Stromal Cell Isolation From Hematopoietic Organs
Summary
Here we present protocols that enable isolation of stromal cells from murine bone, bone marrow, thymus and human thymic tissue compatible with single-cell multiomics.
Abstract
Single-cell sequencing has enabled the mapping of heterogeneous cell populations in the stroma of hematopoietic organs. These methodologies provide a lens through which to study previously unresolved heterogeneity at steady state, as well as changes in cell type representation induced by extrinsic stresses or during aging. Here, we present step-wise protocols for the isolation of high-quality stromal cell populations from murine and human thymus, as well as murine bone and bone marrow. Cells isolated through these protocols are suitable for generating high-quality single-cell multiomics datasets. The impacts of sample digestion, hematopoietic lineage depletion, FACS analysis/sorting, and how these factors influence compatibility with single-cell sequencing are discussed here. With examples of FACS profiles indicating successful and inefficient dissociation and downstream stromal cell yields in post-sequencing analysis, recognizable pointers for users are provided. Considering the specific requirements of stromal cells is crucial for acquiring high-quality and reproducible results that can advance knowledge in the field.
Introduction
In the healthy adult, de novo production of blood cells occurs in the bone marrow and the thymus. Stromal cells at these sites are essential for maintenance of hematopoiesis, but stroma constitutes less than 1% of the tissue1,2,3,4. Obtaining pure isolates of hematopoiesis supporting stroma therefore constitutes a significant challenge, particularly for single-cell multiomics that requires expedient processing to obtain samples of high quality. Components of different digestion cocktails may interfere with certain steps in multiomics analysis5,6. The protocols presented here detail the isolation of a wide variety of stromal cells from bone marrow and thymic tissues.
Perturbations of stromal constituents in both bone marrow and thymus result in profound disruption in blood cell development and can result in malignancies7,8,9. Hematopoiesis supporting stroma is damaged following cytotoxic conditioning and bone marrow transplantation, resulting in reduced secretion of cytokines and growth factors that sustain hematopoietic stem and progenitor cells (HSPCs)2,10,11. Furthermore, aging affects bone marrow and thymus stromal cells likely contributing to aged hematopoietic phenotypes. The thymus is the first organ to undergo extensive age-associated involution. Fat and fibrotic tissue start replacing T cell supportive stroma as early as the onset of puberty12,13. In the bone marrow, adipocyte content increases with age and the vascular and endosteal niches are significantly remodeled14,15,16.
To enable study of hematopoiesis supportive stroma across multiple stress states and in the case of the thymus of both human and murine tissue, we have optimized previously published digestion protocols1,2,8,17,18. These protocols ensure efficient and reproducible isolation of cells, and they are compatible with single-cell RNAsequencing (scRNAseq) and other types of multiomics.
Protocol
All work with human tissue was conducted after approval by the Massachusetts General Hospital Internal Review Board (IRB). All animal procedures were conducted in accordance with the Massachusetts General Hospital Institutional Animal Care and Use Committee (IACUC) guidelines. C57Bl/6 mice, 8-10 weeks old, and both males and females, were used for the present study. The animals were obtained from a commercial source (see Table of Materials).
1. Preparation of murine thymic tissue
- Prepare the following buffers and solutions.
- Medium 199 (M199) with 2% (v/v) fetal bovine serum (FBS) (see Table of Materials).
- Murine dissociation cocktail: 0.5 WU/mL Liberase TM and 6.3 U/mL DNase I in M199 + 2% FBS (see Table of Materials).
- M199 with 2% (w/v) Bovine serum albumin (BSA).
NOTE: FBS containing medium can be switched to BSA to improve the RNA quality.
- Perform the murine thymus dissection
- Euthanize the mouse by CO2 asphyxiation (following institutionally approved protocols) and place it on its back. Wet the fur on the chest by spraying 70% ethanol and proceed to open the chest cavity.
- Carefully dissect the thymus by first making a transverse incision just below the rib cage with surgical scissors. Cut the ribs on each side of the mouse all the way up to the clavicle. Then cut the diaphragm so that the chest wall can be released. Lift the ribs with a pair of forceps to expose the thoracic cavity.
- The thymus is a white, bilobular organ, sitting at the top of the cavity, just above the heart. Hold the thymus with the forceps and gently cut the connective tissue that holds the thymus in place. Put the dissected thymic tissue in the well of a 6-well plate with M199 + 2% FBS on ice.
NOTE: When working with aged or radiation-conditioned mice or mutant strains with thymic defects, the thymus may be so small that a dissection microscope is required to reliably excise the organ.
- The thymus is a white, bilobular organ, sitting at the top of the cavity, just above the heart. Hold the thymus with the forceps and gently cut the connective tissue that holds the thymus in place. Put the dissected thymic tissue in the well of a 6-well plate with M199 + 2% FBS on ice.
- Using a dissection microscope, remove any extrathymic tissue using blunt-ended forceps and micro-spring scissors.
- Perform enzymatic dissociation of murine thymic tissue (30 min in increments of 10 min)
- Place the cleaned thymus in the cap of a 15 mL tube and finely mince the tissue with sterile surgical scissors.
NOTE: Mincing the tissue in the cap of the tube that is to be used for the digestion minimizes tissue loss due to transfer from one container to another. This is very important when working with aged or radiation-conditioned mice or mutant strains with thymic defects. The small thymus size in these settings necessitates the need to reduce the loss of material wherever possible. - Add 2 mL of murine dissociation cocktail to the 15 mL tube, attach the cap containing the thymus pieces, and invert 5 times to ensure that the tissue is resuspended in the dissociation cocktail.
- To prevent leakage, wrap the cap of the tube in paraffin film. Then place the tube horizontally in a 37 °C water bath with 250 rpm shaking. Incubate for 10 min.
