Two-Photon Intravital Microscopy of Glioblastoma in a Murine Model
Summary
We present a novel approach for two-photon microscopy of the tumor delivery of fluorescent-labeled iron oxide nanoparticles to glioblastoma in a mouse model.
Abstract
The delivery of intravenously administered cancer therapeutics to brain tumors is limited by the blood-brain barrier. A method to directly image the accumulation and distribution of macromolecules in brain tumors in vivo would greatly enhance our ability to understand and optimize drug delivery in preclinical models. This protocol describes a method for real-time in vivo tracking of intravenously administered fluorescent-labeled nanoparticles with two-photon intravital microscopy (2P-IVM) in a mouse model of glioblastoma (GBM).
The protocol contains a multi-step description of the procedure, including anesthesia and analgesia of experimental animals, creating a cranial window, GBM cell implantation, placing a head bar, conducting 2P-IVM studies, and post-surgical care for long-term follow-up studies. We show representative 2P-IVM imaging sessions and image analysis, examine the advantages and disadvantages of this technology, and discuss potential applications.
This method can be easily modified and adapted for different research questions in the field of in vivo preclinical brain imaging.
Introduction
Two-photon intravital microscopy (2P-IVM) is a fluorescence imaging technique that allows the visualization of living tissue1.
First developed in the 1990s, 2P-IVM has been used for in vivo analysis of the retina2, kidney3, small intestine4, cochlea5, heart6, trachea7, and the brain in various preclinical models8,9. In the field of neuroscience, 2P-IVM has gained importance as a technique for real-time imaging of the healthy brain in awake animals10, as well as studying diseases of the nervous system such as Alzheimer's11, Parkinson's12 and glioblastoma (GBM)13,14,15,16.
2P-IVM offers an elegant solution for studying the tumor microenvironment during the development of GBM. While some previous studies focused on in vitro17 and ex vivo models18, others implemented orthotopic19 and xenotropic20 in vivo models for examining GBM. Madden et al. performed native imaging of CNS-1 rat glioma cell line in a mouse model13. Using an orthotopic GL261-DsRed murine model, Ricard et al. performed an intravenous administration of a fluorophore to enhance the blood vessels in the tumor region in 2P-IVM14.
Here, we apply 2P-IVM for tracking the tumor delivery of fluorescent-labeled iron oxide nanoparticles (NP) in an orthotopic mouse model of GBM. Using a cranial window, this method allows us to study the real-time spatiotemporal distribution of NPs in the brain in detail.
Protocol
The animal procedure described in this protocol is in accordance with the requirements of the Administrative Panel on Laboratory Animal Care (APLAC).
1. Cell culture
- Preparation of hood
- Wash hands, wear gloves and a lab coat. Turn on the biological safety cabinet and set the sash level to an appropriate opening height. Let the hood purge for 3-5 min. Spray the hood area with 70% ethanol and wipe it down with tissue paper.
- Spray all reagents with 70% ethanol and wipe down with tissue paper. Move the reagents into the hood. Do not put any items on the grill. If a spill occurs in the hood, cover it with wipes, spray it with 70% ethanol, and wipe again.
- After the experiment, carefully close the lids of each item, wipe down all materials, remove any items from the hood, and transfer them to their original location. Spray 70% ethanol in the hood and wipe it down. Shut the sash and turn off the biological safety cabinet.
- Preparation of a growth medium
- Add 50 mL of 100% fetal bovine serum (FBS) to 500 mL Dulbecco’s Modified Eagle Medium (DMEM). Add 5 mL of the antibiotic-antimycotic solution (100x).
- Pass all the reagents through a sterile 500 mL filtering bottle (0.22 µm filter).
- Thawing of cryopreserved cells
- In a laminar flow hood, add 20 mL of the growth medium to a T75 flask. Label the flask with the name of cells, date, and passage number on the flask. In this study, adherent C6 glioma cells were used.
- Thaw cells quickly in a 37 °C water bath. Do not vortex the cells. Thaw and immediately use the cells.
- Transfer the thawed cells to the T75 flask using a 1 mL pipette tip. Be careful not to introduce any bubbles during the transfer process, and avoid any medium residue in the neck of the flask. This could increase the risk of possible contamination.
- Changing the medium
- Change the medium on the day following thawing to remove any residual trypsin or dimethyl sulfoxide (DMSO) (step 1.6). Afterward, change the medium at least two times per week or more, depending on cell confluency level. The medium must be changed one day after passage to eliminate trypsin.
- To change the medium, turn the aspiration apparatus on, attach the aspiration glass pipet, and aspirate all the medium.
- Add 20 mL of the growth medium (step 1.2).
- Passaging the cells
- Load the hood with the items needed in the course of the experiment.
- Use an aspiration glass pipet and aspirate away all media, ensuring the glass pipette is on the non-cell side of the flask. For this step, position the T75 flask vertically.
- Add ~10 mL of room temperature (RT) phosphate-buffered saline (PBS, Ca++ and Mg++ free) or Hanks' Balanced Salt Solution (HBSS, Ca++, and Mg++ free) on the non-cell side. Wash cells briefly with PBS by positioning the T75 flask horizontally with the cell side facing down.
- Immediately place the flask vertically and aspirate the PBS/HBSS. Repeat this washing and aspirating 3 times.
- Add 2 mL of trypsin (recombinant or animal-derived) on the cell side (T75 flask horizontal, cell side facing down). Incubate in a standard tissue culture incubator (37 °C, 5% CO2) for 4 min. Triturate it once after 2 min using a 5 mL pipette.
- After 4 min of total incubation, transfer the solution to a 15 mL conical tube. Add 8 mL of the growth solution to the conical tube (2 mL trypsinized cells + 8 mL of medium = 10 mL in total).
- Use another empty 15 mL conical tube with a filter and transfer the cells via a 25 mL pipette to achieve individual cells.
- Transfer 1/10 (1 mL) of the solution (cell + media + Tryp-LE) to a T75 flask with 20 mL of media. Alternatively, count the cells (step 1.7). Change the media the next day to have a non-trypsin media solution.
