Suspension Culture Production and Purification of Adeno-Associated Virus by Iodixanol Density Gradient Centrifugation for In Vivo Applications
Summary
Adeno-associated virus is produced in suspension cell culture and purified by double iodixanol density gradient centrifugation. Steps are included to increase total virus yield, decrease the risk of virus precipitation, and further concentrate the final virus product. Expected final titers reach 1012 viral particles/mL and are suitable for pre-clinical in vivo use.
Abstract
This protocol describes recombinant adeno-associated virus (rAAV) production and purification by iodixanol density gradient centrifugation, a serotype-agnostic method of purifying AAV first described in 1999. rAAV vectors are widely used in gene therapy applications to deliver transgenes to various human cell types. In this work, the recombinant virus is produced by transfection of Expi293 cells in suspension culture with plasmids encoding the transgene, vector capsid, and adenoviral helper genes. Iodixanol density gradient centrifugation purifies full AAV particles based on particle density. Additionally, three steps are included in this now-ubiquitous methodology in order to increase total virus yield, decrease the risk of precipitation due to contaminating proteins, and further concentrate the final virus product, respectively: precipitation of viral particles from cell media using a solution of polyethylene glycol (PEG) and sodium chloride, the introduction of a second round of iodixanol density gradient centrifugation, and buffer exchange via a centrifugal filter. Using this method, it is possible to consistently achieve titers in the range of 1012 viral particles/mL of exceptional purity for in vivo use.
Introduction
Recombinant adeno-associated viral (rAAV) vectors are widely used tools for the treatment of genetic diseases, including spinal muscular atrophy, retinal dystrophy, and hemophilia A1,2,3. rAAV vectors are engineered to lack viral genes present in wild-type AAV4, a small, non-envelope icosahedral virus with a linear single-stranded 4.7 kb DNA genome. AAV was first discovered in the 1960s as a contaminant of adenovirus preparations5. Despite its small capsid size, which limits the size of the transgene that can be packaged to a maximum of 4.9 kb excluding ITRs6, AAV is useful for transgene delivery because it is non-pathogenic in humans, allows expression of transgene in many dividing and nondividing cell types, and has limited immunogenic effects7.
As members of the genus dependoparvovirus, the production of rAAVs relies on the expression of helper genes present in adenovirus or herpes simplex virus8. Several strategies to produce rAAV have been developed, but production in HEK293 cells transformed with the adenoviral E1A/E1B helper genes is the most established method used today9. The general approach of rAAV production begins with the transfection of HEK293 cells with three plasmids containing the transgene within inverted terminal repeats (ITRs), AAV rep and cap genes, and additional adenoviral helper genes, respectively. Seventy-two hours after transfection, cells are harvested and processed to purify rAAV containing the transgene.
In the development of new rAAV vectors for therapeutic purposes, a major goal is producing vectors with increased transduction efficiency. An increase in the transduction efficiency of target cells would mean a decrease in the necessary clinical dose of rAAV, thus decreasing the likelihood of adverse immunogenic effects ranging from antibody-mediated neutralization to acute toxicities10,11. To improve the transduction efficacy of rAAV vectors, alterations can be made to the packaged genome or to the capsid. Viable methods to tune transduction efficacy via packaged genome design include the incorporation of strong and tissue-specific promoters, thoughtful selection of mRNA processing elements, and coding sequence optimization to improve translation efficiency12. Alterations to the capsid are made with the goal of increasing tropism for target human cell types. Efforts towards developing new rAAV transgene delivery vector capsids are generally characterized by a focus on either rational design of AAV capsids with specific mutations targeting specific cell receptors or directed evolution to identify capsids with tropism for specific cell types from high-complexity combinatorial capsid libraries without targeting one specific receptor (although some groups combine these approaches)13,14,15. In the directed evolution approach, combinatorial capsid libraries are constructed using a particular serotype backbone with mutated variable regions on the capsid exterior16. Combinatorial capsid libraries are often constructed from AAV serotypes not originating in humans, decreasing the risk of preexisting immunity during clinical use10. Therefore, purification methods that can be applied to any serotype are ideal to eliminate the need for serotype-specific optimization for the less commonly used serotypes serving as backbones for these libraries.
Iodixanol density gradient centrifugation is utilized to purify high titers of rAAV with high infectivity17. In this protocol, rAAV is produced in suspension cell culture to decrease the amount of labor needed to produce large titers of AAV. A centrifugation step is also included to clear cell lysate to reduce the presence of contaminating proteins and decrease the risk of virus precipitation. This protocol is a cost-effective method to produce preparations of high-purity rAAV suitable for pre-clinical use.