NOTE: The success of the digestion protocol depends on the incubation being performed at 37 °C and with agitation. The described protocol has been optimized using a shaking water bath. If that is not available, other approaches that enable agitation in a heated setting might work, but we recommend that the protocol be optimized for these settings first. - Place the 15 mL tube in a rack and let the tissue pieces settle. Carefully remove the supernatant and pass it over a 70 µm cell strainer into an ice-cold 50 ml tube.
- Repeat steps 2-4 twice for a total of 3 rounds of digestion. The tissue should be more or less completely dissociated by the end of the last incubation.
- Add 20 mL of M199 + 2% FBS to the collected cell suspension. Spin down at 500 x g for 5 min at 4 °C.
- Remove the supernatant and resuspend in an appropriate volume of M199 + 2% FBS. Then proceed to cell counting.
NOTE: The volume of M199 + 2% FBS to resuspend in will depend on the state of the thymus. For 6-10-week-old mice, we recommend resuspending the tissue in 3-4 mL of M199 + 2% FBS. However, for experiments using aged or radiation-conditioned tissue or mutant strains with thymic defects, the small thymus size will require lower volumes ranging from 0.1-1 mL. - Count cells by diluting the cell suspension 1:2 in 0.4% (w/v) trypan blue solution. Load cells onto a hemocytometer and count under a bright-field microscope. Count unstained cells to determine the number of live cells per milliliter and the number of blue-stained cells to assess sample viability.
- Place the cleaned thymus in the cap of a 15 mL tube and finely mince the tissue with sterile surgical scissors.
- Perform fluorescence activated cell sorting of murine thymic stroma
- Spin down the cells at 500 x g for 5 min at 4 °C. Resuspend cells at a concentration of 5 x 107 cells/mL in M199 + 2% FBS supplemented with 25 U/mL Protector RNase inhibitor (see Table of Materials).
- Add murine Fc Block (see Table of Materials) at a concentration of 2 µg/mL and incubate for 10 min at 4 °C.
- Stain the cells with the following antibodies: CD45-PE/Cy7 (4 µg/mL), Ter119-PE (4 µg/mL), CD31-BUV737 (2 µg/mL), and EpCam-BV711 (2 µg/mL) (see Table of Materials) for 30 min at 4 °C.
- Wash the samples in M199 + 2% BSA and spin down at 500 x g for 5 min at 4 °C.
- Remove supernatant and resuspend cells in M199 + 2% BSA containing a viability dye such as DAPI (0.5 µg/mL) or 7-AAD (1 µg/mL) (see Table of Materials). Proceed to FACS isolation of thymic stromal cells following established protocols19.
2. Preparation of human thymic tissue
- Prepare the following buffers and solutions.
- Medium 199 (M199) with 2% (v/v) FBS.
- M199 with 1.5% (w/v) BSA.
- Human dissociation cocktail: 1 mg/ml Collagenase IV and 2 mg/mL Dispase and 6.3 U/mL DNase I in M199 +1.5% BSA (see Table of Materials).
NOTE: The last digestion step in the preparation of human thymic tissue includes trypsin. It is therefore essential that all steps up until that point are carried out in BSA containing buffer as FBS will inhibit trypsin activity.
- Perform human thymic tissue isolation
- Place the human thymus (collected following previously published method19) in a suitable volume of M199 + 1.5% BSA and store it on ice until processing starts.
- While working with human thymic tissue, handle it as if it is potentially infectious by carrying out the work in a tissue culture hood and using standard precautions per CDC guidelines20.
- Put the tissue in a 10 cm Petri dish with 10 mL of ice-cold M199 + 1.5% BSA. Using sterile forceps and scissors, carefully clean away extrathymic fat or tissue that was damaged as the organ was extracted.
- Using surgical scissors and a pair of forceps, cut the thymus into 1 cm3 cubes and use the plunger of a 10 mL syringe to gently push down on the pieces. This will release some of the developing thymocytes, thus reducing the volume of tissue to be digested.
NOTE: Save the released hematopoietic fraction if subsequent analysis of thymocyte developmental stages is needed. However, do not combine this fraction with the stromal cell fraction generated during enzymatic digestion. It was observed that pooling the two fractions significantly increases the risk of clogs forming in the cell suspension, ultimately reducing the yield of stromal cells. Few stromal cells are lost during the gentle crushing of the tissue.
- Perform enzymatic dissociation of human thymic tissue (90 min in increments of 30 min)
- Transfer approximately 5 mg of the lightly crushed thymic tissue pieces to the cap of a 50 mL conical tube. Then cut the tissue finely using sterile surgical scissors.
- Pipette 8 mL of human dissociation cocktail into the 50 mL tube, attach the cap containing the minced human thymic tissue, and then gently invert the tube 5 times to ensure that all the tissue is mixed in with the enzyme cocktail.
- Secure the cap of the tube with paraffin film to prevent leakage. Incubate the tube horizontally in a shaking (250 rpm) water bath at 37 °C for 30 min.
- Place the 50 mL tube in a rack and let the human thymic tissue pieces settle before removing the supernatant and passing it over a 70 µm cell strainer into a new 50 ml tube placed on ice.
- Repeat steps 2.3.2-1.3.4 for a total of two rounds of digestion with the human dissociation cocktail.
- After the second round of digestion is done, add an additional 8 mL of the human dissociation cocktail to the remaining tissue. Then also pipette 2 mL of 0.25% trypsin into the tissue dissociation tube. Incubate for an additional 30 min at 37 °C, shaking at 250 rpm.
NOTE: It is absolutely essential that the digestion time for human tissue is at least 90 min, as shorter incubation times lead to drastically reduced stromal cell yields. - Once the last incubation step is done, pool the cell suspension and remaining tissue fragments with the supernatant collected in previous steps by passing over a 70 µm cell strainer. Add 3 mL of FBS to the sample to break the trypsin reaction and place on ice.
- Proceed to counting the cells as described in step 1.3.
- Perform fluorescence activated cell sorting of human thymic stroma
- Spin down the cell suspension of human thymus and resuspend at a concentration of 5 x 107 cells/mL in M199 + 2% FBS supplemented with 25 U/mL Protector RNase inhibitor.
