RNA Interference in the Egg Parasitoid, Trichogramma dendrolimi Matsumura
Summary
The manipulation of RNA interference (RNAi) presents a formidable challenge in many parasitoid species with diminutive size, such as Trichogramma wasps. This study delineated an efficient RNAi method in Trichogramma denrolimi. The present methodology provides a robust model for investigating gene regulation in Trichogramma wasps.
Abstract
The egg parasitoids, Trichogramma spp, are recognized as efficient biological control agents against various lepidopteran pests in agriculture and forests. The immature stages of Trichogramma offspring develop within the host egg, exhibiting remarkable diminutiveness (approximately 0.5 mm in adult length). RNA-interference (RNAi) methodology has emerged as a crucial tool for elucidating gene functions in numerous organisms. However, manipulating RNAi in certain small parasitoid species, such as Trichogramma, has generally posed significant challenges. In this study, we present an efficient RNAi method in Trichogramma denrolimi. The outlined procedure encompasses the acquisition and isolation of individual T. dendrolimi specimens from host eggs, the design and synthesis of double-stranded RNA (dsRNA), the in vitro transplantation and cultivation of T. dendrolimi pupae, the micro-injection of dsRNA, and the subsequent assessment of target gene knockdown through RT-qPCR analysis. This study furnishes a comprehensive, visually detailed procedure for conducting RNAi experiments in T. dendrolimi, thereby enabling researchers to investigate the gene regulation in this species. Furthermore, this methodology is adaptable for RNAi studies or micro-injections in other Trichogramma species with minor adjustments, rendering it a valuable reference for conducting RNAi experiments in other endoparasitic species.
Introduction
Trichogramma spp. are a group of egg parasitoids that have been extensively utilized as highly efficient biological control agents against a wide spectrum of lepidopteran pests in agricultural and forest ecosystems worldwide1,2,3,4. The application of mass-reared Trichogramma provides an environmentally friendly approach for the sustainable management of pests5,6,7. Understanding the molecular biology of Trichogramma wasps provides valuable insights into enhancing the mass-rearing efficiency and field performance of these biological control agents8,9by investigating the methodology of gene regulation and genome editing10.
Since the discovery of double-stranded RNA (dsRNA)-mediated specific genetic interference in Caenorhabditis elegans in 1998, the RNA-interference (RNAi) method has evolved into a vital genetic toolkit for exploring the regulatory mechanisms of organisms by suppressing the expression of target genes11. RNAi experiments have become a standard methodology widely applied to study gene function in numerous insect species12,13. Nevertheless, the manipulation of RNAi presents a formidable challenge in many parasitoid species, particularly among those belonging to the endoparasitic Chalcidoidea family14,15,16. The RNAi method has been documented in at least 13 parasitoid species14,15,16,17,18,19. Among these, the RNAi approach has been comprehensively conducted in Nasonia wasps and is applicable throughout the developmental stages, including embryos, larvae, pupae, and adults14,15,16. It is noteworthy that Nasonia wasps are ectoparasitoids, with their offspring developing in the interstitial space between the host pupa and the puparium, enabling their cultivation in vitro and making them tolerate certain treatments, such as micro-injection. Unlike Nasonia wasps, Trichogramma individuals undergo their entire embryonic, larval, and pupal development inner the host egg. The layer at embryo and larva stages (which may impede dsRNA permeability), vulnerability to damage, and the difficulty in surviving in vitro present formidable obstacles20,21,22. Additionally, the diminutive size of Trichogramma individuals, approximately ~0.5 mm in adult or pupal length, renders them exceedingly intricate to manipulate20,21,22.
In the present study, we outline a comprehensive procedure for conducting RNA interference (RNAi) experiments in Trichogramma denrolimi Matsumura. This procedure encompasses the following procedures: (1) the design and synthesis of double-stranded RNA (dsRNA), (2) microinjection of T. denrolimi pupae, (3) the transplantation and in vitro incubation of these pupae, and (4) the detection of target gene knockdown through RT-qPCR analysis. The target gene selected for the RNAi experiment is the ferritin heavy chain homology (Ferhch). FerHCH, an iron-binding protein, contains a ferroxidase center endowed with antioxidant capabilities, facilitating the oxidation of Fe2+ to Fe3+. It plays an indispensable role in the growth and development of various organisms by maintaining redox equilibrium and iron homeostasis. Depletion of FerHCH can result in the overaccumulation of iron, leading to irreversible tissue damage, and often culminating in significant phenotypic alterations, including growth defects, deformities, and mortality23,24. This study offers a step-by-step guide for conducting RNAi in T. denrolimi, which will be invaluable for investigating the gene functions within the broader context of Trichogramma wasps.
Protocol
NOTE: See the Table of Materials for details related to all materials, instruments, software, and reagents used in this protocol.