Protocol
The composition of the solutions and buffers used in this protocol are provided in Table 1.
| Solution | Composition | |
| AAV lysis buffer | 1.2 mL of 5 M NaCl solution | |
| 2 mL of 1 M Tris-HCl pH 8.5 solution | ||
| 80 uL of 1 M MgCl2 solution | ||
| mQ water to 40 mL | ||
| AAV precipitation solution | 40 g PEG 8000 | |
| 50 mL of 5 M NaCl solution | ||
| mQ water to 100 mL | ||
| 15% iodixanol fraction | 7.5 mL OptiPrep | |
| 3 mL of 10X DPBS | ||
| 6 mL of 5 M NaCl solution | ||
| 30 uL of 1 M MgCl2 solution | ||
| mQ to 30 mL | ||
| 25% iodixanol fraction | 12. 5 mL OptiPrep | |
| 3 mL of 10X DPBS | ||
| 30 uL of 1 M MgCl2 solution | ||
| 60 uL phenol red solution | ||
| mQ to 30 mL | ||
| 40% iodixanol fraction | 33.3 mL OptiPrep | |
| 5 mL of 10X DPBS | ||
| 50 uL of 1 M MgCl2 solution | ||
| mQ to 50 mL | ||
| 60% iodixanol fraction | 50 mL OptiPrep | |
| 100 uL phenol red solution | ||
| AAV buffer solution | 8 mL of 5 M NaCl | |
| 20 uL of 10% Pluronic F-68 | ||
| PBS to 200 mL | ||
Table 1: Solution compositions for solutions used in this protocol.
1. Triple transfection of Expi293 cells
- Seed Expi293 cells (see Table of Materials) at an initial density of 5 x 105 viable cells (vc)/mL.
- Allow the cells to incubate at 37 °C with 8% CO2 and a shaker speed of 125 rpm until they reach a density of 3-5 x 106 vc/mL. Monitor cell density and viability using trypan blue exclusion with an automated cell counter (see Table of Materials).
- When cells reach the desired cell density, split them 1 to 10 by adding fresh media so that their total volume is ten times their original volume. If necessary, transfer cells into a bigger flask. Return to incubation.
- Continue to expand cells as described in steps 1.2-1.3 until they reach a density of at least 2 x 106 vc/mL in 250 mL of media in a 1 L flask.
- The day before transfection, dilute cells to 2 x 106 vc/mL in 250 mL of media, discarding excess cells as necessary. Incubate the cells overnight.
- On the day of transfection, centrifugate half of the cells at 58 x g for 5 min at 18 °C. Resuspend the cell pellet in the same volume (125 mL) of fresh medium. The cell viability should be close to 98%.
- Prepare two 50 mL conical tubes. Label one "PEI" and the other "DNA".
- In the conical tube labeled PEI, dilute 1.2 mL of PEI (Polyethylenimine, see Table of Materials) to a total volume of 12.5 mL in OptiMEM media.
- In the conical tube labeled DNA, prepare an equimolar solution of plasmid DNA to a total of 500 µg of DNA in a total volume of 12.5 mL in OptiMEM media.
- Add the DNA solution to the PEI solution, invert several times to thoroughly combine, and let incubate at room temperature for 10 min.
NOTE: It is critical that the DNA-PEI solution is allowed to incubate for the full 10 min in order for PEI to form charged complexes with the DNA. - After the DNA-PEI solution has incubated for 10 min, use a serological pipet to slowly apply the DNA-PEImax solution to the Expi293 cells. Return the transfected cells to the incubator and let them incubate for 72 h (Figure 1).
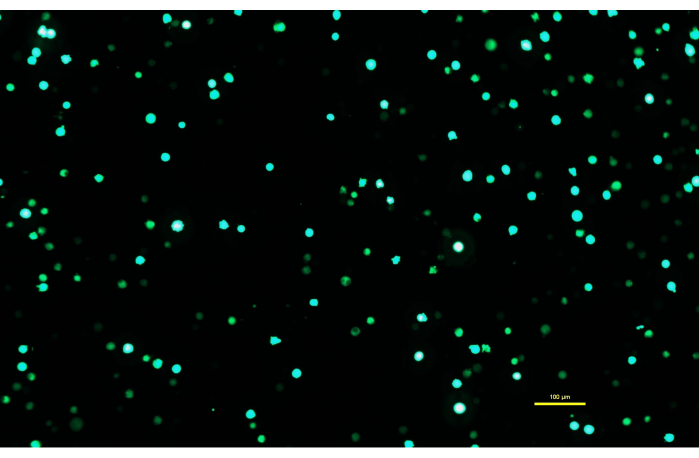
Figure 1: Expi293 cells expressing GFP two days after transfection. After transfection with a plasmid containing a gene for GFP, the Expi293 cells transiently express eGFP. The cell morphology is round. The image was captured with a 15 ms exposure time. Microscope images are acquired using an inverted microscope equipped with epi-fluorescence illumination and a 10x/0.30 objective. Scale bar = 100 µm. Please click here to view a larger version of this figure.
2. rAAV vector purification
- After 72 h, transfer the cells in suspension to two 250 mL conical tubes and spin them down at 415 x g for 10 min at 4 °C.
- Pour the supernatant media into a fresh 500 mL conical tube and store at 0 °C on ice for later processing.
- Resuspend each cell pellet in 10 mL of AAV lysis buffer (Table 1). Pool the two lysates together in one of the tubes. Rinse the other tube with an additional 5 mL of AAV lysis buffer, then add it to the pooled lysates. Freeze at -70 °C.
NOTE: The experiment can be paused at this point. Store the supernatant media at -70 °C rather than on ice. - Add 1:4 volume of AAV precipitation solution to the supernatant media from step 2.2. Invert several times to thoroughly mix and incubate at 0 °C on ice for at least 2 h or overnight.