- Incubate with human Fc Block (5 µg/mL) (see Table of Materials) for 10 min at 4 °C.
- Stain the sample for 30 min at 4 °C with CD45-BV711 (2.5 µg/mL), CD235a-BV711 (2.5 µg/mL), Lineage-cocktail-FITC (3.76 µg/mL), CD66b-FITC (20 µL/100 µL sample), CD8-APC/Cy7 (5 µL/100 µL sample), CD4-BV605 (5 µL/100 µL sample), CD31-PE/Dazzle594 (10 µg/mL), and EpCam-BV421 (2.5 µg/mL) (see Table of Materials).
- Wash the samples in M199 + 1.5% BSA and spin down at 500 x g for 5 min at 4 °C.
- Remove the supernatant and resuspend cells in M199 + 1.5% BSA containing a viability dye such as DAPI (0.5 µg/mL) or 7-AAD (1 µg/mL). Proceed to FACS isolation of thymic stromal cells following established protocols19.
3. Preparation of murine bone and bone marrow tissue
NOTE: Bone and bone marrow fractions are prepared in two separate digestion reactions to obtain maximum purity of stromal cells and optimal dissociation of tissues. The samples can be pooled after the digestion steps to be sorted as one stromal compartment.
- Prepare the following buffers and solutions.
- M199 with 2% (v/v) FBS.
- Murine B&M (bone and marrow) dissociation cocktail: Stemxyme 2 mg/mL, Dispase 1 mg/mL and 10 U/mL DNase I (see Table of Materials) in M199 +2% FBS.
NOTE: Prepare B&M dissociation mix fresh and keep mix on ice until use. - M199 with 0.5% (w/v) BSA.
- Phosphate-buffered saline (PBS) with 0.5% (w/v) BSA.
- M199 with 2% (w/v) Bovine serum albumin (BSA) and 2 mM EDTA.
NOTE: FBS containing medium can be switched to BSA to improve RNA quality.
- Perform murine bone marrow separation
- Euthanize the mouse by CO2 asphyxiation (following institutionally approved protocols). Spray with 70% ethanol to wet the fur.
- Carefully remove the femur, tibia, pelvis, and humerus from each side and place them in a well containing M199 + 2% FBS on ice21,22.
- Remove all muscle, ligament, and cartilage tissue with tissue wipes to minimize damage to the bone and preserve the periosteal layer. Place them in a new well with M199 + 2% FBS on ice.
- Use a scalpel and cut through the growth plates of the long bones to remove the epiphysis and place it in a 10 cm dish with M199 + 2% FBS on ice.
- Flush the bone marrow out of the bones until pale white. Use a 28 G needle and a 10 mL syringe filled with 10 mL M199 + 2% FBS to flush the marrow into a 15 mL tube on ice. Place the flushed bone in the 10 cm dish with the epiphysis.
NOTE: Complete flushing of the bone marrow for entire separation of tissues will result in better sample preparation.
- Perform enzymatic dissociation of murine bone marrow tissue (total 30 min in increments of 10 min)
- Leave the 15 mL tube standing upright on ice until the marrow sinks to the bottom of the tube. Slowly remove the 10 mL M199 + 2% FBS by pipetting.
- Add 4 mL of B&M dissociation cocktail to the 15 mL tube and place it in a 37 °C water bath (no shaking) for 10 min.
- After 5 min, invert the tube three times and place it back in the water bath.
- After the first 10 min, ensure the pellet is at the bottom and collect the supernatant while filtering through a 70 µm cell strainer into an ice-cold 50 mL tube. Add 2 mL of fresh B&M dissociation cocktail to the remaining BM pellet and place it back in the water bath.
- Dissociate any remaining bone marrow pieces by vigorous pipetting using a 1 mL pipette. Collect all the resulting cell suspension in the same tube as in the previous steps. Add 20 mL of M199 + 0.5% BSA + 2 mM EDTA to the collected cell suspension.
NOTE: In step 3.3.5, the buffer added after the final digested sample contains EDTA to aid in inhibiting further enzymatic activity.
- Perform hematopoietic cell depletion of murine bone marrow tissue.
- Centrifuge cells at 500 x g for 5 min at 4 °C. Resuspend in 500 µL of PBS + 0.5% BSA with biotin antibodies (5 mg/mL) targeting CD45, Ter119, CD11b, Gr1, B220, and CD3 (see Table of Materials). Transfer to a round-bottom tube with a cap.
- Incubate on an orbital shaker for 10 min at room temperature in the dark.
- Vortex the magnetic beads thoroughly and add 25 µL of magnetic beads to the tube.
- Incubate on an orbital shaker for 5 min at room temperature in the dark.
- Place the tube in the matching magnet for 2-3 min and collect the supernatant in a fresh tube.
NOTE: The bone marrow can be pooled or kept separate from the bone fraction after this step. If the lineage depletion is successful, the resulting cell suspension should be devoid of red cells.
- Perform enzymatic dissociation of murine bone tissue.
- Break the bones into smaller pieces in the 10 cm dish with the flat cap-end of a 50 mL tube. Filter the supernatant through a 70 µm cell strainer to isolate bone fragments inside the filter.
NOTE: Alternatively, a mortar and pestle can be used for bone fragmentation. This option, however, tends to generate more debris and lower stromal cell viability when the bone is ground rather than broken. The stroma cells are still attached to and in the bone, and the supernatant containing hematopoietic cells is to be discarded. - Use scissors to cut the bone fragments in the cell strainer. This will increase the surface area for a more efficient enzymatic dissociation. Wash the bone with 5 mL of M199 + 2% FBS.
- Place the bone fraction in 5 mL of murine B&M dissociation (see Table of Materials) mix in a new 50 mL tube.
- To prevent leakage, wrap the cap of the tube in paraffin film. Then place the tube horizontally in a 37 °C water bath with 120 rpm shaking for 30 min.
