Summary
The DNAzyme-based nanomachines can be used for highly selective and sensitive detection of nucleic acids. This article describes a detailed protocol for the design of DNAzyme-based nanomachines with a 10-23 core using free software and their application in the detection of an Epstein-Barr virus fragment as an example.
Abstract
DNAzyme-based nanomachines (DNM) for the detection of DNA and RNA sequences (analytes) are multifunctional structures made of oligonucleotides. Their functions include tight analyte binding, highly selective analyte recognition, fluorescent signal amplification by multiple catalytic cleavages of a fluorogenic reporter substrate, and fluorogenic substrate attraction for an increase in sensor response. Functional units are attached to a common DNA scaffold for their cooperative action. The RNA-cleaving 10-23 DNMs feature improved sensitivity in comparison with non-catalytic hybridization probes. The stability of the DNM and the increased chances of substrate recognition are provided by a double-stranded DNA fragment, a tile. DNM can differentiate two analytes with a single nucleotide difference in a folded RNA and a double-stranded DNA and detect analytes at concentrations ~1000 times lower than other protein-free hybridization probes. This article presents the concept behind the diagnostic potential of DNA-nanomachine activity and overviews DNM design, assembly, and application in nucleic acid detection assays.
Introduction
One of the earliest methods for nucleic acid detection is complementary binding of selective oligonucleotides to the analyzed RNA or DNA sequences. This method employs nucleic acid fragments (probes) that can form Watson-Crick base pairs with a targeted DNA or RNA analyte1. The complex is then differentiated from the analyte and the unbound probe by a variety of techniques. Certain methods, such as Northern blotting, in situ hybridization, or qPCR, are based on the phenomenon of complementary binding2. The most common hybridization probes have two significant drawbacks: low sensitivity (they can reach micromolar to nanomolar levels) as well as low selectivity to single nucleotide variations (SNV), including point mutations. The issue requires a sophisticated approach since the solutions to these drawbacks conflict with each other3. To provide the best selectivity, the detecting oligonucleotide should be kept short enough to form unstable hybrids with mismatched analytes. Such short oligonucleotides are not able to bind targeted nucleic acids tightly and unwind their secondary structures, thus often failing to provide a detectable signal. It is especially challenging to detect CG-rich fragments in biological RNA, or double-stranded DNA (dsDNA). On the other hand, long probes are insensitive to SNV3. To break the deadlock, the concept of a binary probe has been developed4.
Binary sensors split a single detection probe into two pieces and specialize its fragments, keeping one of them long to unwind the hairpins or invade the dsDNA. The second binary probe fragment remains short to be sensitive to a mismatched base, thus providing a means for detecting SNV. The signal will be produced if the two binary sensor parts are joined together4. The full complex can be visualized via FRET5, molecular beacon probe6,7, or G-quadruplexes with peroxidase-like activity8,9,10, or else RNA-cleaving11,12,13 DNAzyme. Binary sensors with a 10-23 DNAzyme core have been amply described. They have been adapted to detect single-stranded nucleic acid fragments created synthetically11, or single-stranded amplification products14,15. Detection without amplification is also possible, for example, in the case of 16S rRNA extracted from a cell culture, since this molecule is abundant in cells12,16. Binary sensors with a 10-23 DNAzyme core can also be adapted for non-nucleic acid detection, such as for lead ions17. Nevertheless, low sensitivity is still considered to be one of the major drawbacks of binary 10-23 DNAzyme sensors, and various approaches, including cascades18 or alternative signal-enhancing methods19, have been developed to address this issue. Unfortunately, the above techniques drastically increase the cost and instability of the sensor components, and so they have not come into practice.