1. Collection and maintenance of insect culture
- Adhere a group of ~5,000 eggs of Corcyra cephalonica (Stainton) onto a 9 cm by 16 cm card using a 1:5 (v/v) solution of gum arabic powder and water25,26.
NOTE: Avoid attaching too many host eggs to the card as overcrowding makes it impractical for subsequent transfer and dissection procedures. - To prevent the hatching of host eggs, subject the host egg cards to 30 min of ultraviolet (UV) irradiation (Figure 1-i). Subsequently, cut the paper with inactivated eggs into egg cards measuring 1 cm thick and 8 cm in diameter, each containing approximately 300-500 eggs (Figure 1-ii)25,26,27.
- Introduce a cohort of 80-120 T. denrolimi wasps into a glass tube measuring 2 cm in diameter and 8 cm in length. Seal the tube with cotton.
NOTE: Maintain the wasps at a temperature of 25 ± 1 °C, with a light/dark cycle of 16 h of light and 8 h of darkness and maintain a relative humidity of approximately 75%. - Present a host egg card to the wasps for parasitization (Figure 1-ii). Permit the wasps to deposit their eggs into the host eggs for 6 h, after which promptly remove the wasps.
NOTE: Avoid prolonging the parasitization period excessively as this can lead to overcrowding of T. denrolimi offspring within a host egg, resulting in increased offspring wasp mortality28,29.
2. Synthesis of dsRNA
- Design primer sets containing a T7 promoter for the synthesis of double-stranded RNA (dsRNA). Choose a 300-400 bp segment from the targeted gene (Ferhch) for dsRNA synthesis.
- Acquire commercially synthesized primer sets for the production of dsRNA for the targeted gene and dsGFP for use as a negative control.
- Extract RNA content from 100 T. denrolimi wasps using the referenced kit following the manufacturer's protocol9. Check the quality of RNA content; proceed if the value of OD260/OD280 ranges from 1.8 to 2.09.
- Purify the RNA content with 2 µL of 5x buffer (1 U/50 µL), 1 µL of DNAse (1 U/50 µL), and 6.5 µL of RNA (~100 ng/µL), 0.5 µL of H2O at 42 °C for 2 min.
- Perform reverse transcriptase polymerase chain reaction (RT-PCR) to generate a cDNA template with 10 µL of purified RNA (~100 ng/µL), 4 µL of 5x buffer (1 U/50 µL), 1 µL of Enzyme Mix I (1 U/50 µL), and 4 µL of H2O. Perform RT-PCR using the following settings: 37 °C for 15 min, 85 °C for 5 s. Store the cDNA product at -4 °C until use.
- Perform polymerase chain reaction (PCR) to amplify the dsRNA sequences in accordance with the referenced master mix (10 µL of Taq II [1 U/50 µL], 0.8 µL of forward primer [0.4 µM], 0.8 µL of reverse primer [0.4 µM], 0.8 µL of DNA template [40 ng/µL], and 7.6 µL of H2O). Perform PCR using the following settings: 94 °C for 2 min; 35 cycles with 94 °C for 30 s, 60 °C for 30 s, 72 °C for 30; 72 °C for 2 min.
- Assess the quality of PCR products by electrophoresis on a 2.0% agarose gel5,9 and confirm the target sequence through Sanger sequencing.
- Purify the PCR product using the Gel Extraction Kit as per the manufacturer's guidelines9,23.
- Utilize an RNAi System to synthesize dsRNA for the target gene with 10 µL of 2x buffer (0.02 U/µL), 8 µL of DNA template (75 ng/µL), and 2 µL of enzyme mix (1 U/50 µL) with the following settings: 37 °C for 30 min, 70 °C for 10 min, and 25 °C for 20 min. Elute the synthesized dsRNA with 30 µL of Nuclease-Free Water.
- Assess the quality of the dsRNA product by visualizing it on a 1.0% agarose gel5,9. Dilute the dsRNA to 7,000 ng/µL. Check the quality of the dsRNA product; proceed if the value of OD260/OD280 ranges from 1.8 to 2.09. Store the dsRNA product at -20 °C until use.
3. Transplantation of T. denrolimi pupae
- Cultivate the parasitized host eggs for approximately 8 days at 25 ± 1°C, maintaining a 16 h light/8 h dark cycle and a relative humidity of ~75%. The parasitized host eggs will blacken after 5 days30 (Figure 1-iv,v).
- Transfer the host egg card to a dissecting microscope. Employ a pair of tungsten needles (Figure 1-vi) to meticulously remove the chorion from the host eggs (Figure 1-vii) and retrieve the pupa from within (Figure 1-viii)5,17,18.
NOTE: The tip of the dissecting needle should be less than 0.05 mm. - Prepare a clean plate for the substrate. Pour out 10-15 mL of a 15 g/L agar solution, allowing it to naturally cool (Figure 2-i).