- Freezing the medium and the cells
- Thaw 100% FBS in a 4 °C refrigerator. Do not use a water bath or heat since this may damage the proteins in FBS.
- To prepare 50 mL of freezing media, mix 45 mL of DMEM, 5 mL of FBS, and 5 mL of DMSO. Aliquot it to 1-2 mL containers to prevent intermittent thawing and protein damage. Store them in -20 ° or -80 °C freezer.
- For the remaining 9 mL of solution (step 1.5), add 1 mL of medium to reach 10 mL in total. Centrifuge at 300 x g for 4 min.
- Remove supernatant and resuspend in 1 mL of freezing medium (see above). Place the cells in a freezing container in -80 °C freezer. Move the cells to liquid N2 for long-term storage after 1 or 2 days.
- Counting the cells
- For the remaining 9 mL (step 1.5), add 1 mL of media to reach 10 mL in total. Centrifuge at 300 x g for 4 min.
- Remove the supernatant and resuspend in 4 mL of HBSS. Resuspend 10 µL of cells in 80 µL of HBSS + 10 µL of 0.4% Trypan Blue, dilution factor = 10.
- Take 17 µL of the solution and count four 4 x 4 squares in a hemocytometer. Average counts for the four 4 x 4 squares and multiply by 104x dilution factor to get cells/mL.
2. Surgery
NOTE: It is recommended to perform the surgery by two researchers, where one person is responsible for preparing the cells, mixing the dental cement, and generally assisting in the procedure, while the second person focuses on remaining sterile. Having a second manipulator to assist with the surgical procedure considerably reduces the likelihood of contamination occurring. Following best surgical practices would reduce the chances of post-operative complications. Figure 1A provides an overview of the components of the cranial window.
- General preparations
- Confirm that the procedure is approved by the local animal welfare institutional guidelines before starting.
NOTE: This study used 5-month-old female NSG mice (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) (n = 5). - Before surgery, autoclave all surgical instruments and ensure that all necessary supplies are available. Print the anesthesia and surgery records.
- Ensure that upon arrival, the animals have at least 1 week of acclimatization to the animal husbandry room to reduce additional post-operative distress.
- On the day of surgery, ensure that all devices (microscope, heating pad, sterilizer, drill, vacuum, etc.) are ready to use. Confirm that the anesthesia machine contains enough isoflurane and refill if necessary. For new users, the cranial window procedure can take up to 2 h per animal; therefore, having enough isoflurane is crucial.
- Place the glass windows and head bars in alcohol and prepare a container with saline. This will ensure a smooth workflow without any major breaches in sterility and keep the time under anesthesia for each animal as short as possible.
- Open all surgical materials on a sterile surface on the working station. For example, the inside of a sterile gauze packaging can be used for this purpose. Use the tips-only technique. When not using surgical instruments, place the tips on a sterile surface, such as the inside of the packaging of sterile gauze.
- When performing multiple surgeries, place all surgical instruments in the sterilizer in between surgeries to disinfect them. When performing surgeries on more than 5 animals, use a new set of autoclaved instruments. Switch out the drill tip when it becomes blunt for a new one to avoid tissue trauma.
- Confirm that the procedure is approved by the local animal welfare institutional guidelines before starting.
- Anesthesia and preparations for surgery
NOTE: The anesthesia setup is identical for the surgery, as well as for imaging.- Anesthetize the mouse using isoflurane (3%-5% for induction) in an anesthetic chamber. After ensuring adequate anesthetic depth by testing the pedal withdrawal reflex (foot pad pinch on both hind feet), transfer the animal to a preparation working station. Maintain anesthesia over a nose cone (1%-2%). Here, apply an eye ointment to prevent corneal damage. Regularly control the loss of reflexes during surgery by checking the paw reflex.
- If any identification of the animal is required, use an ear punch tool or scissors to mark the ears or use a marker to mark the tail.
- Remove the fur covering the skull between the ears and eyes with a depilatory cream. Leave the cream as long as indicated (30-60 s) by the manufacturer to avoid skin irritation. Make sure that the cream comes in contact with the hair roots by applying it against the direction of hair growth. Avoid that the depilatory cream comes in contact with the eyes. Remove any cream and hair remnants by cleaning the area with saline. Alternatively, use clippers.
- To avoid any intra- and post-operative pain, infection, or swelling, administer non-steroidal anti-inflammatory drugs (NSAIDs), opioids, anti-inflammatory agents, and antibiotics. Administer carprofen (5-20 mg/kg, subcutaneous [SQ]), buprenorphine – sustained release (1 mg/kg SQ), cefazolin (20 mg/kg SQ), and dexamethasone (0.2 mg/kg SQ) before surgery. Administer approximately 0.3 mL of 0.9% saline SQ to avoid dehydration.
- Following, scrub the area by alternately swabbing povidone-iodine and isopropyl alcohol three times in circular motions from the middle of the skull to the periphery.
- Craniotomy procedure
- Transfer the animal to the surgical table and place it in ventral recumbency on a heating pad (~37 °C) to ensure isothermal conditions during the procedure. Hypothermia in rodents significantly reduces survival rates and prolongs the post-operative recovery phase.
- Place the head in the stereotactic frame by positioning the ear bars and incisor bars. For the ear bars, find the zygomatic arch and insert the bars behind the arches. Start by securing the contralateral bar with the dominant hand (e.g., secure the left bar with your right hand if you are right-handed) and then secure the opposite side. Adjust the positions of the ear and incisor bars by turning the screws if required.
- Reapply eye ointment if necessary, and use a piece of aluminum foil to cover the eyes. This will prevent any damage caused by the bright microscope light and UV light used to cure the cyanoacrylate glue later (step 2.5).
- Before incision, check again for toe pinch reflex; if necessary, adjust anesthesia accordingly. Connect the animal to the anesthesia monitoring device by positioning the sensor on the hind paw. Measuring the heart rate and blood oxygen concentration would help reduce mortality and improve post-operative recovery. Additionally, acquire the respiratory rate by visually inspecting the chest movement.
