymerize into a 3D "dome" shape and prevent the single cells or organoid fragments from sinking to the bottom of the plate.
Using this protocol, gastric organoids can be identified within 10 days of single cell seeding. Experience shows that very few new gastric organoids form beyond day 10, and, in fact, the overall number of organoids may slightly decrease from day 10-20. This is true for organoids generated from both the gastric body and antrum. However, the total number of organoids formed from gastric antral biopsies is significantly higher than from gastric body biopsies. Furthermore, the growth of antral organoids greatly surpasses body organoids between days 10-20 after single cell seeding. This difference may be attributed to variations in Wnt sensitivity. A recent study demonstrated that gastric body PDOs exhibit better growth with lower Wnt activation, whereas gastric antrum PDOs thrive with higher Wnt activation20. The location of gastric biopsies used to generate gastric PDOs is not typically specified in the literature. Such differences should be considered in future studies utilizing gastric PDOs generated from gastric biopsies of different stomach areas.
Another crucial aspect of this protocol is the establishment of a standardized number of single cells to seed per "dome"/well for reliably generating gastric PDOs. While a previous study reported a standardized number of gastric glands to seed for PDO generation, no previous studies using a single cell digest method have mentioned the number of cells seeded or if the number was consistent across different PDO lines. Failing to standardize the number of cells seeded can result in a highly variable number of stem cells being seeded per "dome"/well. Since stem cells are the primary source of gastric organoid formation, this could lead to variable rates of organoid formation and growth. Therefore, utilizing a non-standardized number of single cells could confound interpretations of formation or growth comparisons across different gastric PDO lines. Here, it is demonstrated that standardizing the number of cells seeded to 105 cells per "dome"/well reliably generates gastric PDOs from biopsies of both the body and antral regions of the stomach.
This protocol was optimized for the use of fresh gastric biopsy tissue. Consequently, the success of this protocol may vary when using frozen tissue or tissue preserved by other means. Additionally, there is no data on how long fresh biopsies maintain viability, as the biopsy tissue is processed as soon as possible after removal from a patient's stomach. Presumably, the longer the fresh tissue sits before processing, the fewer viable cells will be isolated.
Passaging gastric PDOs via fragmentation, as described in this protocol, provides an easy method for routine passaging. While gastric PDOs have been successfully passaged up to 4 times using this technique, there have been no attempts to determine how many times they can be passaged while maintaining steady growth and viability. Some reports indicate that the growth of gastric organoids may slow after 5 or more passages21,22, while others have observed reliable growth for up to 10 passages23.
The gastric organoid media used in this protocol contains Wnt-3A, noggin, and R-spondin derived from conditioned media of L-WRN cells. To produce the conditioned media, a protocol described by Miyoshi and Stappenbeck is utilized16. Alternatively, Wnt-3A, noggin, and R-spondin can be purchased separately as recombinant proteins. Purchasing the individual components separately is ideal to avoid potential batch effects that may arise when using conditioned media from L-WRN cells. However, buying the recombinant proteins is expensive and may be cost-prohibitive for researchers who frequently work with organoids.
Given the growing use of gastric PDOs in various applications, it is timely and necessary to establish standardized approaches for generating benign gastric PDOs. The protocol described here offers a reliable method for future investigations utilizing gastric PDOs. In our experience, this protocol has successfully generated organoids from biopsies of benign gastric mucosa over 90% of the time.
Divulgations
The authors have nothing to disclose.
Acknowledgements
University of Pennsylvania Genomic Medicine T32 HG009495 (KHB), NCI R21 CA267949 (BWK), Men & BRCA Program at the Basser Center for BRCA (KHB, BWK), DeGregorio Family Foundation Grant Award (BWK).
Materials
| 0.25% Trypsin-EDTA | Gibco | 25200-056 | |
| A83-01 | R&D Systems | 2939 | |
| Advanced DMEM/F12 | Gibco | 12634-010 | |
| Amphotericin B | Invitrogen | 15290018 | |
| B27 | Invitrogen | 17504044 | |
| BZ-X710 | Keyence | n/a | |
| cellSens | Olympus | n/a | |
| Collagenase III | Worthington | LS004182 | |
| Dispase II | Sigma | D4693-1G | |
| Dithiothreitol (DTT) | EMSCO/Fisher | BP1725 | |
| DPBS | Gibco | 14200-075 | |
| Fungin | InvivoGen | NC9326704 | |
| Gastrin I | Sigma Aldrich | G9145 | |
| Gentamicin | Invitrogen | 1570060 | |
| Glutamax | Gibco | 35050-061 | |
| hEGF | Peprotech | AF-100-15 | |
| HEPES | Invitrogen | 15630080 | |
| hFGF-10 | Peprotech | 100-26 | |
| L-WRN Cell Line | ATCC | CRL-3276 | |
| Matrigel | Corning | 47743-715 | |
| Metronidazole | MP Biomedicals | 155710 | |
| N2 Supplement | Invitrogen | 17502048 | |
| Noggin ELISA Kit | Novus Biologicals | NBP2-80296 | |
| Pen Strep | Gibco | 15140-122 | |
| RPMI 1640 | Gibco | 11875-085 | |
| R-Spondin ELISA Kit | R&D Systems | DY4120-05 | |
| Wnt-3a ELISA Kit | R&D Systems | DY1324B-05 | |
| Y-27632 | Sigma Aldrich | Y0503 |
References
- Drost, J., Clevers, H. Organoids in cancer research. Nature Reviews Cancer. 18 (7), 407-418 (2018).