NOTE: Mix the agar solution with 0.5 g of streptomycin to inhibit the growth of contaminants in vitro. - Employ a disinfected graver to etch several grooves measuring 0.2-0.3 mm in depth and 0.4-0.8 mm in width (Figure 2-ii).
- Use a small brush (Figure 2-iii) to transplant a pupa from the dissected host egg into one of the grooves on the agar substrate (Figure 2-iv).
- Repeat steps 3.4 and 3.5, transplanting 100-300 pupae individually into the grooves one by one (Figure 2-v).
4. Micro-injecting dsRNA into T. denrolimi pupa
- Use a glass needle puller to pull a glass capillary (Figure 3-i,ii). Set the Heat à 280, the Velocity à 170, the Delay à 250, and the Pull à 30 on a needle puller (Figure 3-iii). Prepare multiple capillary glass needles for microinjection (Figure 3-iv,v).
- Bevel the tip of the glass needle using an abrasive plate (Figure 3-vi).
NOTE: Ensure that the injection needle's tip remains sharp; otherwise, it may result in pupal mortality or impede reagent flow through the needle (Figure 3-vii). - Load approximately 2 µL of the dsRNA injection solution with 7,000 ng/µL into the prepared injection needle using a micro-injection pump (Figure 3-vii).
- Transfer the agar substrate containing hundreds of pupae onto the platform of a compound microscope (Figure 3-viii).
- Carefully insert the injection needle into the abdomen of a T. denrolimi pupa at a ~30° angle to the abdomen under the lens (Figure 3-ix).
- Gradually inject ~5 nL of the injection solution (step 4.3) into the T. denrolimi pupa as gently as possible.
NOTE: The T. denrolimi pupa may slightly swell as the dsRNA solution is injected. Injecting an excessive amount of solution into the pupa or using needles with overly large openings may result in premature pupal death. Change the needles when they become clogged after injecting several to tens of pupae. - Repeat steps 4.5 and 4.6, injecting dsRNA into 600 pupae one by one.
NOTE: For reliable RT-qPCR analysis, RNA content should be isolated from at least 100 pupae. Inject at least 600 pupae for three replicates in the dsRNA treatment and three replicates in the dsGFP negative control9.
5. Incubation of T. denrolimi pupae
- Transfer the substrate containing injected pupae to an incubator at 25 ± 1 °C with a 16 h/8 h light/dark cycle and ~75% RH.
- Incubate approximately 700 T. denrolimi pupae per treatment for either 24 h or 48 h. Extract RNA content from a group of 100 pupae per replicate9.
NOTE: Remove the shrunk pupae (dead pupae) from the samples; otherwise, it may result in errors in the detection of gene expression. - Incubate ~50 T. denrolimi pupae per treatment until the wasps emerge (Figure 3-x). Record the emergence rate and deformity rate.
6. Detection of targeted gene expression
- Design the primer set for the target gene and reference genes for RT-qPCR using a design tool.
NOTE: Ensure that the sequences of the RT-qPCR primers do not overlap with the targeted region of dsRNA. - Employ the gene forkhead box O (FoxO) as the reference gene in the RT-qPCR analysis; the primers of FoxO have been reported previously9.
- Obtain commercially synthesized primer sets. Perform RT-qPCR with 10 µL of Taq II (1 U/50 µL), 0.8 µL of forward primer (0.4 µM), 0.8 µL of reverse primer (0.4 µM), 0.8 µL of template (10 ng/µL), and 7.6 µL of H2O. Perform RT-qPCR using the following settings: 95 °C for 30 s; 35 cycles with 95 °C for 5 s, 57 °C for 30 s, 72 °C for 30; 95 °C for 10 s; 60 °C for 5 s, and 95 °C for 5 s.
- Calculate the expression value of the targeted gene by using the 2-ΔΔCt method9,25.
- Employ the Kruskal-Wallis test to analyze the expression of the target gene under the influence of different treatments (dsFerhch, dsGFP, non-injection).
NOTE: Avoid using parametric tests (e.g., t-test) for post-hoc comparisons as the data's heteroscedasticity and non-normality may lead to erroneous conclusions25.
Representative Results
The emergence rate of T. denrolimi pupae injected with dsFerhch was significantly lower than that of those injected with dsGFP or those without injection (Table 1). Among the emerged wasps, 51.85% of T. denrolimi wasps subjected to dsFerhch developed deformed small wings. The deformed wasps were not observed in the wasps injected with dsGFP or without any injection (Table 1). Moreover, the abdomens of T. denrolimi pupae injected with dsFerhch exhibited melanism which is the phenotype of abnormal iron metabolism23. The melanism was not observed in T. denrolimi pupae injected with dsGFP or those without any injection (Figure 4).