- Cover the body of the animal with a drape to avoid hypothermia and contamination. Perform one final povidone-iodine swab before starting the surgery.
- Put on gloves and a clean lab coat.
NOTE: Using sterile gloves is encouraged due to the invasiveness of the procedure and to lower the risk of any post-operative infection and inflammation resulting in morbidity and loss of image quality in 2P-IVM (section 5). - Lift the skin on the skull and create an incision by holding the scissors between the right eye and ear and cutting towards the left side of the skull following the incision marks. Remove the resulting round skin flap.
NOTE: Depending on the region of interest in the brain and the window size, the incision site and diameter can vary. The size of the incision needs to be bigger than the diameter of the head to accommodate room for mounting (step 2.5). If needed, the skin surrounding the incision can be gently dissected to create more space. - Remove the periosteum by gently scratching the skull surface with a scalpel blade or a microcurrette. Exercise caution when removing the periosteum around the cranial sutures since those areas are fragile and more prone to bleeding. Use saline and a vacuum to keep the surgical area clean from debris. Scratch the periosteum to ensure better adherence to the cyanoacrylate glue and dental cement (step 2.5)
- Identify the region of the brain to perform the cell injection. For this, use an atlas of the stereotactic coordinates of the mouse brain. Per the brain coordinates, mark the position of the cranial window by using a biopsy punch in the same diameter as the window, a surgical pen, or an autoclaved pencil.
- Hold the drill in the dominant hand and any other instrument (syringe, forceps) in the non-dominant hand. Avoid prolonged drilling in the same spot. The drill should be used in bursts to avoid overheating. During these breaks, apply cold saline to the calvarium to keep the surgical view clean and to prevent overheating. Use the sensory feedback from the drill tip to detect when the skull has been perforated completely.
- Use cold saline, in combination with a vacuum, to clean the skull surface. Do not use gauze or a cotton tip applicator after opening the calvarium since remnants of cotton threads may lead to a foreign body reaction upon sealing the brain window.
- While drilling, ensure that the trajectory and diameter are the same size as those of the glass coverslip. Do this by positioning the glass coverslip on top of the skull and confirming that the glass coverslip will fit exactly inside the cranial window.
- Once it is possible to depress the skull flap by gently pressing on it, use forceps to carefully remove the fragment from the surgical field (Figure 1B, left). If it is not yet possible to depress the skull, continue drilling in the areas of the cranial window that are still connected to the rest of the skull and reevaluate.
- If minor bleeding occurs, use a hemostatic sponge combined with light pressure or alternatively, ice cold saline, to help reduce blood loss.
- Cell implantation
- Prepare and count the cells to be implanted as described in (step 1.7). Ensure that the cell number is 200,000 cells/µL.
NOTE: Suspending the cells in a membrane matrix that solidifies at RT is recommended. This will improve the success rate of implanted cells in the brain parenchyma. - Transfer the cells in a container with ice to the surgical room. Use a gastight microliter syringe. Before aspiration, keep the syringe on ice to avoid temperature differences.
- After aspirating 1 µL, position the syringe inside the stereotactic frame.
NOTE: An automated stereotactic coordinate device showing the positions in the X, Y, and Z axes can be used to save time. It is also possible to manually calculate the positions based on lambda. Injecting more than 1.5 µL is not recommended. This will ensure that the injected area does not overfill, causing unsuccessful implantation, growth outside the brain parenchyma, or metastases. - Perform implantation in the V2MM region (visual cortex 2, mediomedial part) of the brain, which corresponds to approximately medial to lateral (M-L): -1.2 to -1.6 mm, and dorsal to ventral (D-V): -2.6 to -3.5 mm coordinates.
- For two-photon imaging (step 4.6), implant the cells in the superficial brain areas so that the tumor mass can be visualized through the cranial window later on. Inject 0.5 µL (100,000 cells) at anterior to posterior (A-P): 0.8 mm depth. Move the needle slowly, in a stepwise manner, when entering the brain and wait for 30 s after injection (Figure 1B, middle column).
- Use the same approach when retracting the needle and exiting the brain tissue. Avoid injecting close to the ventricles since the cerebrospinal fluid might encourage metastasis in the central and peripheral nervous systems.
- Prepare and count the cells to be implanted as described in (step 1.7). Ensure that the cell number is 200,000 cells/µL.
- Closure of the cranial window
- Move the head bar and glass coverslip from alcohol to a saline solution. Use a vacuum tip or forceps to navigate those components to the surgical site.
- Position the glass coverslip inside the cranial window and place a small amount of cyanoacrylate glue between the skull and the glass coverslip. UV-activated cyanoacrylate glue reduces the curing time and avoids accidental displacement. If any glue spills on the glass coverslip, remove it with a cotton tip applicator or by gently scratching it off with the blunt side of a scalpel before it cures completely.
CAUTION: UV light is harmful to the eyes and skin. Avoid eye and skin contact while curing the glue. - After securing the glass coverslip in the cranial window, apply the head bar. Mix the two-component dental cement, position the head bar, and apply the cement to the surrounding area, ensuring contact between the skull and the head bar. Carefully clean any superfluous cement with the tip of a syringe, a scalpel, or a drill tip.
NOTE: Dental cement solidifies very quickly, so it is advised to apply it in a timely manner. - After sealing the cranial window, identify if any skull areas between the dental cement and skin are still exposed. If that is the case, apply surgical glue to cover up the skull by closing the skin. Alternatively, perform a single suture with an absorbable suture material.
3. Post-surgical recovery and tumor growth
- Recovery
- Monitor the animal on a heated pad in a recovery cage until it regains full consciousness. House the animals separately post-surgery to reduce the risk of injury.
- Administer a recovery diet, such as commercially available water gels with electrolytes or sugars. Alternatively, supply moistened standard lab chow to ensure easy nutrition and avoid dehydration and hypoglycemia.