- Centrifugate the incubated solution at 3000 x g for 1 h at 4 °C.
- Discard the supernatant and resuspend the pellet containing viral precipitate in 5 mL of AAV lysis buffer.
- Thaw the cell lysate at 37 °C in a water bath.
- Pool the viral precipitate with the cell lysate. This is the crude lysate. Rinse the centrifugation tube that contained the viral precipitate with an additional 5 mL of AAV lysis buffer and pool with the crude lysate.
- Freeze the crude lysate to -70 °C, then thaw at 37 °C. Repeat this cycle once more.
NOTE: The crude lysate should not be left at 37 °C for any longer than is necessary to thaw it. - Once thawed for the third time, immediately add 4 µL of benzonase to the crude lysate, invert to mix, and incubate for 30 min at 37 °C.
- Centrifugate the crude lysate for 10 min at 650 x g at 18 °C.
- Transfer the supernatant to a clean 50 mL conical tube. This is the crude virus. Discard the pellet.
- Centrifugate the crude virus for an additional 30 min at 3000 x g at 18 °C to clear contaminating proteins.
- Transfer the supernatant to a clean 50 mL conical tube. This is the clarified virus.
NOTE: The experiment can be paused at this point. Freeze down the clarified virus to -70 °C. - Set up the multichannel peristaltic pump with four peristaltic tubes (see Table of Materials). Attach capillaries to both ends of each tube.
- Place the capillaries on the input side of the pump in a beaker filled with deionized water. Place the capillaries on the output side of the pump in an empty beaker. Run the peristaltic pump at 25.0 rpm to flush the tubing with deionized (DI) water.
- When thoroughly flushed, empty the peristaltic tubing by running the pump until the tubing is filled only with air. Lay all capillaries on a clean lint free wipe.
- Place four ultracentrifuge tubes in a rack on the output side of the pump.
- Use a 10 mL serological pipet to carefully dispense 10 mL of clarified virus into each ultracentrifuge tube. Take care not to introduce air bubbles.
NOTE: The volume of clarified virus in each tube can be stretched to up to 12 mL per tube, if necessary. Reduce the amount of 60% iodixanol appropriately in step 2.25 below. - The clarified virus is underlaid with iodixanol fractions (Table 1) from lowest density to highest density using the peristaltic pump. The 15% fraction is added first, followed by the 25% fraction, the 40% fraction, and finally the 60% fraction. Transfer 22 mL (5.5 mL per ultracentrifuge tube) of the 15% iodixanol fraction into a clean 50 mL conical tube. Place the capillaries on the input side of the pump into the tube, taking care that all capillaries are touching the bottom of the tube.
- Start the pump and allow the tubing to fill with the iodixanol fraction. When the iodixanol fraction reaches the ends of the capillaries on the output side of the pump, stop the pump.
- Insert one output capillary into each ultracentrifuge tubes with clarified virus, taking care that the capillaries are touching the bottom of each ultracentrifuge tube.
NOTE: It is of critical importance that there is no air left in the capillaries. Iodixanol may flow through the peristaltic tubing at slightly different rates, so ensure that each individual output capillary is completely filled with iodixanol before inserting it into the clarified virus. It may be necessary to stop and start the pump several times. - Start the pump and allow the ultracentrifuge tubes to fill. When the last of the 15% fraction is about to be taken into the input capillaries, stop the pump. It is critical that no air bubbles enter the peristaltic tubing.
NOTE: If air bubbles enter the input capillaries, the pump can be briefly run in the reverse direction to push them back out. - Transfer 22 mL (5.5 mL per ultracentrifuge tube) of the 25% iodixanol fraction into the 50 mL conical tube. Take care that all capillaries are touching the bottom of the tube and start the pump. When the last of the 25% fraction is about to be taken into the input capillaries, stop the pump.
- Repeat step 2.24 with 20 mL (5 mL per ultracentrifuge tube) of the 40% iodixanol fraction and then with 24 mL (6 mL per ultracentrifuge tube) of the 60% fraction.
- If there is still unfilled volume left in the ultracentrifuge tube, continue to add more of the 60% fraction until the ultracentrifuge tubes are completely filled with liquid.
- Fill each tube until the lysate makes a dome over the top but does not overflow from the tube. Stop the pump and carefully remove each output capillary, taking care not to disturb the iodixanol gradient.
- Cap each ultracentrifuge tube with a spacer and load it into a Type 70 Ti rotor (see Table of Materials).
NOTE: Ensure that the rotor is appropriately balanced. Do not attempt to operate the ultracentrifuge without proper training. - Load the Type 70 Ti rotor into the ultracentrifuge and centrifugate at 489,000 x g for 1 h at 18 °C.
- After centrifugation, unload the rotor from the ultracentrifuge. Use needle nose pliers to carefully remove each ultracentrifuge tube from the rotor, taking care not to disturb the iodixanol gradient.
- Use a support ring stand with clamp to secure one ultracentrifuge tube.
- Attach a 20 GA needle to a 5 mL syringe and set aside. Use a lint-free wipe to remove the cap from the ultracentrifuge tube.