RT-qPCR analysis showed that the expression of Ferhch de T. denrolimi pupae, 24 h after dsFerhch injection (0.6460 ± 0.0056), was significantly downregulated in comparison to those injected with dsGFP (1.4514 ± 0.5402; P = 0.0415). However, it did not differ significantly from the expression in pupae without any injection (1.0000 ± 0.1953; P = 0.8725). Ferhch expression in T. denrolimi pupae, 48 h post dsFerhch injection (0.5170 ± 0.0575), was significantly downregulated compared to pupae injected with dsGFP (0.8515 ± 0.0233; P = 0.0182) and those without any injection (1.0548 ± 0.3006; P = 0.0113) (Figure 5).
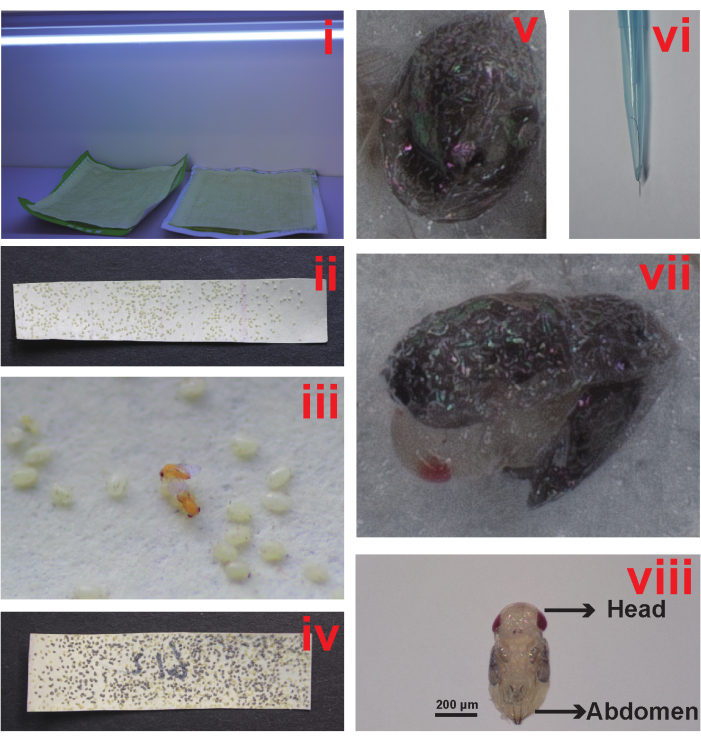
Figure 1: Collection and maintenance of Trichogramma denrolimi culture. (i) Expose the host egg cards to ultraviolet (UV) irradiation. (ii) Cut the paper with inactivated eggs into egg cards. (iii) Present a host egg card to the wasps for parasitization. (iv) Cultivate the parasitized host eggs for 8 days. (v) The parasitized host eggs will blacken after 5 days. (vi) The dissecting needle with a 0.04 mm tip. (vii) Remove the chorion from the host eggs. (viii) T. denrolimi pupa. Please click here to view a larger version of this figure.
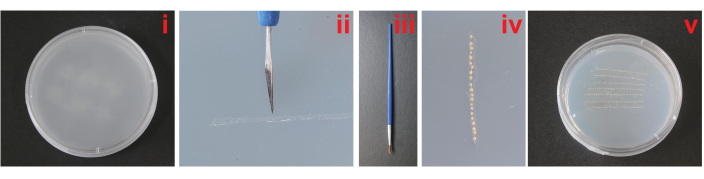
Figure 2: Transplantation and incubation of Trichogramma denrolimi pupae. (i) The agar substrate. (ii) Employ a disinfected graver to etch several grooves on the substrate. (iii) Use a small brush to (iv) transplant the pupae from the dissected host egg into a groove on the substrate. (v) Transplanting 100-300 pupae individually into the grooves one by one. Please click here to view a larger version of this figure.
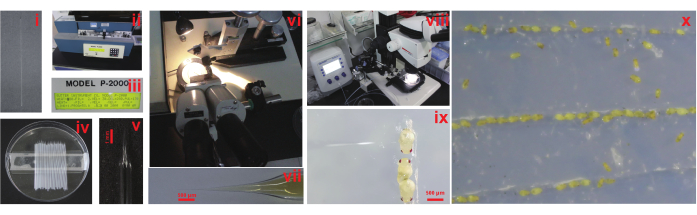
Figure 3: Procedure of micro-injection. (i) Glass capillary. (ii) Glass needle puller. (iii) Settings for needle puller. (iv) Prepare multiple capillary glass needles for microinjection. (v) The tip of the capillary glass needle. (vi) Bevel the tip of the glass needle using an abrasive plate. (vii) Load approximately 2 µL of the dsRNA injection solution. (viii) Compound microscope. (ix) Insert the injection needle into the abdomen and inject the injection solution. (x) Emergence of wasps. Please click here to view a larger version of this figure.