- Monitoring
- Upon recovery, monitor the animal daily for signs of pain, distress, or infection. If needed, administer analgesics, anti-inflammatory drugs, or antibiotics. If an animal reaches the study endpoint, euthanize it humanely. Following the final imaging session, euthanize the mouse by carbon dioxide asphyxiation, followed by cervical dislocation.
NOTE: Examples of early euthanasia criteria include but are not limited to significant weight loss (>20%), neurological disorders, dyspnea, excessive bleeding during surgery, or pronounced stereotypical behavior. Inspect the cranial window and clean it with saline or alcohol when necessary. - To track tumor growth and plan the imaging time points, perform whole-body bioluminescence imaging (BLI). For this, inject 150 mg/kg D-luciferin intraperitoneally and image the animal after 5-15 min.
- Upon recovery, monitor the animal daily for signs of pain, distress, or infection. If needed, administer analgesics, anti-inflammatory drugs, or antibiotics. If an animal reaches the study endpoint, euthanize it humanely. Following the final imaging session, euthanize the mouse by carbon dioxide asphyxiation, followed by cervical dislocation.
4. Nanoparticle synthesis
- Particle preparation
- Add 11.17 mL of Ferumoxytol (6 mmol Fe in total) to a solution containing 50 mL of 5 M NaOH, 20 mL of deionized water (DI water) and 20 mL of epichlorohydrin. Observe phase segregation at this stage. Incubate the mixture at RT under gentle shaking for 24 h.
- After 24 h, the solution becomes uniform. Remove excess epichlorohydrin using dialysis tubing (12,000-14,000 Da cutoff) against water for 3 days.
- After dialysis, transfer the solution remaining in the tube into a glass bottle (total volume ~110 mL). Add 30 mL of ammonia hydroxide, and stir the mixture at 37 °C for 18-20 h.
- After stirring, repeat dialysis against water for 3 days. Transfer the solution remaining in the dialysis tubing into a new glass bottle with a total volume ~110 mL.
- Fluorescein isothiocyanate (FITC) conjugation
- Take out 27.5 mL of amine-functionalized particles for FITC conjugation. Concentrate the particles using a 10 kDa ultracentrifugation filter and wash with a pH 9 (Na2CO3/NaHCO3) buffer at 5752 x g at 25 °C for 10 min.
- Add 4 mL of the solution into the filter and ultracentrifuge the tube at 5752 x g at 25 °C for 10 min. Discard the filtrate and refill the filter with buffer for a second round of washing with the same protocol.
- Discard the filtrate and collect the solution in the filter using a pipet. Estimate the molar concentration of the particle by the mass concentration of Ferumoxytol, assuming the diameter of each particle is 5 nm.
- Dissolve FITC in anhydrous dimethyl sulfoxide (DMSO) at 10 mg/mL. Slowly add 1.4 mL of DMSO-dissolved FITC into the concentrated amine-functionalized particle. The molar ratio of FITC: particle is 20:1. After that, incubate the solution at 4 °C for 8 h in the dark.
- Quench the reaction by adding excess NH3.H2O (the final concentration of NH3.H2O is 50 mM), then letting the solution stay at 4 °C in the dark for 2 h. Wash away excess FITC using Na2CO3/NaHCO3 (pH 9) buffer through a 10 kDa filter at 5752 x g at 25 °C for 10 min. The final pH of the solution is adjusted to 7.4 using HCl aqueous solution.
5. 2P-IVM
NOTE: For the 2P-IVM sessions, a Prairie Ultima IV microscope with a custom stage (Figure 2 and Supplemental Coding File 1) that allows adjusting the position of the cranial window horizontally and vertically was used. Fiji software was used for post-processing and image analysis. This way, the laser beam can be adjusted to hit the glass at a 90 ° angle, reducing artifacts and improving imaging quality.
- Imaging session
- Turn on the Ti-Sapphire laser. Turn on the main system switch and Prairie View software.
- Anesthetize the animal, as described above (step 2.2). Perform imaging sessions for up to 2 h per animal. Keep the imaging time to a minimum to reduce the risk of anesthesia complications and associated morbidity or mortality.
- Immobilize the mouse under the two-photon microscope using a custom stage (Figure 1B, right column). Place the animal in a prone position on a heated pad designed for rodent surgery and secure it lightly using tape while paying attention to not constrict the thorax. Place a drop of water on the cranial window and adjust the focus.
- Acquire heart rate and blood oxygenation to monitor the animals' vital parameters. Position the sensor on the hind paw and keep the monitoring device outside the two-photon imaging chamber.
- Once the animal is on the microscope stage, adjust the head position. Using the binoculars and widefield fluorescent illumination, set the red fluorescent protein (RFP) filter and find the desired imaging field of view (Figure 1B, right column).
- Once sample orientation and field of view are determined, switch the microscope from widefield mode to light scanning microscopy mode (LSM).
- Make sure the Ti-Sapphire laser is mode-locked at 920 nm, and all shutters are open. Bring the GaAsP photomultiplier tube detectors (PMT) voltages up to 500-600 V. Use two PMTs with filter sets with band passes at 595/50 (RFP) and 525/50.
- Press Live Image and slowly modify the pockel cell to increase the laser power at the sample until an image is visible.
- Once a clear image is achieved, use the software and the motorized stage to set the top and bottom of an image stack within the desired sample area. Beware of the step size and take into consideration that the Z-axis adjusts the depth of the lens.
- With increasing depth, the images will get darker. Increase the pockels/PMT gain slowly to keep the image bright. Be careful not to use too much power as it can lead to phototoxicity and tissue damage.
- Set a save path and start the Z series. It will automatically traverse to the starting and ending positions using the set pockel/PMT settings. Once the stack is complete, use the playback to look over the stack for quality. Once acquiring the necessary images is complete, turn off the Ti-Sapphire laser.
- Move the animal to a recovery cage, as described above (step 3.1).
- Exit Prairie view and ensure all the data is saved/transferred. Shut down the computer and shut down all hardware.