- Penetrate the wall of the ultracentrifuge tube with the needle about 3 mm below the 40%-60% iodixanol interface (Figure 2).
- Slowly aspirate the interface and part of the 40% fraction. Avoid aspirating the top of the 40% fraction, for a total draw of about 4 mL.
- Holding one finger over the open top of the ultracentrifuge tube, pull out the syringe and transfer the aspirated AAV fraction to a 50 mL conical tube. Discard the syringe in a sharps container. Discard the ultracentrifuge tube.
- Repeat steps 2.31-2.35 for each ultracentrifuge tube.
- Dilute the aspirated AAV fraction approximately two-fold in AAV lysis buffer to a volume of 40 mL.
- Flush the peristaltic tubing as described in steps 2.16-2.17.
NOTE: The experiment can be paused at this point. Store the diluted AAV fraction at 0 °C overnight. - Load 20 mL each of the diluted AAV fraction into two new ultracentrifuge tubes.
- For the second round of iodixanol density gradient centrifugation, the diluted AAV fraction is underlaid as described above using only 10 mL (5 mL per ultracentrifuge tube) of the 40% fraction and 14 mL (7 mL per ultracentrifuge tube) of the 60% fraction. Repeat steps 2.29-2.36 for ultracentrifugation and aspiration.
NOTE: Before storing the peristaltic pump and tubing, flush out the tubing with DI water as described in steps 2.16-2.17.
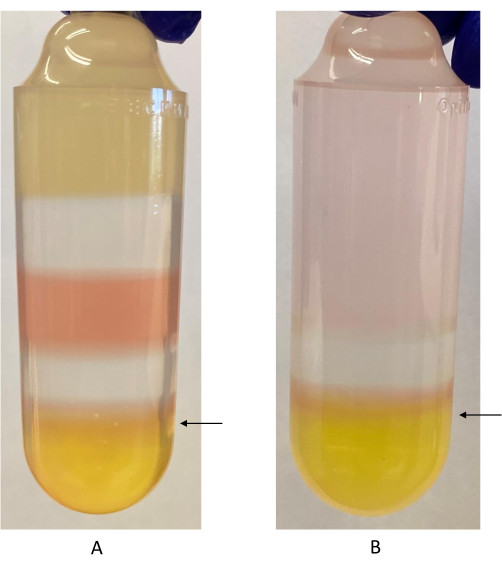
Figure 2: Iodixanol gradient with 40%-60% iodixanol interface labeled. (A) First iodixanol gradient. Phenol red is used in the 40% iodixanol and 60% iodixanol fractions. It appears as a different color because of the difference in pH between the two fractions. The arrow indicates where the syringe should be inserted to harvest the rAAV fraction, just below the 40%-60% iodixanol interface. (B) Second iodixanol gradient. Only the 40% and 60% iodixanol fractions are used in this step. The arrow indicates where the syringe should be inserted to harvest the rAAV fraction. Please click here to view a larger version of this figure.
3. Buffer exchange and virus concentration
- After obtaining the AAV fraction from the second round of iodixanol density gradient centrifugation, dilute it two-fold with AAV buffer solution.
- Equilibrate the centrifugal filter by adding 20 mL of AAV buffer solution to the top of the filter. Centrifugate the apparatus at 3000 x g at 18 °C for 5 min and discard flow through.
- Add the diluted AAV fraction to the centrifugal filter. Centrifugate the apparatus at 3000 x g at 18 °C for 5 min and discard flow through.
- Add 20 mL of AAV buffer solution to the top of the filter. Centrifugate the apparatus at 3000 x g at 18 °C for 5 min and discard flow through.
NOTE: If the centrifugal filter membrane becomes blocked, causing the solution to flow through inefficiently, carefully use a P200 micropipette to draw up and down the solution in the top of the filter. Take extreme care not to touch the filter with the micropipette tip. - Repeat step 3.4 twice more.
NOTE: Ensure that the AAV is not overconcentrated by maintaining at least 1 mL of volume in the top of the filter. It may be necessary to adjust centrifugation times to prevent overconcentration. If AAV is overconcentrated, precipitation can occur. - For a final titer in the range of 1012 vg/mL, spin down the virus to a final volume of about 1 mL.
NOTE: Depending on the serotype backbone used and the packaging efficiency of the virus, you may have to concentrate the virus in a smaller volume. - Use a p1000 micropipette to transfer the concentrated AAV from the top of the centrifugal filter into a microcentrifuge tube. Use a p200 micropipette to wash the centrifugal filter with 50 µL of AAV buffer to collect any remaining virus and pool with the rest of the concentrated AAV.
- Set aside a 2 µL aliquot of the concentrated AAV for titering. See Supplementary Table 1 to view ITR primers that can be used for qPCR.
- Pass the concentrated AAV through a 0.2 µm syringe filter to sterilize it.
NOTE: When sterilizing a small volume, take care to select a syringe filter with a small diameter in order to minimize virus loss. Using a low-protein binding filter of a small diameter will result in minimal viral loss (data not shown). - The sterilized AAV can be used immediately to transduce cells or stored at 4 °C for use within four weeks. It should be stored at -70 °C for longer-term storage.