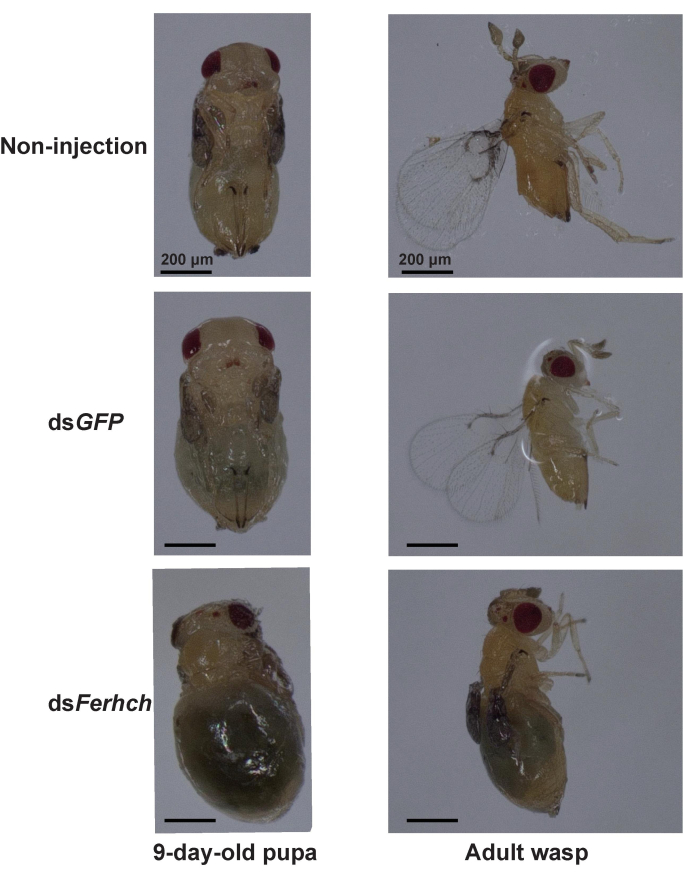
Figure 4: Morphological characteristics of Trichogramma denrolimi pupa and wasp. The pupa and wasp injected with dsFerhch exhibit melanism with blackened body color. The wasp injected with dsFerhch shows a pair of deformed small wings. The melanism and deformed wings are not observed in the pupa and wasp injected with dsGFP or those without any injection. Scale bars = 200 µm. Please click here to view a larger version of this figure.
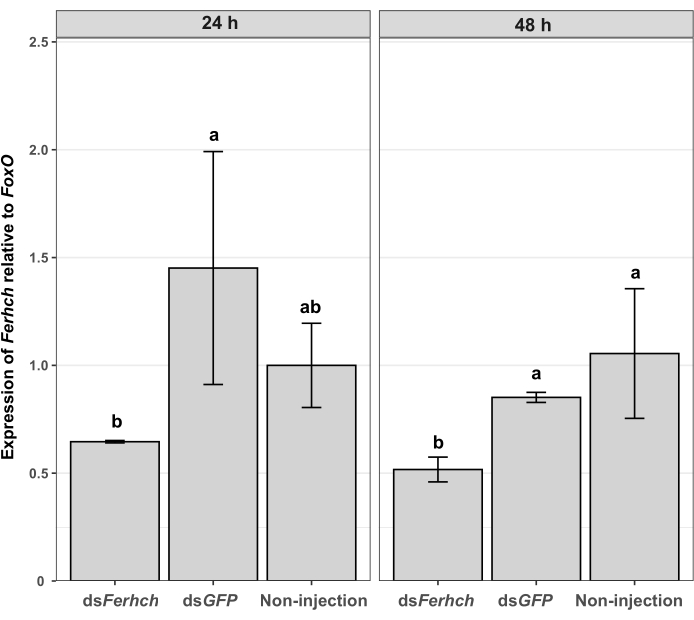
Figure 5: Expression of target gene Ferhch relative to FoxO at 24 h and 48 h post dsFerhch or dsGFP injection, or non-injection. Error bars indicate the standard errors. Different lowercase letters indicate a significant difference at P < 0.05. Please click here to view a larger version of this figure.
| Variables | dsFerhch | dsGFP | Non-injection | χ2 | P |
| Emergence rate | 54.00% (27/50) b | 78.00% (39/50) a | 90.00% (45/50) a | 17.46 | <.001 |
| Deformity rate | 51.85% (14/27) A | 0% (0/39) B | 0% (0/45) B | 49.84 | <.001 |
Table 1: The emergence and deformity of Trichogramma denrolimi injected by dsFerhch, dsGFP, or without injection. Different lowercase letters indicate significant differences in emergence rate at P < 0.05. Different uppercase letters indicate significant differences in deformity rate at P < 0.05. The values of χ2 and P listed in the table are estimated by the χ2 independent test and indicate the total effect of three treatment levels on the emergence rate and deformity rate.