The experiment described in this work uses 10-23 DNAzyme-based DNA-nanosensors referred to as DNA-nanomachines (DNM)20,21. These structures include binary sensors and also (1) DNA facilitators flanking the binary probe, unwinding the structured regions and invading into double-stranded DNAs, and (2) a DNA tile that holds all the DNM parts together in close proximity and increases the chances of signal formation (Figure 1). Altogether, the 10-23 DNAzyme-based DNMs decrease the limit of detection (LOD) down to the picomolar range21; they can be used to detect 16rRNA in cell lysates22 or report the presence of viral RNA without amplification23,24. At the same time, the DNMs remain sensitive to the SNV and can even be used to genotype alleles in heterozygous organisms25. The DNM method can be used as a complementary technique to any amplification type for product visualization or identification of the product in a complex mixture with high selectivity. The DNMs, unlike conventional TaqMan and beacon probes, do not require heating of the sample and so can be used in end-point detection protocols, making the overall detection cost-effective.
This article describes the in silico development of a DNM and demonstrates procedures for assembly and functional characterization of DNM. The work was performed using a synthetic fragment of Epstein-Barr virus (EBV) corresponding to the positions 13972-14154 of the viral DNA (OR652423.1) (see Table of materials).
Protocol
1. DNM design
- Select a unique region within the genome of interest.
NOTE: This work uses a region of the Epstein-Barr virus (EBV) genome in positions 13972-14154 (OR652423.1). - Create a primer set for the amplification of the selected fragment, for example, via Primer3 tool embedded in Ugene26.
- Open the UNAFold web tool27 (see Table of Materials) and insert the selected region. Change the folding temperature to 55 °C, and ionic conditions to 250 mM of monovalent ions, and 200 mM of Mg2+.
- Open the resulting secondary structure of the ssDNA fragment and select a stable hairpin. Locate the DNA-nanomachines' binding site in the unstructured regions or, alternatively, on the loops of the hairpin structures to achieve a stronger binding of the target.
- If the targeted analyte site is folded in a stem-loop structure, place Arm 1 and 2 as follows: Ensure that Arm 2 binds one side of the stem, the loop, and 3-5 nt of the second stem side; Arm 1 binds a fragment of the second stem side.
- Open the DinaMelt tool28 (see Table of Materials) and analyze the sequences of the arms and their reverse-complement sequences. Use the DinaMelt tool to make sure that the Tm of Arms 2 and 3 is above 65 °C: at least 10 °C above the assay temperature (55 °C).
- Keep the Tm of Arm 1 at least 1-3 °C above the reaction temperature (55 °C) if high recognition selectivity is to be achieved29.
- Combine Arms 1 and 2 sequences with the DNAzyme core's halves and the F-sub binding fragments13.
- Analyze the secondary structure of the designed DNA strands using UNAFold27.
NOTE: 55 °C (assay temperature), 200 mM Mg2+, 250 mM monovalent ions (HEPES, Na+, K+).- Use the design only if the folding ΔG is lower than – 4 kcal/mol. Try to avoid long CG-rich sequences. If the secondary structure is too stable, introduce a mutation to the Arm to increase the folding ΔG30. Alternatively, placing Arm 3 2-3 nt away from Arm 2 is another strategy to change the Arm 3 sequence to avoid stable intermolecular structures.
- Create a random sequence for the DNA tile fragment as long as Arm 3. Combine the Arm 3 sequence with the DNA tile sequence via (dT)4-6 or ethylene glycol (e.g., triethylene glycol (TEG) or hexaethylene glycol (HEG)). The DNM version presented in the experiment uses (dT)6.
- Link Arm 2 with the sequence complementary to the DNA tile fragment selected in step 1.8 via a linker.
- Analyze the secondary structure of the designed DNA strands using the UNAFold web app. Ensure that the folding energy of each DNA strand is above 4 kJ/mol at 55 °C, and avoid long CG-enriched sequences. Preferably, change the DNA tile sequence.
- Draw the structure using any available vector graphics software. This article uses Biorender and Pixelmator Pro for visualization (see Table of Materials). Verify accuracy in 5'->3' directions for each strand.
NOTE: Obtain the sequences from a reliable commercial vendor.
2. Functional test of the DNM
- Preparation of buffer and reagents
- Prepare the reaction buffer composed of 50 mM HEPES (pH 7.5-7.8), 50 mM NaCl, 150 mM KCl, and 200 mM MgCl2. Fill with the deionized water up to 50 mL.