Representative Results
This method can be used to obtain titers of at least 1012 viral particles per mL. A titer can be obtained (Figure 3) by qPCR using the ITR primers provided in Supplementary Table 1, by ddPCR, or by any other titering method. Suboptimal titers could result from using a cap gene encoding a capsid with poor packaging efficiency.
Another possible source of suboptimal results is the poor transduction efficiency of Expi293 cells. It is recommended to have cells at a density of 3-4 x 106 vc/mL on the day of transfection and that the cell viability is close to 98%. In the present study, the authors obtained a titer of 1.07 x 1012 vg following this protocol. This yield aligns with previous yields obtained from packaging AAVrh7418.
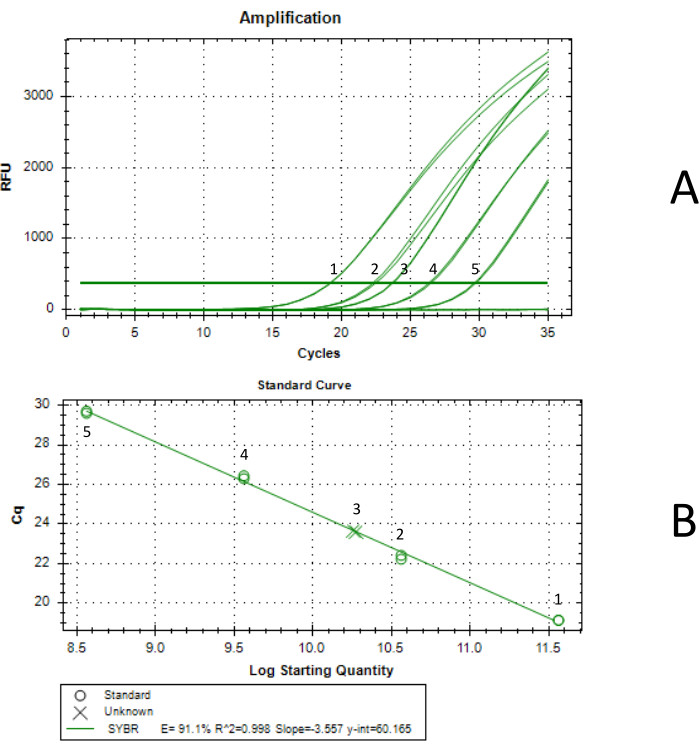
Figure 3: Titer determination by qPCR. Curve 1 was generated with a standard concentration of 3.66 x 1011 vg/mL, curve 2 was generated with a standard concentration of 3.66 x 1010 vg/mL, curve 3 was the final concentrated AAV sample, curve 4 was generated with a standard concentration of 3.66 x 109 vg/mL, and curve 5 was generated with a standard concentration of 3.66 x 108 vg/mL. Each qPCR reaction was performed in duplicate. (A) qPCR amplification curve. Standards were generated by serial dilution of an AAV standard tittered by ddPCR. The concentration of the undiluted standard was 3.66 x 1012 vg/mL. (B) Standard curve generated by qPCR. The produced AAV was determined to have a concentration of 1.85 x 1011 vg/mL in 5.75 mL, for a total yield of 1.07 x 1012 vg. Please click here to view a larger version of this figure.
Supplementary Table 1: Forward and reverse primer sequences for AAV inverted terminal repeats (ITRs). Please click here to download this File.
Discussion
The double iodixanol density gradient purification protocol is the universal method because it is applicable to any AAV mutant variants, regardless of their receptor specificity. Early methods of AAV purification relied on particle density and included isopycnic centrifugation in CsCl and continuous sucrose density gradient centrifugation19. Later, serotype-specific approaches were developed, which made use of monoclonal antibodies bound to Sepharose columns20. A novel density-based purification method using a discontinuous iodixanol gradient was developed in 1999 and yielded AAV isolates with higher infectivity than those isolated from CsCl gradients21. Methods to purify AAV continued to be developed into the early 2000s, as some groups used high-performance liquid chromatography to purify rAAV with recovery from crude lysate exceeding 70%22,23,24. These methods are still used in large-scale manufacturing processes25. Despite the development of chromatography methods for isolating AAV, purification by iodixanol density gradient centrifugation is still widely used due to its lower cost and serotype agnosticism26,27.
While this method is well suited for the time-efficient pre-clinical production of rAAV, it is very limited in its scalability and ability to be adapted for cGMP manufacturing25. Thus, as described above, other purification methods are preferred for large-scale production. Additionally, the transgene capacity of rAAV vectors is limited. The size limit for an AAV genome that can be packaged intact has been found to be about 5.2 kb, for a maximum transgene size of 4.9 kb, accounting for ITRs6. This complicates the expression of proteins whose cDNA exceeds this capacity. These proteins cannot be expressed by the system described here without modification of the transgene itself or the use of a split vector system. Chamberlain et al. describe several methods to express transgenes larger than 4.9 kb28.