Discussion
Trichogramma wasps are recognized as effective biological control agents, specifically targeting a range of lepidopteran pests in agriculture and forestry1. These diminutive wasps undergo their immature stages within the host egg, a characteristic that presents challenges in conducting RNAi experiments5,18. This study offers a comprehensive visual guide for conducting RNAi experiments in T. denrolimi. Given the shared biological traits among Trichogramma species, the procedure can be readily adapted for RNAi studies or micro-injections in other Trichogramma species and endoparasitic species with some adjustments.
The procedure introduces several innovative approaches, facilitating efficient micro-injections of dsRNA content, streamlining the RT-qPCR process for T. denrolimi, and optimizing the transplantation and in vitro incubation of T. denrolimi pupae. According to this study, the emergence rate of transplanted pupae reached 90% without any injection. When the transplanted pupae were subjected to dsGFP injection, the emergence rate of these pupae was 78% (Table 1). Successful RNAi experiments in Trichogramma hinge significantly on precise micro-injections and the careful incubation of pupae in vitro. The susceptibility of isolated Trichogramma pupae to damage during injection may result from oversized micro-injection needles, hasty injection techniques, or microbial contamination on the agar substrate. The detailed visual method presented here also offers insights into the microinjection technique in T. denrolimi, which is not only essential for other genetic editing tools (e.g., CRISPR-Cas9 system) but also for conducting various physiological experiments (e.g., artificial transinfection of symbionts and the delivery of inhibitors or activators of physiological responses)1,14,15,16.
For RT-qPCR analysis, approximately 1,500 pupae were injected in this study. Given the small size of T. denrolimi, a minimum of 100 pupae per replicate is necessary to ensure sufficient RNA quality and quantity for RT-qPCR analysis9. Consequently, injecting dsRNA into hundreds of pupae is imperative. Additionally, improvements in the RNA isolation procedure (e.g., employing a specific RNA extraction kit, refining PCR programs) are required to obtain eligible RNA content from a reduced number of Trichogramma individuals per replicate. An alternative approach involves the delivery of dsRNA through soaking or feeding, which may be more convenient for obtaining a substantial number of individuals subjected to dsRNA. Additionally, it is essential to note that the current RNAi procedure is applicable only at the pupal stage of T. denrolimi. Future research should place particular emphasis on the development of efficient RNAi methods for the embryo and larva stages15,16.
In summary, this study has delineated an efficient RNAi method in T. denrolimi. For decades, gene functions within the entire Trichogramma genus have been rarely explored. The present methodology provides a robust model for investigating gene regulations during developmental and physiological activities in Trichogramma wasps. Future research should emphasize the development of genetic tools, such as genome editing and RNA interference in Trichogramma wasps. The depth exploration of the molecular biology of Trichogramma wasps offers valuable insights for improving the mass-rearing efficiency and field performance of these biological control agents for the control of pests8,9.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This research was funded by the Projects of the National Natural Science Foundation of China (32172476, 32102275), the Agricultural Science and Technology Innovation Program (CAAS-ZDRW202203, CAAS-ZDRW202108), and Central Funds Guiding the Local Science and Technology Development (XZ202301YD0042C).
Materials
| 2x ES Taq MasterMix (Dye) | Cowin Biotech, China | CW0690H | To amplify the dsRNA sequences |
| 20x PBS Buffer, DEPC treated (7.2-7.6) | Sangon Biotech, China | B540627-0500 | To dilute dsRNA |
| Agar strip | Shishi Globe Agar Industries Co.,Ltd, China | n/a | To make culture medium |
| Ampicillin sodium | Sangon Biotech, China | A610028 | To make culture medium |
| Bioer Constant temperature metal bath | BIOER, China | MB-102 | To synthesis dsRNA |