- After the digestion, add 25 mL of M199 + 0.5% BSA + 2 mM EDTA and filter the supernatant containing the stromal cells through a 70 µm cell strainer into an ice-cold 50 ml tube.
NOTE: Check that the resulting bone fragments have been visibly diminished by the digestion. In step 3.5.5, the buffer added after the final digested sample contains EDTA to aid in inhibiting further enzymatic activity.
- Break the bones into smaller pieces in the 10 cm dish with the flat cap-end of a 50 mL tube. Filter the supernatant through a 70 µm cell strainer to isolate bone fragments inside the filter.
- Perform fluorescence activated cell sorting of murine bone and bone marrow stroma.
- Spin down the cells at 500 x g for 5 min at 4 °C.
- Resuspend in murine Fc Block at a concentration of 10 µg/mL in PBS + 0.5% BSA + 2 mM EDTA and incubate for 10 min at 4 °C.
- Stain the cells (up to 10 million cells/100 µL total staining volume) with the following antibodies: CD45-PE/Cy7 (1 µg/mL), Ter119-PE/Cy7 (1 µg/mL), CD31-BV421 (0.66 µg/mL), CD140a-APC (2 µg/mL), Sca1-AF700 (0.66 µg/mL), CD51-PE (2 µg/mL), CD105-PE/dazzle (2 µg/mL) (see Table of Materials).
- Leave Calcein-AM to equilibrate to room temperature and resuspend in 50 µL of DMSO. Prepare fresh for every use. Keep at room temperature after resuspension. Pre-dilute the calcein in PBS (0.1 mg/mL) before adding to the sample (20 µg/mL) already resuspended in the antibody staining cocktail and incubate for 20-30 min at 4 °C in the dark.
- Wash the samples in PBS + 0.5% BSA + 2 mM EDTA and centrifuge at 500 x g for 5 min at 4 °C.
- Remove the supernatant and resuspend the cells in PBS + 0.5% BSA + 2 mM EDTA. Proceed to FACS isolation of bone and bone marrow stromal cells following established protocols19.
Representative Results
These protocols yield reproducible stromal cell varieties from the thymus and bone marrow suitable for flow cytometric analysis, as well as single-cell multiomics, such as scRNA sequencing. Murine thymic tissue undergoes significant remodeling in response to stressors, such as the cytotoxic conditioning that precedes bone marrow transplantation or the natural aging process. As a consequence, thymic cellularity is drastically reduced in both of these settings (Figure 1A). While a thymus from an 8-week-old wild-type mouse contains approximately 100 million cells, the cellularity of a 2-year-old mouse can be expected to be half of that, and a thymus 4 days post-irradiation and bone marrow transplantation can be as low as 5,00,000 cells (Figure 1A).
Cytotoxic conditioning will preferentially ablate hematopoietic cells, so the stromal compartment will be proportionally enriched (Figure 1B). However, the overall low cellularity will make isolating sufficient numbers of thymic stromal cells challenging. It may, therefore, be necessary to pool animals to obtain cell numbers compatible with downstream multiomics applications and to minimize the loss of material wherever possible. To perform multiomics on thymic stromal cells, fluorescence-activated cell sorting (FACS) of live, single, CD45-Ter119- cells (Figure 1C) will ensure that sufficient numbers of cells can be sorted from most stress conditions.
To preserve the heterogeneity of the stroma compartment, we typically do not sort on a single positive stroma marker but gate out hematopoietic cells. Including positive stromal cell markers is still recommended for analysis to ensure that proper tissue dissociation has been achieved. However, FACS isolation is imperfect, and as determined by scRNAseq, 30%-50% of the sorted cells will be of hematopoietic origin (Figure 1D,E). This can be improved upon by magnetically depleting CD45+ cells prior to FACS; however, this is not recommended for conditions of reduced thymic cellularity (Figure 1A) as the loss of material that comes with the extra processing involved in magnetic depletion results in stroma yields too low for downstream applications.
For the isolation of human thymic stroma, the most critical step is the enzymatic digestion. If the dissociation step is rushed, the overall stromal cell yield, as well as stromal cell subtype representation, will be severely diminished (Figure 2A). It is also recommended to always include the markers of thymic endothelium and epithelium to ensure that tissue digestion has been optimal, as simply gating negative for hematopoietic markers will not be sufficient to gauge actual stromal cell release. This is exemplified in Figure 2A, where the percentage of cells negative for hematopoietic markers is higher in the sample digested for 30 min compared to the sample incubated for 90 min. However, as seen in the consecutive gate, the yield of endothelial and epithelial cells is very poor in the shorter digestion protocol (Figure 2A).
For FACS isolation of human thymic stromal cells for multiomics applications, staining with a hematopoietic lineage antibody cocktail, as well as CD4 and CD8 antibodies is suggested, in addition to the pan-hematopoietic marker CD45 and the erythroid marker CD235a (Figure 2B) to improve stromal cell enrichment. Thus, for human multiomics analysis of thymic stroma, live, single, CD45-CD235a-Lineage-CD4-CD8- cells should be FACS sorted (Figure 2B). As seen for scRNAseq of murine samples, this still results in contamination of 30%-50% hematopoietic cells, but this still gives acceptable resolution of the human thymus stromal compartment (Figure 2C,D).
For bone and BM stroma, the same strategy was used as for thymic tissue; stromal cells are isolated by gating on cells negative for hematopoietic markers, and further stromal cell markers are included as digestion controls (Figure 3). The dissociation of calcified tissue like bone results in a significant accumulation of debris. The addition of calcein as a viability dye will allow the exclusion of most of this debris, which otherwise falsely inflates sorted numbers and decreases gated population frequencies (Figure 3). This is exemplified in the FACS plots comparing gating on all cells and just gating on Calcein+ (Figure 3). As seen for human thymic stromal cells, the percentage of hematopoietic marker-negative cells is not a reliable readout for bone and BM tissue dissociation efficiency (Figure 3). While the CD45-Ter119- gate can contain an abundance of cells in a sample where the digestion step failed, further analysis of typical bone and BM stromal cell markers CD31, CD140a, and CD51 clearly demonstrate poor stroma release (Figure 3 and Figure 4).