This method is particularly useful for the production of high-complexity combinatorial capsid libraries containing AAV capsids with varied biochemical properties due to mutations present on the capsid surface. If the rAAV preparation is destined for injection in animals, especially in non-human primates, it is critical to limit endotoxin contamination. Great care must be taken to separate areas used to grow bacterial cultures from rAAV production to prevent bacterial contamination during all steps. Perform all work with human cells and crude AAV in a biosafety cabinet using disposable plasticware. This prevents bacterial contamination and is a necessity for working with AAV, as it is a BSL1 agent.
If a lab does not have access to a CO2 incubator with a shaker, adherent cell culture with HEK293 cells can be used in place of Expi293 suspension cell culture. Follow manufacturer instructions for subculturing and use the transfection protocol described in Crosson et al., 201817.
Critical steps in this protocol are Expi293 transfection, preparation of the iodixanol gradient, and aspiration of the iodixanol fraction containing AAV. Errors during any of these steps can result in suboptimal rAAV yields or failure to produce rAAV.
Transfection of the Expi293 cells is a critical step in the production of rAAV using this protocol. On the day of transfection, cells are resuspended in half a volume of fresh media to provide them with the nutrients necessary to recover from transfection29; transfection is a very taxing process for the cells. However, the conditioned media is not entirely discarded because it contains growth factors and other metabolites important for cell maintenance and growth. It is critical that PEI and DNA incubate for a full ten minutes before being applied to cells in order to form a charged complex30. Without forming a complex with PEI, DNA will not be efficiently uptaken by cells.
When producing rAAV, the proportion of virus found in the media depends on the particular serotype's interactions with Expi293 cell receptors. Some serotypes are released into cell media at higher rates than others31, necessitating that viral particles be precipitated from cell media to capture the highest yield possible and prevent the loss of any variants based on a lack of affinity for Expi293 cell receptors. In contrast, the serotype AAV2 has strong receptor interactions with heparan sulfate proteoglycans present in 293 cells, so very little packaged virus is found in the media32. When producing a derivative of AAV2, omit the PEG precipitation step. For the production of combinatorial capsid libraries or rAAV of alternate serotypes, precipitation of viral particles with PEG is appropriate.
It is critical that 1 M sodium chloride is present in the 15% iodixanol fraction to disassociate aggregated AAV particles during ultracentrifugation. The omission of NaCl in this fraction can lead to undesired outcomes, such as decreases in transduction efficiency and immunogenicity33. Finally, take great care in aspirating AAV from the iodixanol gradient. Aspirating too much of the iodixanol fraction can result in the presence of empty capsids in the final product17.
This method can be used to package rAAV combinatorial capsid libraries for use in downstream-directed evolution experiments to identify capsids with higher tropism for certain human cell types. rAAV is stable at 4 °C for short-term use and at -70 °C for long-term storage34. Currently, the recombinant AAV is in use in five FDA-approved medications treating conditions including Duchenne muscular dystrophy35,36,37. There are several additional rAAV therapies in clinical and pre-clinical trials38,39,40. Further research on this vector is thus critical to the development of additional gene therapies.
Divulgations
The authors have nothing to disclose.
Acknowledgements
None.
Materials
| 5810 R benchtop centrifuge | Eppendorf | 22625501 | |
| 8-channel peristaltic pump | Watson-Marlow | 020.3708.00A | |
| Automated cell counter | NanoEntek | EVE-MC | |
| Avanti J-E high-speed centrifuge | Beckman Coulter | 369001 | |
| Benzonase | Thermo Scientific | 88701 | |
| Biological safety cabinet | Labconco | 322491101 | |
| CO2 incubator with shaker | Set at 8% CO2 and 37 °C | ||
| Conical centrifuge tubes | Thermo Scientific | 339652 | 50 mL |
| Conical centrifuge tubes | Thermo Scientific | 339650 | 15 mL |
| Disposable micro-pipets | Fisherbrand | 21-164-2G | Capillaries |
| Dulbecco's phosphate buffered saline without CaCl2 and MgCl2 (DPBS) (10x) | Sigma-Aldrich | D1408 | |
| ECLIPSE Ts2R-FL inverted microscope | Nikon | ||
| Expi293 Expression Medium | Gibco | A1435101 | |
| Expi293F cells | Gibco | A14527 | |
| Filter tips | USA Scientific | 1126-7810 | 1000 µL |