- Prepare 10 µM aliquots of Dzb-Tile and Tile-Arm3 oligonucleotides. Prepare 1 µM solution of the Dza, a 100 µM F-sub, and 1 nM, 10 nM, 100 nM analytes in RNase/free water or sterile deionized water. The details of the oligonucleotide sequences are provided in the Table of Materials.
- DNM assembly
- Thaw the oligonucleotide solutions completely before using. Gently mix by tapping (do not intensively vortex). Spin down (rapidly, for a few seconds) the solution using a microcentrifuge.
- Mix 1 µM each of Dzb-Tile and Tile-Arm3 in 200 µL of the reaction buffer in 0.5 mL microcentrifuge tubes.
- Mix the tube gently and spin down the solution.
- Wrap the lid of the closed tube with paraffin film. Alternatively, use screw cap tubes.
- Incubate the tube into a 500 mL beaker with boiling water for 2 min, then turn the heater off and let the temperature passively cool down overnight.
- Prepare the 12% native PAGE: Mix 1 mL of 10х TBE buffer, 3 mL of 40% AA:BA, deionized water up to 10 mL, 50 µL of APS, and 5 µL of TEMED (see Table of Materials). Move the mixture to the gel cassette and let it polymerize.
- Mix 1 µL of each sample, namely, the assembled DNM, Dzb-Tile, and Tile-arm3, with 1 µL of 4x loading dye and load them into the gel.
- Put the cassette into the chamber, fill it with 1x TBE buffer, and let the gel run for 90 min at 80 V.
- Dye the gel with Ethidium bromide and visualize it using a gel-documenting system (see Table of Materials).
- LOD assay
- Prepare 160 µL of 200 nM F-sub in the reaction buffer. This is done to assess the basic background of the substrate stability.
- Prepare 7 aliquots of 160 µL of preassembled DNM, F-sub, and Dza at concentrations of 20 nM of DNM and Dza and 200 nM of F-sub in the reaction buffer. Out of the seven tubes, keep tube #1 as the blank background to assess the spontaneous assembly of the DNAzyme core.
- Add the analyte into tubes #2-7 at the final concentrations of 1 pM, 10 pM, 100 pM, 200 pM, 500 pM and 1000 pM.
- Gently mix, spin down the tubes, and classify the solutions into three 50 µL portions into a black 96-well plate. Seal the plate with an optically transparent film.
- Incubate the plate in a water bath at 55 °C for 1 h. Spin down the plate.
- Measure fluorescence at 525 nm (λex = 495 nm). Calculate the mean and the standard deviation for each point.
- Calculate the blank-to-basic ratio by dividing the signal of the tube that contains F-sub and DNM by the signal of the tube that contains F-sub only. Ensure the ratio is within the 1.1-1.5 range.
- Calculate the signal-to-blank ratio by dividing the signal of the tube that contains F-sub, DNM, and the analyte to the signal of the tube that contains F-sub and DNM only. Consider the signal as true positive if it is higher than the background average plus three standard deviations.
- Repeat the experiment twice and ensure the standard deviation between the analytical repeats is less than 0.5.
Representative Results
The aim of the first experiment was to show the assembly of the DNM before the synthetic fragment of the analyte. All the constituent DNM strands were added to the reaction buffer and assembled in the beaker. The assembled DNM complex was assessed for its correct size and homogeneity by native PAGE. Native PAGE shows the assembled DNM with the analyte in lane 1 and two DNM strands in lanes 2 and 4. If the DNA nanomachine were not assembled, 2 or 3 separate bands would be visible instead of a single low-mobility band (lane 2 in Figure 2A).
The second part of the Protocol implies the functional characterization of the DNM performance and detection of target nucleic acids. In this study, the synthetic fragment of EBV 13972-14154 was used. For this purpose, the preassembled DNMs' fragments, together with the Dza, were incubated with various concentrations of the analyte for 1 h at 55 °C. Two control fluorescent signal values were measured that of the preassembled DNMs' fragments and Dzb without the analyte (blank), and that of the background from F-sub (basic). Three technical measurements were made. For statistics, three independent trials have been conducted as well. It is noteworthy that the magnesium concentration in this study is higher than what can be found in literature30. High levels of magnesium ions increase the signal in all the testing tubes, as they promote a spontaneous F-sub cleavage31. We optimized the reaction buffer composition during the preliminary stages of technology development and did not repeat this process with every study undertaken.