| Borosilicate glass capillary | WPI, USA | 1B100-4 | To pull capillary glass needle |
| Clean bench | Airtech, China | SW-CJ-1FD | To extract RNA |
| Double distilled water | Sangon Biotech, China | A500197-0500 | To dilute cDNA |
| Environmental Testing chamber | Panasonic, Japan | MLR-352H-PC | To culture T. denrolimi |
| Eppendorf Centrifuge | Eppendrof, Germany | 5418R | To store RNA content |
| Eppendorf FemtoJet 4i | Eppendrof, Germany | FemtoJet 4i | To inject T. denrolimi |
| Eppendorf Refrigerated Centrifuge | Eppendrof, Germany | 5810R | Centrifuge |
| Ethanol solution (75%, RNase-free) | Aladdin, China | M052131-500ml | To extract RNA |
| Gel Extraction Kit | Omega, USA | D25000-02 | To extract cDNA |
| GUM Arabic | Solarbio, China | CG5991-500g | To make egg card |
| Isopropyl alchohol | Aladdin, China | 80109218 | To extract RNA |
| Laser-Based Micropipette Puller | SUTTER, USA | P-2000 | To pull capillary glass needle |
| Microloader | Eppendrof, Germany | 20 µL | To load dsRNA |
| Multi-sample tissue grinder | LICHEN, China | LC-TG-24 | To grind T. denrolimi |
| Needle Grinder | SUTTER, USA | BV-10-E | To grind capillary glass needle |
| Nuclease-Free Water | Sangon Biotech, China | To dilute RNA | |
| OLYMPUS Microscope | OLYMPUS, Japan | XZX16 | To observe T. denrolimi |
| PCR machine | Bio-rad, USA | S-1000 | For DNA amplification |
| PowerPac Basic | Bio-rad, USA | PowerPacTM Basic | To detect the quality of dsRNA |
| Primer of dsGFP (Forward) | [TAATACGACTCACTATAGGG] ACAAACCAAGGCAAGTAATA |
||
| Primer of dsGFP (Reverse) | [TAATACGACTCACTATAGGG] CAGAGGCATCTTCAACG |
||
| Primer of Ferhch for qPCR (Forward) | TGAAGAGATTCTGCGTTCTGCT | ||
| Primer of Ferhch for qPCR (Reverse) | CTGTAGGAACATCAGCAGGCTT | ||
| Primer of Ferhch for RNAi (Reverse) | [TAATACGACTCACTATAGGG]AG TAGCCATCATCTTTCC |
||
| Primer of Ferhch for RNAi(Forward) | [TAATACGACTCACTATAGGG] ACACTGTCAATCGTCCTG |
||
| Primer of FoxO for qPCR (Forward) | CTACGCCGATCTCATAACGC | ||
| Primer of FoxO for qPCR (Reverse) | TGCTGTCGCCCTTGTCCT | ||
| PrimeScript RT reagent Kit with gDNA Eraser (Perfect Real Time) | TaKaRa, Japan | RR047A | |
| Quantitative Real-time PCR | Bio-rad, USA | CFX 96 Touch | To perform reverse transcriptase polymerase chain reaction (RT-PCR) |
| Real-time PCR (TaqMan) Primer and Probes Design Tool | https://www.genscript.com/tools/real-time-pcr-taqman-primer-design-tool/ | ||
| T7 RiBoMAX Express RNAi System | Promega, USA | P1700 | To synthesis dsRNA in vitro |
TB Green Premix Ex TaqTM  (Til RnaseH Plus) (Til RnaseH Plus) |
TaKaRa, Japan | RR820A | To perform RT-qPCR |
| Trichloromethane | KESHI, China | GB/T682-2002 | To extract RNA |
| TRIzol Reagent | Ambion, USA | 15596018 | To extract total RNA content from samples |
| Ultra-low Temperature Freezer | Thermo, USA | Forma 911 |
References
- Zang, L. S., Wang, S., Zhang, F., Desneux, N. Biological control with Trichogramma in China: History, present status and perspectives. Annu Rev Entomol. 66, 463-484 (2020).
- Zhou, J. C., et al. Optimal clutch size for quality control of bisexual and Wolbachia-infected thelytokous lines of Trichogramma dendrolimi Matsumura (Hymenoptera: Trichogrammatidae) mass reared on eggs of a substitutive host, Antheraea pernyi Guérin-Méneville (Lepidoptera: Saturniidae). Pest Manag Sci. 76 (8), 2635-2644 (2020).
- Li, T. H., et al. Current status of the biological control of the fall armyworm Spodoptera frugiperda by egg parasitoids. J Pest Sci. 96, 1345-1363 (2023).
- Zhou, J. C., et al. Effects of temperature and superparasitism on quality and characteristics of thelytokous Wolbachia-infected Trichogramma dendrolimi Matsumura (Hymenoptera: Trichogrammatidae) during mass rearing. Sci Rep. 9 (1), 18114 (2019).
- Zhou, J. C., et al. Penetrance during Wolbachia-mediated parthenogenesis of Trichogramma wasps is reduced by continuous oviposition, associated with exhaustion of Wolbachia titers in ovary and offspring eggs. Pest Manag Sci. 78 (7), 3080-3089 (2022).
- Huang, N. X., et al. Long-term and large-scale releases of Trichogramma promote pesticide decrease in maize in northeastern China. Entomol Gen. 40 (4), 331-335 (2020).
- Wang, P., et al. Performance of Trichogramma japonicum as a vector of Beauveria bassiana for parasitizing eggs of rice striped stem borer, Chilo suppressalis. Entomol Gen. 41 (2), 147-155 (2021).