| Filter tips | USA Scientific | 1120-8810 | 200 µL |
| Filter tips | USA Scientific | 1120-1810 | 20 µL |
| Filter tips | USA Scientific | 1121-3810 | 10 µL |
| Hypodermic needles | Tyco Healthcare | 820112 | 20 GA x 1-1/2 A |
| Ice bucket with lid | VWR | 10146-184 | |
| JS-5.3 rotor | Beckman Coulter | 368690 | |
| Magnesium chloride solution (1 M) | Millipore Sigma | M1028-100ML | |
| Metal stand and clamp | Fisherbrand | 05-769-6Q | |
| Microcentrifuge tubes | Eppendorf | 22600028 | 1.5 mL |
| Needle nose pliers | |||
| Optima XE-90 ultracentrifuge | Beckman Coulter | A94471 | |
| Opti-MEM I Reduced-Serum Medium | Gibco | 31985062 | |
| OptiPrep density gradient media (iodixanol) | Serumwerk | AXS-1114542 | 60% iodixanol solution |
| P1000 Pipet | Gilson | F144059M | |
| P2 Pipet | Gilson | F144054M | |
| P20 Pipet | Gilson | F144056M | |
| P200 Pipet | Gilson | F144058M | |
| Phenol red solution | Sigma-Aldrich | P0290 | |
| Phosphate buffered saline (PBS) | Sigma-Aldrich | P4474 | |
| Pipet-Aid XP pipette controller | Drummond Scientific | 4-000-101 | |
| Plasmid pCapsid | De novo or Addgene, etc. | N/A | We used pACGrh74. |
| Plasmid pHelper | Addgene | 112867 | |
| Plasmid pTransgene | De novo or Addgene, etc. | N/A | We used pdsAAV-GFP. |
| Pluronic F-68 polyol solution (10%) | Mp Biomedicals | 92750049 | |
| Polyethylene glycol 8000 | Research Products International | P48080-500.0 | |
| Polyethylenimine HCl Max (PEI-Max) | Polysciences | NC1038561 | Dilute in water to 40 μM |
| Polypropylene centrifuge tubes, sterile | Corning | 431123 | 500 mL |
| Polypropylene centrifuge tubes, sterile | Corning | 430776 | 250 mL |
| Polypropylene Optiseal tubes | Beckman Coulter | 361625 | |
| Serological pipettes | Alkali Scientific | SP250-B | 50 mL |
| Serological pipettes | Alkali Scientific | SP225-B | 25 mL |
| Serological pipettes | Alkali Scientific | SP210-B | 10 mL |
| Serological pipettes | Alkali Scientific | SP205-B | 5 mL |
| Shaker flasks | Fisherbrand | PBV1000 | 1 L |
| Shaker flasks | Fisherbrand | PBV50-0 | 500 mL |
| Shaker flasks | Fisherbrand | PBV250 | 250 mL |
| Shaker flasks | Fisherbrand | PBV12-5 | 125 mL |
| Sodium chloride solution (5 M) | Fisher Scientific | NC1752640 | |
| Sterile syringes | Fisherbrand | 14-955-458 | 5 mL |
| Syringe filter | Millipore | SLGV013SL | 0.22 micron |
| Tris-HCl pH 8.5 (1 M) | Kd Medical | RGE3363 | |
| Trypan blue solution | Gibco | 15250061 | |
| Tube rack assembly | Beckman Coulter | 361646 | |
| Tube spacers (x4) | Beckman Coulter | 361669 | |
| Tubing for peristaltic pump | Fisher Scientific | 14190516 | |
| Type 70 Ti fixed-angle titanium rotor | Beckman Coulter | 337922 | |
| Ultra low temperature freezer | Set at -70 °C | ||
| Vivaspin 20 centrifugal concentrator | Sartorius | VS2041 | |
| Water bath | Set at 37 °C |
References
- Strauss, K. A., et al. Onasemnogene abeparvovec for presymptomatic infants with three copies of SMN2 at risk for spinal muscular atrophy: the Phase III SPR1NT trial. Nat Med. 28 (7), 1390-1397 (2022).
- Fuller-Carter, P. I., Basiri, H., Harvey, A. R., Carvalho, L. S. Focused update on AAV-based gene therapy clinical trials for inherited retinal degeneration. BioDrugs. 34 (6), 763-781 (2020).
- George, L. A., et al. Multiyear factor VIII expression after AAV gene transfer for hemophilia A. N Engl J Med. 385 (21), 1961-1973 (2021).
- Naso, M. F., Tomkowicz, B., Perry, W. L., Strohl, W. R. Adeno-Associated Virus (AAV) as a vector for gene therapy. Biodrugs. 31 (4), 317-334 (2017).
- Atchison, R. W., Casto, B. C., Hammon, W. M. c. D. Adenovirus-associated defective virus particles. Science. 149 (3685), 754-756 (1965).
- Wu, Z., Yang, H., Colosi, P. Effect of genome size on AAV vector packaging. Mol Ther. 18 (1), 80-86 (2010).
- Samulski, R. J., Muzyczka, N. AAV-mediated gene therapy for research and therapeutic purposes. Annu Rev Virol. 1 (1), 427-451 (2014).
- Zolotukhin, S. Production of recombinant adeno-associated virus vectors. Hum Gene Ther. 16 (5), 551-557 (2005).
- Penaud-Budloo, M., François, A., Clément, N., Ayuso, E. Pharmacology of recombinant adeno-associated virus production. Mol Ther – Methods Clin Dev. 8, 166-180 (2018).
- Costa-Verdera, H., et al. Understanding and Tackling immune responses to adeno-associated viral vectors. Hum Gene Ther. 34 (17-18), 836-852 (2023).
- Ertl, H. C. J. Mitigating serious adverse events in gene therapy with AAV Vectors: Vector dose and immunosuppression. Drugs. 83 (4), 287-298 (2023).
- Pupo, A., et al. AAV vectors: The Rubik’s cube of human gene therapy. Mol Ther. 30 (12), 3515-3541 (2022).
- Marsic, D., et al. Vector design tour de force: Integrating combinatorial and rational approaches to derive novel adeno-associated virus variants. Mol Ther. 22 (11), 1900-1909 (2014).