- Post-processing with FIJI
NOTE: Fiji is an open-source software focused on biological image analysis21.- Download FIJI with respect to the operating system. Unzip the folder contents and run the .exe file.
- Drag the .env file gathered from the two-photon imaging to the dialogue box. Split the channels into different image boxes. This will create two different images with different channels, one containing the cell signal and the other the particle signal.
- Copy one of the images and click on File > New > Internal Clipboard to create another image. Rename one of the images as Source and the other as Copy.
- Use the copy image and click on Process > Enhance contrast in the dialogue box. Click Normalize and OK and then Process > Smooth. This image is used to create a mask and not for analysis. Click on Image > Adjust > Threshold in the dialogue box. Use the sliding scale and method that is appropriate to extract, then click Apply.
- Use Process > Binary > Erode to erode single pixels in the background and click Process > Binary > Dilate to add back pixels to the image.
- Click on Process > Image Calculator in the dialogue box, select the source image as the first and select the second image as Copy. Use AND to create the intersection of both images. Repeat the same step for the other channel image.
- For signal analysis outside of the vascular region, open an unaltered copied image and delete the vascular area of the signal with the Freehand ROI tool.
- Set desired parameters by going to Analyze > Set Measurements. Make sure Area, Integrated Density, and Mean Grey Value are checked.
- Now analyze by Analyze > Measure. A window with the measurements will pop up. Copy the data into a spreadsheet.
- Now select a small area of the image that has no fluorescence. This will be the background.
- Click Analyze > Measure for that region. Copy data into a spreadsheet.
- Save the resulting image as File > Save as > Analyze file type for remeasurements or publication purposes.
Representative Results
Here, we performed cranial window surgery and engrafted C6 cells in an NSG mouse model of GBM (n = 5). A proper seal between all components involved in the creation of the window (Figure 1A) will ensure the windows' durability for long-term imaging and, additionally, reduce morbidity. Using the stage adapted for in vivo 2P-IVM (Figure 2), we could image animals under anesthesia for up to 2 h without any major motion artifacts. Approximately 10 min after intravenous application of the nanoparticles in GBM-bearing mice, 2P-IVM shows the green, fluorescent signal of vessels in the region of the tumor, consistent with intra-vascular localization of the FITC-labeled nanoparticles. Only a small amount of green-fluorescent signal is noted outside of the blood vessels, indicating the beginning extravasation of NPs (Figure 3A). An increase in the FITC signal originating from the extravascular space is seen, which is in line with advanced extravasation (Figure 3B).
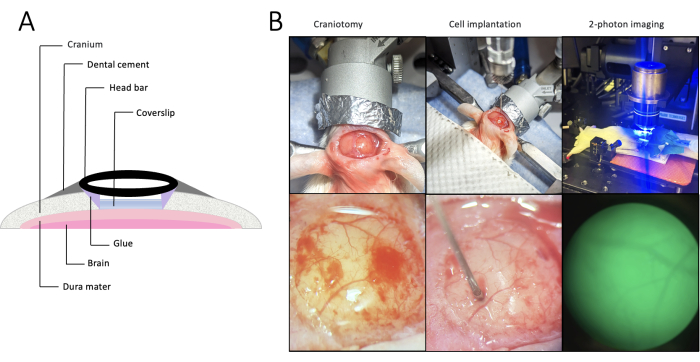
Figure 1. Cranial window placement, craniotomy, implantation, and imaging. (A) Cross-section of the anatomical placement of a cranial window. Since the head bar is metal-free, it is possible to perform magnetic resonance imaging or magnetic particle imaging in addition to two-photon intravital microscopy. (B) Overview (upper row) and a detailed view (lower row) of the craniotomy (left), cell implantation (middle), and 2-photon intravital microscopy (right). Upon opening the skull, superficial bleeding occurs (left) and is quickly reabsorbed (middle). Please click here to view a larger version of this figure.
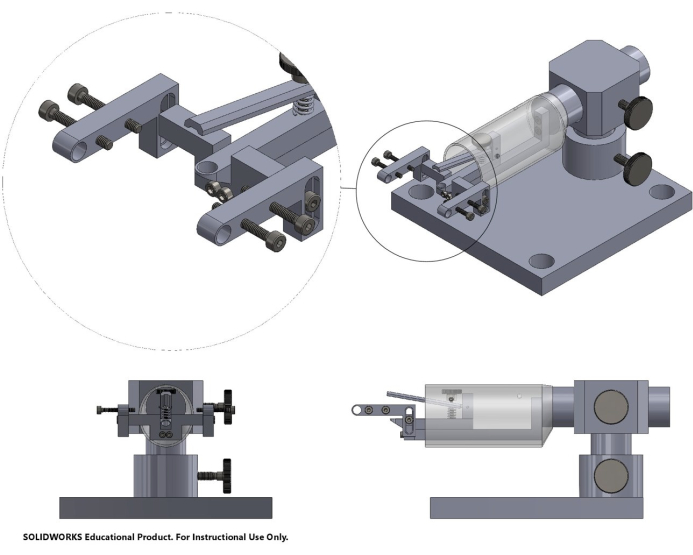
Figure 2. Computer-assisted design (CAD) of the stage. CAD of the stage used for in vivo 2-photon intravital microscopy, including a bite bar, head bar stabilization, and screws for height and angle adjustments. The 3D CAD file can be found in Supplemental Coding File 1. Please click here to view a larger version of this figure.