- Ning, S. F., et al. The identification and expression pattern of the sex determination genes and their sex-specific variants in the egg parasitoid Trichogramma dendrolimi Matsumura (Hymenoptera: Trichogrammatidae). Front Physiol. 14, 1243753 (2023).
- Huo, L. X., et al. Selection and evaluation of RT-qPCR reference genes for expression analysis in the tiny egg parasitoid wasp, Trichogramma dendrolimi Matsumura (Hymenoptera: Trichogrammatidae). J Asia Pac Entomol. 25 (2), 101883 (2022).
- Leung, K., et al. Next-generation biological control: the need for integrating genetics and genomics. Biol Rev Camb Philos Soc. 95 (6), 1838-1854 (2020).
- Fire, A., et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 391 (6669), 806-811 (1998).
- Leonard, S. P., et al. Engineered symbionts activate honey bee immunity and limit pathogens. Science. 367 (6477), 573-576 (2020).
- Sun, Y., et al. Silencing an essential gene involved in infestation and digestion in grain aphid through plant-mediated RNA interference generates aphid-resistant wheat plants. Plant Biotechnol J. 17 (5), 852-854 (2019).
- Li, M., Bui, M., Akbari, O. S. Embryo Microinjection and Transplantation Technique for Nasonia vitripennis Genome Manipulation. J Vis Exp. 130, e56990 (2017).
- Dalla Benetta, E., Chaverra-Rodriguez, D., Rasgon, J. L., Akbari, O. S. Pupal and Adult Injections for RNAi and CRISPR Gene Editing in Nasonia vitripennis. J Vis Exp. 166, e61892 (2020).
- Lynch, J. A., Desplan, C. A method for parental RNA interference in the wasp Nasonia vitripennis. Nat Protoc. 1 (1), 486-494 (2006).
- Zhu, K. Y., Palli, S. R. Mechanisms, Applications, and Challenges of Insect RNA Interference. Annu Rev Entomol. 65, 293-311 (2020).
- Colinet, D., et al. Development of RNAi in a Drosophila endoparasitoid wasp and demonstration of its efficiency in impairing venom protein production. J Insect Physiol. 63, 56-61 (2014).
- Wang, B., et al. A digestive tract expressing α-amylase influences the adult lifespan of Pteromalus puparum revealed through RNAi and rescue analyses. Pest Manag Sci. 75 (12), 3346-3355 (2019).
- Zhou, J. C., et al. Wolbachia-infected Trichogramma dendrolimi is outcompeted by its uninfected counterpart in superparasitism but does not have developmental delay. Pest Manag Sci. 79 (3), 1005-1017 (2023).
- Zhou, J. C., et al. Posterior concentration of Wolbachia during the early embryogenesis of the host dynamically shapes the tissue tropism of Wolbachia in host Trichogramma wasps. Front Cell Infect Microbiol. 13, 1198428 (2023).
- Lee, T. Y. The development of Trichogramma evanescens Westw. and its influence on the embryonic development of its host, Attacus cynthia ricini Boisd. Acta Entomol Sin Z. 11 (1), 89-119 (1961).
- Shen, Y., Chen, Y. Z., Zhang, C. X. RNAi-mediated silencing of genes in the brown planthopper Nilaparvata lugens affects survival, growth and female fecundity. Pest Manag Sci. 77 (1), 365-377 (2021).
- Wu, S., Yin, S., Zhou, B. Molecular physiology of iron trafficking in Drosophila melanogaster. Curr Res Insect Sci. 50, 100888 (2022).
- Zhou, J. C., et al. Wolbachia-Driven Memory Loss in a Parasitic Wasp Increases Superparasitism to Enhance Horizontal Transmission. mBio. 13 (6), e0236222 (2022).
- Ning, S. F., Zhou, J. C., Liu, Q. Q., Zhao, Q., Dong, H. Gradual, temperature-induced change of secondary sexual characteristics in Trichogramma pretiosum infected with parthenogenesis-inducing Wolbachia. PeerJ. 7, e7567 (2019).
- Zhang, C., et al. Decreased Wolbachia titers cause gradual change in masculinization of intersex individuals of thelytokous Trichogramma dendrolimi. Entomol Gen. 42 (5), 751-759 (2022).
- Zhang, X., et al. Multi-parasitism: A promising approach to simultaneously produce Trichogramma chilonis and T. dendrolimi on eggs of Antheraea pernyi. Entomol Gen. 41 (6), 627-636 (2021).
- Zhou, J. C., et al. Effects of Thelytokous parthenogenesis-inducing Wolbachia on the fitness of Trichogramma dendrolimi Matsumura (Hymenoptera: Trichogrammatidae) in superparasitised and single-parasitised hosts. Front Ecol Evol. 9, 730664 (2021).
- Flanders, S. E. Notes on the life history and anatomy of Trichogramma. Ann Entomol Soc Am. 30 (2), 304-308 (1937).