- Corrò, C., Novellasdemunt, L., Li, V. S. A brief history of organoids. American Journal of Physiology-Cell Physiology. 319 (1), C151-C165 (2020).
- Zhao, Z., et al. Organoids. Nature Reviews Methods Primers. 2 (1), 94 (2022).
- Weeber, F., et al. Preserved genetic diversity in organoids cultured from biopsies of human colorectal cancer metastases. Proceedings of the National Academy of Sciences. 112 (43), 13308-13311 (2015).
- Boretto, M., et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nature Cell Biology. 21 (8), 1041-1051 (2019).
- Lo, Y. H., Karlsson, K., Kuo, C. J. Applications of organoids for cancer biology and precision medicine. Nature Cancer. 1 (8), 761-773 (2020).
- Grönholm, M., et al. Patient-derived organoids for precision cancer immunotherapy. Cancer research. 81 (12), 3149-3155 (2021).
- Yan, H. H., et al. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell. 23 (6), 882-897 (2018).
- Yoon, C., et al. Patient-derived organoids from locally advanced gastric adenocarcinomas can predict resistance to neoadjuvant chemotherapy. Journal of Gastrointestinal Surgery. 27 (4), 666-676 (2023).
- Miao, X., et al. Establishment of gastric cancer organoid and its application in individualized therapy. Oncology Letters. 24 (6), 1-8 (2022).
- Pompaiah, M., Bartfeld, S. Gastric organoids: an emerging model system to study Helicobacter pylori pathogenesis. Molecular Pathogenesis and Signal Transduction by Helicobacter pylori. 400, 149-168 (2017).
- Schlaermann, P., et al. A novel human gastric primary cell culture system for modelling Helicobacter pylori infection in vitro. Gut. 65 (2), 202-213 (2016).
- Bartfeld, S., et al. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology. 148 (1), 126-136 (2015).
- Seidlitz, T., Koo, B. K., Stange, D. E. Gastric organoids-an in vitro model system for the study of gastric development and road to personalized medicine. Cell Death & Differentiation. 28 (1), 68-83 (2021).
- Bartfeld, S., Clevers, H. Organoids as model for infectious diseases: culture of human and murine stomach organoids and microinjection of Helicobacter pylori. Journal of Visualized Experiments. 105, e53359 (2015).
- Miyoshi, H., Stappenbeck, T. S. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nature Protocols. 8 (12), 2471-2482 (2013).
- Yang, H. J., et al. Sample collection methods in upper gastrointestinal research. Journal of Korean Medical Science. 38 (32), e255 (2023).
- Kim, S., et al. Comparison of cell and organoid-level analysis of patient-derived 3D organoids to evaluate tumor cell growth dynamics and drug response. SLAS DISCOVERY: Advancing the Science of Drug Discovery. 25 (7), 744-754 (2020).
- Maru, Y., Tanaka, N., Itami, M., Hippo, Y. Efficient use of patient-derived organoids as a preclinical model for gynecologic tumors. Gynecologic Oncology. 154 (1), 189-198 (2019).
- McGowan, K. P., Delgado, E., Hibdon, E. S., Samuelson, L. C. Differential sensitivity to Wnt signaling gradients in human gastric organoids derived from corpus and antrum. American Journal of Physiology-Gastrointestinal and Liver Physiology. 325 (2), G158-G173 (2023).
- Busslinger, G. A., et al. Human gastrointestinal epithelia of the esophagus, stomach, and duodenum resolved at single-cell resolution. Cell Reports. 34 (10), 108819 (2021).
- Yang, R., et al. A quick and reliable image-based AI algorithm for evaluating cellular senescence of gastric organoids. Cancer Biology & Medicine. 20 (7), 519 (2023).
- Skubleny, D., et al. Murine and Human gastric tissue establishes organoids after 48 hours of cold ischemia time during shipment. Biomedicines. 11 (1), 151 (2023).