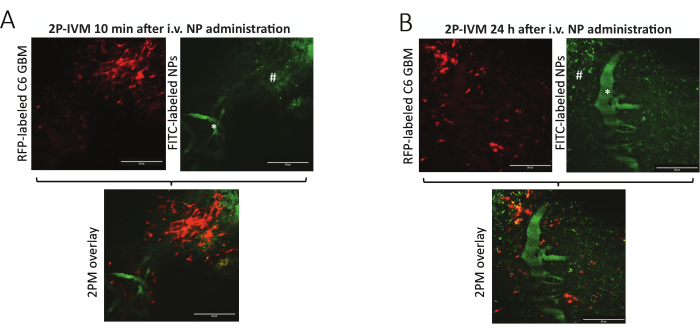
Figure 3. In vivo 2-photon intravital microscopy images. Images acquired (A) 10 min and (B) 24 h after intravenous nanoparticle administration. While the GBM cells emit fluorescent signals in the red spectrum (left), the nanoparticles in the brain tissue are visualized in green (right). # indicates extravasated NPs, * indicates a blood vessel. Only a limited number of particles have undergone extravasation and are visible in the vicinity of the tumor cells 10 min after NP administration. An abundance of particles is visible in the region of GBM cells, suggesting extravasation 24 h following NP administration. The nanoparticles consist of fluorescein isothiocyanate (FITC) and iron oxide nanoparticles (Ferumoxytol). The scale bars represent 100 µm. Abbreviations: 2P-IVM: 2-photon intravital microscopy, RFP: red fluorescent protein, GBM: glioblastoma, FITC: fluorescein isothiocyanate, NP: nanoparticles. Please click here to view a larger version of this figure.
Supplemental Coding File 1: 3D CAD file of the stage. Please click here to download this File.
Discussion
We present a method for real-time in vivo NP tracking using 2P-IVM through a cranial window to evaluate the tumor delivery of fluorescent-labeled iron oxide NPs. The surgical technique for this procedure requires a steady hand and advanced experimental surgical skills. It is advisable to practice using carcasses or phantoms before moving forward to live animal experience. As an alternative, Hoeferlin et al. implemented a robotic drill to reduce thermal damage, minimize surgical technique variability, and standardize surgery22.
The size of the window represents another critical parameter. A larger window would enable imaging of a bigger portion of the tumor with 2P-IVM. In the literature, sizes between 3-7 mm have been described23. However, larger windows cannot accommodate for the brain curvature, which can lead to increased regional pressure24. To overcome this restraint, a so called "glass skull" can be used instead25. In comparison to traditional glass windows, this method uses curved glass, accommodating the brain curvature and thus allowing for the creation of bigger windows while reducing focal pressure on the cortex. This type of curved glass is not commercially available, and it only has limited applications in 2P-IVM, since the curved surface causes reflection artifacts, limiting the area of the window that could be imaged. Another alternative is a silicon-based window24. On one hand, this method offers more flexibility regarding the size and form of the window to be created. In addition, comparable results in terms of window failure and inflammation rates to classic glass windows have been reported. On the other hand, 2-photon imaging quality in polymer-based windows has been shown to decrease faster than in glass windows, making it not feasible for long-term applications26. A thinned skull cranial window represents another alternative. While being less invasive than the classical glass window, it does not allow for GBM cell implantation using the same technique as described here. Additionally, it is difficult to achieve a consistent skull thickness, and as a result, this can cause significant artifacts in 2P-IVM27.
The cell line and amount of GBM cells being implanted are other important considerations when planning the study timeline. The C6 rat glioma cell line is one of the most widely used cell lines in GBM research. In rats and mice, cell numbers between 5 x 104 and 5 x 106 have been reported for intracranial implantations27,28,29,30,31. As a general rule, when implanting a higher number of cells, faster tumor growth is to be expected. In this protocol, 1 x 105 cells were implanted and imaging was performed on days 11 and 12 after implantation. Xin et al. implanted 1 x 105 C6 cells in BALB/c nude mice and reported detecting advanced GBM in MRI on day 7 post-implantation and an increased mortality after 15 days30. In comparison, Jia et al. used a higher number of cells (1 x 106) and the same mouse strain and detected a small tumor mass at day 7 in MRI with a slightly disrupted blood-brain barrier (BBB), as demonstrated by a pale Evans blue stain in the GBM tissue32. On day 14, the Evans blue stain was stronger than on day 7, indicating that the BBB was highly disrupted. In turn, the tumor growth also affects how long the animals can be kept in the experiment. This is an important consideration of animal welfare for longitudinal imaging studies. Chronic cranial windows have been reported to be suitable for imaging for up to 6 months after implantation26.
The nanoparticles used in this protocol consist of iron oxide and fluorophore components. Possible applications include the investigation of the tumor delivery of novel therapeutic drugs, cellular therapeutics, and interventions on the tumor vasculature and tumor microenvironment. Different drug candidates and combination therapies can be evaluated on a cellular and molecular level. The iron oxide component of the NPs allows for multimodal imaging with MRI or magnetic particle imaging in addition to 2P-IVM. While MRI represents a clinically relevant imaging modality, its anatomical resolution is inferior to that of intravital microscopy33.
This method also has certain limitations. The brain coordinates according to a mouse brain atlas are standardized for C57BL/6J mice and must be adjusted depending on the mouse strain, sex, and age. Moreover, with two-photon imaging, only a limited depth of approximately 450 µm can be accessed9. Therefore, only partial characterization of the tumor is possible with 2P-IVM, and regional differences in the tumor characteristics could be missed. Additionally, only two time points following NP administration were included. Future studies, including more time points after intravenous administration, will allow for a more detailed analysis of the spatiotemporal behavior of the NPs in the tumor microenvironment.
To conclude, we evaluated the tumor delivery of fluorescent-labeled iron oxide nanoparticles using 2P-IVM in a mouse model of GBM. This method can be easily modified to fit various areas of research and represents an important tool for in vivo brain imaging in the field of neuroscience.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We would like to thank the Stanford Wu Tsai Neuroscience Microscopy Service, the Stanford Center for Innovations in In Vivo Imaging (SCi3) – small animal imaging center, NIH S10 Shared Instrumentation Grant (S10RR026917-01, PI Michael Moseley, Ph.D.), and Stanford Preclinical Imaging Facility at Porter Drive for providing the equipment and infrastructure for this project. This work was supported by a grant from the National Institute for Child Health and Human Development, grant number R01HD103638. We would like to thank the Schnitzer Group, Stanford University; the Zuo lab, University of Santa Cruz; and the Neurovascular Imaging Laboratory, Boston Photonic Center, University of Boston, for educational discussions on two-photon imaging and cranial window models.