For FACS isolation of murine bone and BM stromal cells, using the gating strategy is suggested as in Figure 3, where we only sort CD45-Ter119-Calcein+ but monitor the forward and side scatter properties and stromal cell marker expression of the sorted population to ensure optimal digestion (Figure 3A). The combination of hematopoietic lineage-positive magnetic cell depletion of the bone marrow prior to FACS and sorting on the shown gates results in single-cell sequencing of cells that are >90% stroma. An example of such a scRNA seq run (Figure 5A,B) illustrates this low hematopoietic contamination after transcriptome-based annotation of the analyzed cells (examples of hematopoietic marker genes Figure 5C). This is in stark contrast to the thymic stromal preparations where magnetic cell depletion was not performed before the sorting step due to the limited starting material in post-transplant thymic tissue (Figure 1F).
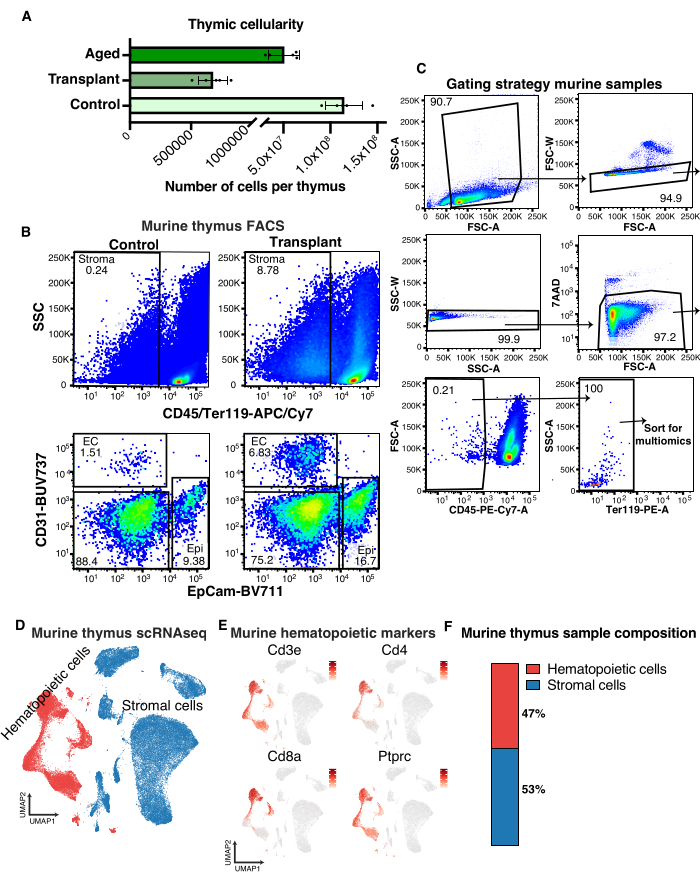
Figure 1: Flow cytometric and single-cell RNA sequencing analysis of murine thymic stroma. (A) Quantification of thymocyte numbers in 8-week-old mice (Control), 8-week-old mice lethally irradiated and transplanted 4 days prior (Transplant) and 2-year-old mice (Aged). (B) Representative flow cytometry plots demonstrating the enrichment in stromal cell subtypes 4 days following lethal irradiation and transplantation. (C) Flow cytometry gating strategy to sort murine thymic stroma for single-cell multiomics analysis. (D) UMAP of murine thymus indicating stroma (blue) and hematopoietic cells (red). (E) UMAPs showing murine hematopoietic marker genes in thymus samples. (F) Quantification of murine stromal and hematopoietic cells in thymus scRNAseq samples. Please click here to view a larger version of this figure.
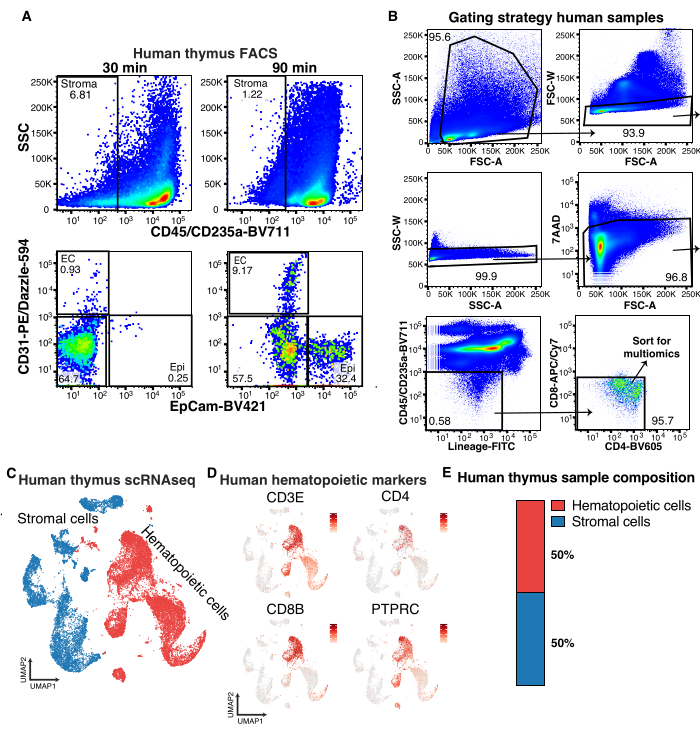
Figure 2: Flow cytometric and single-cell RNA sequencing analysis of human thymic stroma. (A) FACS plots showing representative results of the impact of different digestion times on the release of human thymic stromal cell populations. (B) Flow cytometry gating strategy to sort human thymic stroma for single-cell multiomics analysis. (C) UMAP of human thymus indicating stroma (blue) and hematopoietic cells (red). (D) UMAPs showing human hematopoietic marker genes in thymus samples. (E) Quantification of human stromal and hematopoietic cells in thymus scRNAseq samples. Please click here to view a larger version of this figure.