- Grimm, D., Zolotukhin, S. E Pluribus Unum: 50 Years of research, millions of viruses, and one goal-tailored acceleration of AAV evolution. Mol Ther. 23 (12), 1819-1831 (2015).
- Biswas, M., et al. Engineering and in vitro selection of a novel AAV3B variant with high hepatocyte tropism and reduced seroreactivity. Mol Ther – Methods Clin Dev. 19, 347-361 (2020).
- Perabo, L., et al. In vitro selection of viral vectors with modified tropism: the adeno-associated virus display. Mol Ther. 8 (1), 151-157 (2003).
- Crosson, S. M., Dib, P., Smith, J. K., Zolotukhin, S. Helper-free production of laboratory grade AAV and purification by iodixanol density gradient centrifugation. Mol Ther – Methods Clin Dev. 10, 1-7 (2018).
- Chan, C., Harris, K. K., Zolotukhin, S., Keeler, G. D. Rational design of AAV-rh74, AAV3B, and AAV8 with limited liver targeting. Viruses. 15 (11), 2168 (2023).
- Schmidt, O. W., Cooney, M. K., Foy, H. M. Adeno-associated virus in adenovirus type 3 conjunctivitis. Infect Immun. 11 (6), 1362-1370 (1975).
- Grimm, D., Kern, A., Rittner, K., Kleinschmidt, J. A. Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum Gene Ther. 9 (18), 2745-2760 (1998).
- Zolotukhin, S., et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 6 (6), 973-985 (1999).
- Clark, K. R., Liu, X., Mcgrath, J. P., Johnson, P. R. Highly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild-type viruses. Hum Gene Ther. 10 (6), 1031-1039 (1999).
- Debelak, D., et al. Cation-exchange high-performance liquid chromatography of recombinant adeno-associated virus type 2. J Chromatogr B Biomed Sci App. 740 (2), 195-202 (2000).
- Burova, E., Ioffe, E. Chromatographic purification of recombinant adenoviral and adeno-associated viral vectors: methods and implications. Gene Ther. 12 (1), S5-S17 (2005).
- Adams, B., Bak, H., Tustian, A. D. Moving from the bench towards a large scale, industrial platform process for adeno-associated viral vector purification. Biotechnol Bioeng. 117 (10), 3199-3211 (2020).
- Grieger, J. C., Choi, V. W., Samulski, R. J. Production and characterization of adeno-associated viral vectors. Nat Protoc. 1 (3), 1412-1428 (2006).
- Florea, M., et al. High-efficiency purification of divergent AAV serotypes using AAVX affinity chromatography. Mol Ther Methods Clin Dev. 28, 146-159 (2022).
- Chamberlain, K., Riyad, J. M., Weber, T. Expressing transgenes that exceed the packaging capacity of adeno-associated virus capsids. Hum Gene Ther Methods. 27 (1), 1-12 (2016).
- Green, E. A., Hamaker, N. K., Lee, K. H. Comparison of vector elements and process conditions in transient and stable suspension HEK293 platforms using SARS-CoV-2 receptor binding domain as a model protein. BMC Biotechnol. 23 (1), 7 (2023).
- Erbacher, P., Zou, S., Bettinger, T., Steffan, A. M., Remy, J. S. Chitosan-based vector/DNA complexes for gene delivery: Biophysical characteristics and transfection ability. Pharm Res. 15 (9), 1332-1339 (1998).
- Vandenberghe, L. H., et al. Efficient serotype-dependent release of functional vector into the culture medium during adeno-associated virus manufacturing. Hum Gene Ther. 21 (10), 1251-1257 (2010).
- Summerford, C., Samulski, R. J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 72 (2), 1438-1445 (1998).
- Wright, J. F., et al. Identification of factors that contribute to recombinant AAV2 particle aggregation and methods to prevent its occurrence during vector purification and formulation. Mol Ther. 12 (1), 171-178 (2005).
- Gruntman, A. M., et al. Stability and compatibility of recombinant adeno-associated virus under conditions commonly encountered in human gene therapy trials. Hum Gene Ther Methods. 26 (2), 71-76 (2015).
- Srivastava, A. Rationale and strategies for the development of safe and effective optimized AAV vectors for human gene therapy. Mol Ther Nucleic Acids. 32, 949-959 (2023).
- Mullard, A. FDA approves first gene therapy for Duchenne muscular dystrophy, despite internal objections. Nat Rev Drug Discov. 22 (8), 610-610 (2023).
- Center for Biologics Evaluation and Research. Approved Cellular and Gene Therapy Products. US Food Drug Adm. , (2023).
- Kang, L., et al. AAV vectors applied to the treatment of CNS disorders: Clinical status and challenges. J Control Release Off J Control Release Soc. 355, 458-473 (2023).
- De Wolf, D., Singh, K., Chuah, M. K., VandenDriessche, T. Hemophilia gene therapy: The end of the beginning. Hum Gene Ther. 34 (17-18), 782-792 (2023).
- Simons, E. J., Trapani, I. The opportunities and challenges of gene therapy for treatment of inherited forms of vision and hearing loss. Hum Gene Ther. 34 (17-18), 808-820 (2023).