Materials
| 0.9% sodium chloride infusion solution | Baxter Corp | 533-JB1301P | |
Dulbecco's Modified Eagle Medium |
Invitrogen | 11965-092 | |
| 1 mL syringes | BD | Luer-Lok syringe, REF309628 | |
| 10% FBS | Thermo fisher | Cytiva SH30910.03HI | |
| 10% DMSO | Sigma-Aldrich | D8418-50ML | |
| 2-photon microscope | Prairie Technologies, Bruker | Prairie Ultima IV | |
| Alcohol applicators, 70% | Medline Industries, LP | MDS093810 | |
| Alcohol, spray bottle | Decon Labs Inc | Decon SaniHol, 04-355-122 | |
| Aluminum foil | Reynold Brands | Reynold Wrap non-stick aluminum foil | |
| Anesthesia machine | Patterson Scientific | SAS3 | |
| Anesthesia monitoring | Kent Scientific | MouseSTAT Jr. Rodent Pulsoxymeter | |
| Antibiotic-Antimycotic (100x), liquid | Invitrogen | 15240-096 | |
| Betadine applicators | Professional Disposables International, Inc | S41125 | |
| Biopsy punch, 5 mm | Miltex | Size 5 | |
| Buprenorphine sustained release | Zoo Pharm | Bup SR Lab, 1.0 mg/mL | A generic drug can be used instead. |
| C6 rat glioma cell line | ATCC (American Tissue Culture Collection) | CCL-107 | |
| Cannulas | BD | 16 G, 1.1/2”, 30 G, 1” | |
| Carprofen | Pfizer | Rimadyl, 50 mg/ml | A generic drug can be used instead. |
| Cefazoline | Sagent Pharmaceuticals | 25021-101-10, 1 g/vial | A generic drug can be used instead. |
| Cell strainer, 40 µm | Fisher Scientific | 87711 | |
| Cotton tip applicators, 6” | Dyad Medical Sourcing, LLC | HCS1005 | |
| Dental cement | Stoelting Co | 51459 | Dental cement kit, clear, 2 components |
| Dexamethasone | Bimeda | 138RX, 2 mg/mL | A generic drug can be used instead. |
| DietGel | ClearH2O | Recovery, 72-06-502 | |
| Drape | Cardinal Health | Bio Shield Wrap | |
| Drill | Saeyang Microtech | Escort Pro, B08350 | |
| Drill tips | Hager & Meissinger GmbH | REF310104001001005 | Size 005, US 1/4 |
| FIJI imaging analysis software | National Institute of Health | https://imagej.net/software/fiji/ | |
| Forceps | Fisher Scientific | 13-812-41 | |
| Gauze | Fisher HealthCare | Sterile Cotton Gauze Pad, 4 x 4”, 22-415-469 | |
| Gelfoam | Ethicon Inc. | Surgifoam absorbable gelatin sponge, Ref. 1972 | |
| Germinator | Cellpoint Scientific | Germinator 500, No. 11688 | |
| Glass coverslips, 5 mm diameter | Fisher Scientific | Menzel Cover glass | |
| Gloves, non-sterile | Fisher Scientific | Nitrile powder-free medical examination gloves | |
| Gloves, sterile | Medline Industries, LP | MDS104070 | |
| Hair removal cream | Church & Dwight | Nair Hair remover lotion | |
| Hamilton syringe | Hamilton Company Inc | Gastight #1701, 10 µL | |
| HBSS without Ca, Mg | Fisher Scientific | PI88284 | |
| Head bar | Hongway | 5 mm inner diameter O-rings | |
| Heating pad | Stoelting Co. | Rodent warmer X2 | |
| Insulin syringes | Exel International Medical Products | 29G x 1/2″ | |
| Iron oxide nanoparticles | Covis Pharma GmbH | Feraheme ferumoxytol injection, 510 mg/17 mL, 59338077501 | |
| Isoflurane | Dechra | 26675-46-7 | |
| Mice | Jackson Laboratories | NSG, Strain 005557 | |
| Microscope (surgery) | Seiler Medical | Seiler IQ Q-100-220 | |
| Nanoparticles | Custom | Iron oxide nanoparticles (Ferumoxytol) labeled with fluorescein isothiocyanate | |
| Ophtalmic ointment | Major pharmaceuticals | Lubrifresh P.M. nighttime ointment, 203964 | |
| Oxygen | Linde Gas & Equipment Inc. | High Pressure Steel K Style Cylinder, 249CF, 2000PSIG, CGA 540 | |
| Plastic cups | Georgia-Pacirif Consumer Products | Dixie Portion Cup, 2 oz., Plastic, Clear, PK2400 | |
| Polyethylene tubing | Braintree Scientific | 50-195-5494 | |
| Scale | Ohaus Corp | CR2200 | |
| Scalpel | Integra Life Sciences Production Corp | Integra Miltex Stainless steel disposable scalpel | |
| Scissors | Fisher Scientific | 13-804-18 | |
| Sealant | Henkel Corp | Loctite 4014 | |
| Single use lab gown | High Tech Conversions | 17-444-081 | |
| Stereotactic frame | Stoelting Co. | Stoelting New Standard TM | |
| Sterile Vacuum Bottle Top Filtration Systems | Fisher Scientific | S2GPU05RE, MilliporeSigma NO.:S2GPU05RE | |
| Styrofoam box | N/A | N/A | |
| Surgical gloves | Cardinal Health | 19-163-108 | |
| Surgical glue, 3M Vetbond tissues adhesive | 3M Animal Care Prodcuts | 1469SB | |
| Tail vein cathether | Custom | Consists of two 30 G cannulas connected with sillicone tubing | |
| TrypLE Express (1x), no phenol red | Invitrogen | 12604-039 | |
| Ultraviolett torch | Spring sunshine technology | Consciot |
References
- Denk, W., Strickler, J. H., Webb, W. W. Two-photon laser scanning fluorescence microscopy. Science. 248 (4951), 73-76 (1990).