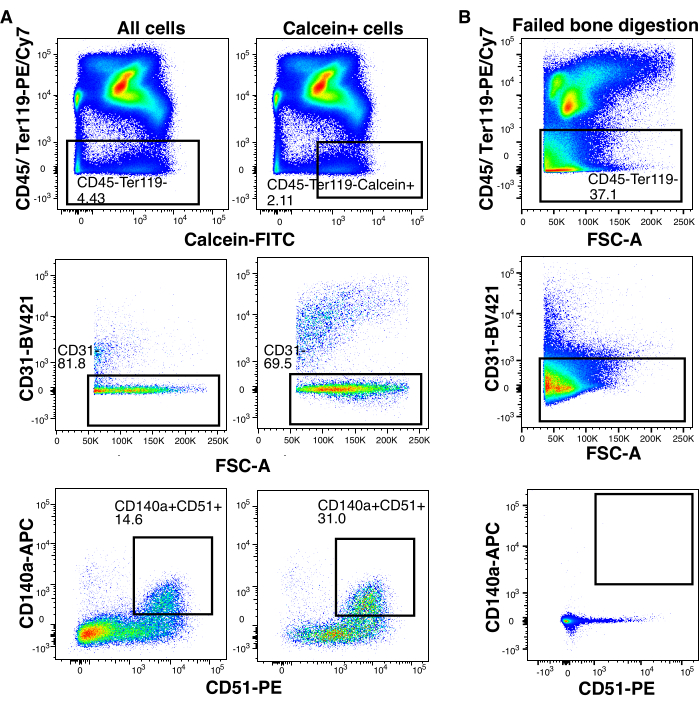
Figure 3: Murine bone marrow stromal markers and Calcein are helpful to estimate dissociation efficiency. (A) Representative flow cytometry analysis of CD45–Ter119– murine bone marrow stromal cells gated on all cells and Calcein+ cells. With subsequent CD31– gating showing a higher proportion of CD140a+CD51+ cells when selecting Calcein+ cells. (B) Flow cytometry analysis representative of a failed bone digestion with missing CD140a+CD51+ cells. Please click here to view a larger version of this figure.
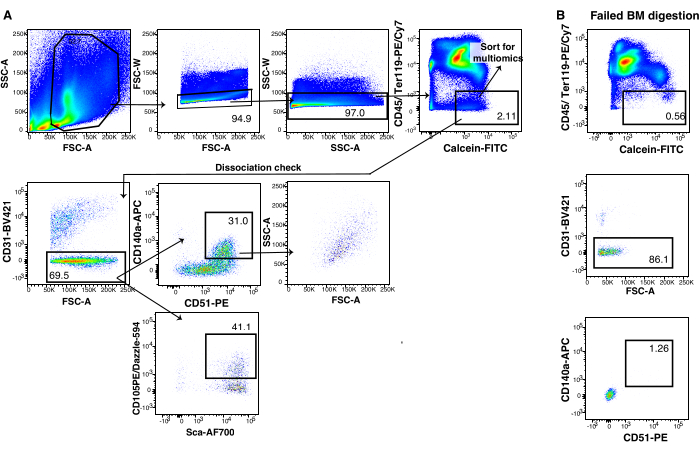
Figure 4: Bone marrow stroma gating strategy. (A) Flow cytometry gating strategy to sort murine bone marrow stroma for single-cell multiomics. (B) Flow cytometry analysis representative of a failed bone marrow digestion with missing CD140a+CD51+ cells. Please click here to view a larger version of this figure.
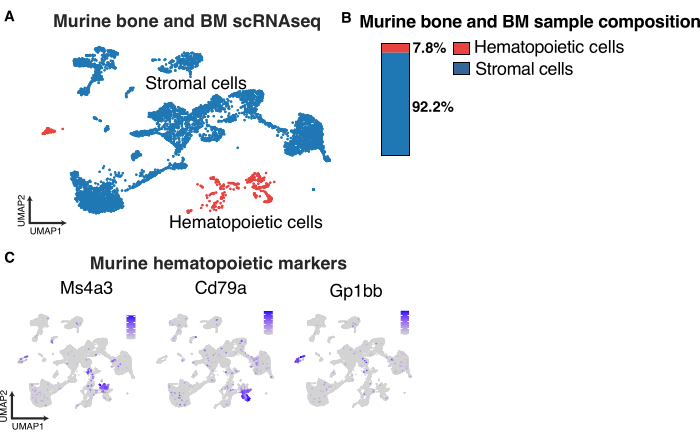
Figure 5: Bone marrow single-cell RNA sequencing with stromal and hematopoietic cell composition. (A) UMAP of murine bone and bone marrow indicating stroma (blue) and hematopoietic cells (red). (B) Quantification of stromal and hematopoietic cells in murine bone and bone marrow samples. (C) UMAP of hematopoietic marker genes in murine bone marrow samples. Please click here to view a larger version of this figure.
Discussion
Stromal cells in hematopoietic organs are critical for normal blood production and hematopoietic stroma perturbations can result in severe impairments in hematopoietic maintenance and response to stress9,23,24. Insight into hematopoietic stromal cells is essential for understanding hematological diseases7,9,10,24 and for the ability to develop therapeutics to combat them25,26. Reproducibly separating hematopoietic stroma from parenchyma is however a challenge, particularly with the advent of single-cell multiomics which require high-quality sample preparations while maximizing stromal cell yield and variety. The protocols described here have been optimized to be compatible with most downstream multiomics applications while meeting the challenges of isolating hematopoietic stromal cells1,2,19.
The first issue to consider for isolation of hematopoietic stromal cells is dissociation conditions. Optimal dissociation is critical to release stroma cells from the tissue to reflect full heterogeneity of the stroma compartment1,2. Choosing the right dissociation mix depends on the experimental scope and downstream protocols. We would recommend preparing dissociation mixes as freshly as possible and keeping them on ice until use. Some components are hard to dissolve such as dispase and might require more time for pipet mixing. Vortexing is not recommended as this may disrupt the native conformation of the digestion enzymes, ultimately leading to their inactivation. Importantly, EDTA will inhibit enzymatic activity and should be avoided during active digestion steps, but can be employed to stop the reaction if the enzyme containing buffer cannot be removed promptly. In addition to the enzyme mixture itself, two points are critical to efficient dissociation of tissue, (1) thorough crushing and cutting to increase surface area and, (2) sufficient incubation time with the digestion enzymes. Human thymic tissue is for instance significantly harder to dissociate than murine tissue and digestion times must therefore be longer. If adjustment is not made for human tissue, stromal yield and cell variety will be decreased (Figure 2A). Dissociation enzymes require an optimal temperature of 37 °C which inevitably influences gene expression27. Although cold or lower temperature dissociation would be desirable to conserve cell states, currently there are no commercially available cold-active dissociation enzyme combinations suitable for bone and bone marrow. For any tissue that requires digestion, the heterogeneity and the percent contribution acquired from different cell types will invariably change with the digestion time, enzyme cocktail composition and processing. Downstream of these initial steps, heterogeneity and representation will be further limited by filtration steps such as nozzle sizes during sorting and capture of challenging cell-types (e.g., adipocytes from stroma) and fluidic device channel diameters. Thus, when comparing datasets, we should be mindful that these differences in protocols can impact the representation of subpopulations. It logically follows that we must be vigilant about keeping processing steps equal when measuring the impact of perturbations in stromal systems.
While the cost of sequencing has been and is decreasing, single cell technologies are often limited by the number of cells that can be analyzed. To optimize the capacity of each reagent kit, hematopoietic cell magnetic depletion was employed whenever possible. This enables the avoidance of a high number of contaminant cells which are much more numerous than stroma in both bone marrow and thymic sample fractions. However, if the starting material is limited, as in the case of post-transplant thymic tissue, the increased, non-specific loss of cells that magnetic depletion strategies invariably incur, may make this inadvisable. In addition, during preparation and digestion of the tissues, crushing, cutting and enzymatic release of extracellular matrix components generates a large amount of debris. This type of contaminant, if left in the sample for analysis, can cause clogging issues in downstream processing, cause negative wells for single cell index sorting and highly inflate parent gate percentages in analysis. Calcein staining was used to gate positive for cells/live cells. Calcein comes in different colors which makes it highly adaptable to your staining matrix. When working with calcein be careful to not subject the dye to repeated freeze-thaw cycles (i.e., always prepare fresh and keep at room temperature after dissolving).
The ability to multiplex samples based on antibodies barcoded with DNA oligos28 allows increased utility of single cell sequencing reactions on platforms such as 10x Genomics. The hashtagging antibodies designed for sample multiplexing come with different targets for cell surface molecules or the nuclear membrane and specificity for both mouse and human. While the cell hashtagging system has been used successfully for multiple tissues, experiments combining digestion enzyme mixes with subsequent use of hashtags can lead to drastically reduced recovery. We have attempted to include additional washing steps post-dissociation and added EDTA to inhibit the activity of the digestion enzymes. However, even with these steps, expect lower hashtag identification than hematopoietic cells. It was found that with enzymatic digestion protocols, the hashtag capture rates were between 30%-70%. This occurs despite the fact that the same antibody clone with a fluorochrome conjugate labeled >90% of stromal cells in the same population. Any experimental design pairing enzymatic digestion of tissue with hashtagging should be piloted and caution taken to ensure enzymatic inhibition.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We were supported with expert technical assistance by the HSCI-CRM Flow Cytometry facility at Massachusetts General Hospital and the Bauer Core Facility at Harvard University. T.K and K. G were supported by the Swedish Research Council and C.M. by the German Research Foundation. We thank Sergey Isaev and I-Hsiu Lee for assistance in analysis of single-cell RNA sequencing data.
Materials
| 0.25% Trypsin-EDTA | Thermo Fisher Scientific | 25200-072 | |
| 7AAD (7-aminoactinomycin D) | BD Biosciences | 559925 | |
| Anti-Human Lineage Cocktail 3-FITC | BD Biosciences | 643510 | |
| Bovine Serum Albumin | Millipore Sigma | A9647 | |
| C57Bl/6 mice | Jackson | 664 | Males or females, 8-12 weeks old |
| Calcein | Fisher Scientific | 65-0853-78 | |
| Collagenase IV | Millipore Sigma | C5138 | |
| Corning Sterile Cell Strainers, White, Mesh Size: 70 µm | Fisher Scientific | 08-771-2 | |
| DAPI (4',6-Diamidino-2-Phenylindole, Dilactate) | Biolegend | 422801 | |