- Wang, Z., McCracken, S., Williams, P. R. Transpupillary two-photon in vivo imaging of the mouse retina. J Vis Exp. 168, 61970 (2021).
- Zhang, K., et al. In vivo two-photon microscopy reveals the contribution of Sox9+ to kidney regeneration in a mouse model with extracellular vesicle treatment. J Biol Chem. 295 (34), 12203 (2020).
- Jang, W. H., et al. Two-photon microscopy of Paneth cells in the small intestine of live mice. Sci Rep. 8 (1), 14174 (2018).
- Ihler, F., Bertlich, M., Weiss, B., Dietzel, S., Canis, M. Two-photon microscopy allows imaging and characterization of cochlear microvasculature in vivo. Biomed Res Int. 2015, 154272 (2015).
- Matsuura, R., et al. Intravital imaging with two-photon microscopy reveals cellular dynamics in the ischeamia-reperfused rat heart. Sci Rep. 8 (1), 15991 (2018).
- Veres, T. Z., et al. Intubation-free in vivo imaging of the tracheal mucosa using two-photon microscopy. Sci Rep. 7 (1), 694 (2017).
- Zong, W., et al. Large-scale two-photon calcium imaging in freely moving mice. Cell. 185 (7), 1240-1256.e30 (2022).
- Takasaki, K., Abbasi-Asl, R., Waters, J. Superficial bound of the depth limit of two-photon imaging in mouse brain. eNeuro. 17 (1), (2019).
- Birkner, A., Konnerth, A. Deep two-photon imaging in vivo with a red-shifted calcium indicator. Methods Mol Biol. 1929, 15-26 (1929).
- Busche, M. A. In vivo two-photon calcium imaging of hippocampal neurons in Alzheimer mouse models. Methods Mol Biol. 1750, 341-351 (2018).
- Li, L., et al. A sensitive two-photon probe to selectively detect monoamine oxidase B activity in Parkinson’s disease models. Nat Commun. 5, 3276 (2014).
- Madden, K. S., Zettel, M. L., Majewska, A. K., Brown, E. B. Brain tumor imaging: live imaging of glioma by two-photon microscopy. Cold Spring Harb Protoc. 2013 (3), (2013).
- Ricard, C., et al. Phenotypic dynamics of microglial and monocyte-derived cells in glioblastoma-bearing mice. Sci Rep. 6, 26381 (2016).
- Soubéran, A., et al. Effects of VEGF blockade on the dynamics of the inflammatory landscape in glioblastoma-bearing mice. J Neuroinflammation. 16 (1), 191 (2019).
- Zhang, W., et al. Real-time vivo reveals specific nanoparticle target binding in a syngeneic glioma mouse model. Nanoscale. 14 (15), 5678-5688 (2022).
- Wartchow, K. M., et al. Treatment with cyclic AMP activators reduces glioblastoma growth and invasion as assessed by two-photon microscopy. Cells. 10 (3), 556 (2021).
- Jiang, L. W., et al. Label-free detection of fibrillar collagen deposition associated with vascular elements in glioblastoma multiforme by using multiphoton microscopy. J Microsc. 265 (2), 207-213 (2017).
- Chen, Z., Ross, J. L., Hambardzumyan, D. Intravital 2-photon imaging reveals distinct morphology and infiltrative properties of glioblastoma-associated macrophages. Proc Natl Acad Sci U S A. 116 (28), 14254-14259 (2019).
- Zhang, Y. S., et al. Labeling human mesenchymal stem cells with gold nanocages for in vitro and in vivo tracking by two-photon microscopy and photoacoustic microscopy. Theranostics. 3 (8), 532-543 (2013).
- Schindelin, J., et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 9 (7), 676-682 (2012).
- Hoeferlin, G. F., Menendez, D. M., Krebs, O. K., Capadona, J. R., Shoffstall, A. J. Assessment of thermal damage from robot-drilled craniotomy for cranial window surgery in mice. J Vis Exp. 189, 64188 (2022).
- Holtmaat, A., et al. high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat Protoc. 4 (8), 1128-1144 (2009).
- Heo, C., et al. A soft, transparent, freely accessible cranial window for chronic imaging and electrophysiology. Sci Rep. 6, 27818 (2016).
- Kim, T. H., et al. Long-term optical access to an estimated one million neurons in the live mouse cortex. Cell Rep. 17 (12), 3385-3394 (2016).
- Kılıç, K., et al. Chronic cranial windows for long term multimodal neurovascular imaging in mice. Front Physiol. 11, 612678 (2021).
- Helm, P. J., Ottersen, O. P., Nase, G. Analysis of optical properties of the mouse cranium-implications for in vivo multi photon laser scanning microscopy. J Neurosci Methods. 178 (2), 316-322 (2009).
- Lu, W., Sun, Q., Wan, J., She, Z., Jiang, X. G. Cationic albumin-conjugated pegylated nanoparticles allow gene delivery into brain tumors via intravenous administration. Cancer Res. 66 (24), 11878-11887 (2006).
- Zhang, B., et al. LDLR-mediated peptide-22-conjugated nanoparticles for dual-targeting therapy of brain glioma. Biomaterials. 34 (36), 9171-9182 (2013).
- Xin, H., et al. Enhanced anti-glioblastoma efficacy by PTX-loaded PEGylated poly(ɛ-caprolactone) nanoparticles: In vitro and in vivo evaluation. Int J Pharm. 402 (1-2), 238-247 (2010).
- Valable, S., et al. In vivo MRI tracking of exogenous monocytes/macrophages targeting brain tumors in a rat model of glioma. Neuroimage. 40 (2), 972 (2008).
- Jia, Y., et al. Phototheranostics: Active targeting of orthotopic glioma using biomimetic proteolipid nanoparticles. ACS Nano. 13 (1), 386-398 (2021).
- Asan, L., et al. Cellular correlates of gray matter volume changes in magnetic resonance morphometry identified by two-photon microscopy. Sci Rep. 11, 4234 (2021).