| Dispase II | Thermo Fisher Scientific | 17105041 | |
| Dnase I Solution | Thermo Fisher Scientific | 90083 | 2500 U/mL |
| Easysep mouse streptavidin RapidSpheres Isolation kit | StemCell Technologies | 19860 | |
| Fetal Bovine Serum | Gibco | A31605-01 | Qualified One Shot |
| Human Fc Block | BD Biosciences | 564220 | |
| Liberase TM | Millipore Sigma | 5401127001 | Research Grade |
| Medium 199 | Gibco | 12350 | |
| Mouse anti-human CD235a-BV77 | BD Biosciences | 740785 | |
| Mouse anti-human CD31-PE/Dazzle594 | Biolegend | 303130 | |
| Mouse anti-human CD45-BV77 | Biolegend | 304050 | |
| Mouse anti-human CD4-BV605 | BD Biosciences | 562658 | |
| Mouse anti-human CD66b-FITC | BD Biosciences | 555724 | |
| Mouse anti-human CD8-APC/Cy7 | BD Biosciences | 557760 | |
| Mouse anti-human EpCam-BV421 | Biolegend | 324220 | |
| Protector RNase Inhibitor | Millipore Sigma | 3335402001 | |
| Rat anti-mouse CD105-PE /dazzle594 | Biolegend | 120424 | |
| Rat anti-mouse CD11b-Biotin | Biolegend | 101204 | |
| Rat anti-mouse CD140a-APC | Fisher Scientific | 17-1401-81 | |
| Rat Anti-Mouse CD16/CD32 (Mouse BD Fc Block) | BD Biosciences | 553142 | |
| Rat anti-mouse CD31-BUV737 | BD Biosciences | 612802 | |
| Rat anti-mouse CD31-BV421 | Biolegend | 102424 | |
| Rat anti-mouse CD3-Biotin | Biolegend | 100244 | |
| Rat anti-mouse CD45.2-Biotin | Biolegend | 109804 | |
| Rat anti-mouse CD45-PE/Cy7 | Biolegend | 103114 | |
| Rat anti-mouse CD45-PE/Cy7 | Biolegend | 103114 | |
| Rat anti-mouse CD45R/B220-Biotin | Biolegend | 103204 | |
| Rat anti-mouse CD51-PE | Biolegend | 104106 | |
| Rat anti-mouse EpCam-BV711 | BD Biosciences | 563134 | |
| Rat anti-mouse Ly-6A/E(Sca-1)-AF700 | Biolegend | 108142 | |
| Rat anti-mouse Ly-6G/Ly-6C(Gr1)-Biotin | Biolegend | 108404 | |
| Rat anti-mouse Ter119-Biotin | Biolegend | 116204 | |
| Rat anti-mouse Ter119-PE | Biolegend | 116208 | |
| Rat anti-mouse Ter119-PE/Cy7 | Biolegend | 116222 | |
| Stemxyme | Worthington Biochemical | LS004107 |
References
- Baryawno, N., et al. A cellular taxonomy of the bone marrow stroma in homeostasis and leukemia. Cell. 177 (7), 1915-1932 (2019).
- Severe, N., et al. Stress-induced changes in bone marrow stromal cell populations revealed through single-cell protein expression mapping. Cell Stem Cell. 25 (4), 570-583 (2019).
- Han, J., Zuniga-Pflucker, J. C. A 2020 view of thymus stromal cells in t cell development. J Immunol. 206 (2), 249-256 (2021).
- Park, J. E., et al. A cell atlas of human thymic development defines t cell repertoire formation. Science. 367 (6480), (2020).
- Denisenko, E., et al. Systematic assessment of tissue dissociation and storage biases in single-cell and single-nucleus rna-seq workflows. Genome Biol. 21 (1), 130 (2020).
- Lischetti, U., et al. Dynamic thresholding and tissue dissociation optimization for cite-seq identifies differential surface protein abundance in metastatic melanoma. Commun Biol. 6 (1), 830 (2023).
- Ding, L., Saunders, T. L., Enikolopov, G., Morrison, S. J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 481 (7382), 457-462 (2012).
- Mendez-Ferrer, S., et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 466 (7308), 829-834 (2010).
- Raaijmakers, M. H., et al. progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 464 (7290), 852-857 (2010).
- Himburg, H. A., et al. Distinct bone marrow sources of pleiotrophin control hematopoietic stem cell maintenance and regeneration. Cell Stem Cell. 23 (3), 370-381 (2018).
- Zhou, B. O., et al. marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting scf. Nat Cell Biol. 19 (8), 891-903 (2017).
- Steinmann, G. G. Changes in the human thymus during aging. Curr Top Pathol. 75, 43-88 (1986).
- Steinmann, G. G., Klaus, B., Muller-Hermelink, H. K. The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand J Immunol. 22 (5), 563-575 (1985).
- Ambrosi, T. H., et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell. 20 (6), 771-784 (2017).
- Ho, Y. H., et al. Remodeling of bone marrow hematopoietic stem cell niches promotes myeloid cell expansion during premature or physiological aging. Cell Stem Cell. 25 (3), 407-418 (2019).
- Kusumbe, A. P., et al. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature. 532 (7599), 380-384 (2016).
- Seach, N., Wong, K., Hammett, M., Boyd, R. L., Chidgey, A. P. Purified enzymes improve isolation and characterization of the adult thymic epithelium. J Immunol Methods. 385 (1-2), 23-34 (2012).
- Stoeckle, C., et al. Isolation of myeloid dendritic cells and epithelial cells from human thymus. J Vis Exp. (79), e50951 (2013).
- Gustafsson, K., Scadden, D. T. Isolation of thymus stromal cells from human and murine tissue. Methods Mol Biol. 2567, 191-201 (2023).
- Amend, S. R., Valkenburg, K. C., Pienta, K. J. Murine hind limb long bone dissection and bone marrow isolation. J Vis Exp. (110), e53936 (2016).
- Zhu, H., et al. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat Protoc. 5 (3), 550-560 (2010).
- Calvi, L. M., et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 425 (6960), 841-846 (2003).
- Kode, A., et al. Leukaemogenesis induced by an activating beta-catenin mutation in osteoblasts. Nature. 506 (7487), 240-244 (2014).
- Agarwal, P., et al. Mesenchymal niche-specific expression of cxcl12 controls quiescence of treatment-resistant leukemia stem cells. Cell Stem Cell. 24 (5), 769-784 (2019).
- Duarte, D., et al. Inhibition of endosteal vascular niche remodeling rescues hematopoietic stem cell loss in aml. Cell Stem Cell. 22 (1), 64-77 (2018).
- O’flanagan, C. H., et al. Dissociation of solid tumor tissues with cold active protease for single-cell rna-seq minimizes conserved collagenase-associated stress responses. Genome Biol. 20 (1), 210 (2019).
- Stoeckius, M., et al. Cell hashing with barcoded antibodies enables multiplexing and doublet detection for single cell genomics. Genome Biol. 19 (1), 224 (2018).