After 1 h incubation, the sample fluorescence was measured using a spectrofluorometer (see Table of Materials). To confirm the detection of the analyzed DNA by the DNM, we compared the fluorescent signal of the sensor assembled with the analyzed DNA and that of the negative control, which does not contain any analyte added. The signal-to-blank ratio (a ratio between the mean relative fluorescence units of the wells containing the analyte and the wells with no analyte) should be no less than 1.5 to confirm successful detection32. The blank-to-basic ratio (fluorescence of sensor response in the absence of analyte divided by that of the F-sub only control) should be in the range of 1.1-1.5; this ratio can be adjusted by changing DNM's and Dzb concentrations (the typical concentration range for each is 5-20 nM). In our practice, LOD is quantified as the intersection of the trendline and three standard deviations above the mean blank (Figure 2B). The DNM is effective if the LOD is <100 pM33. The DNM has to be redesigned if the LOD is higher than 100 pM.
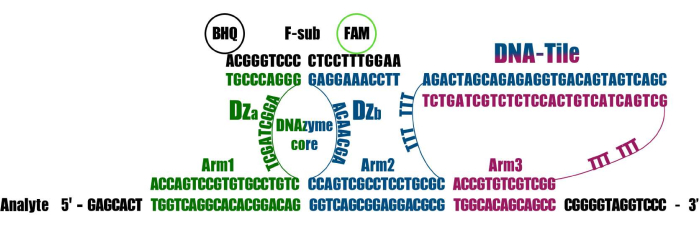
Figure 1: The three arm DNM used in this study. Three oligonucleotides, Dza, Dzb-Tile, and Tile-Arm3, are marked as green, blue, and purple, respectively. Please click here to view a larger version of this figure.
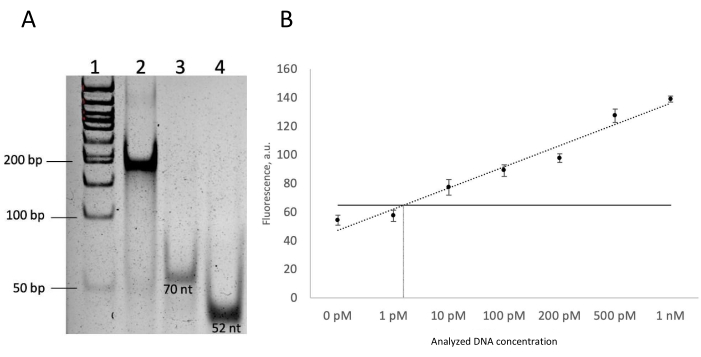
Figure 2: DNM characterization. (A) Assembly of the DNM via native electrophoresis in 12% polyacrylamide gel, 80 V, 1 h, using a 50 bp+ DNA ladder. (B) The limit of detection of synthetic DNA after 60 min incubation at 55 °C. The horizontal line stands for the threshold, and the vertical line is the LOD. The average values of three independent measurements are presented with one standard deviation. Please click here to view a larger version of this figure.
Discussion
The design of DNA machines is straightforward but requires some experience in designing hybridization probes or functional DNA nanostructures. It is appropriate to keep the analyte fragment as short as possible to diminish the number of possible secondary structures and simplify DNM invasion to the secondary structure. The CG content should preferably be below 60% to avoid stable intramolecular structures. Successful assembly of the DNM is achieved at slow cooling rates. In some cases, DNMs can be spontaneously assembled in the tube, and the annealing step can be eliminated. DNM assembly can be performed in a thermal cycler with 0.1-0.3 °C/min or in large volumes of cooling water. In the case of improper DNM assembly, we suggest slowing down the temperature change during the annealing step.
To measure the LOD, at least seven analyte concentration points are needed: the 0 pM analyte concentration, which is F-sub + DNM, and six different analyte concentrations, for example, in the range of 1 nM to 1 pM to observe the linear dependence (Figure 2B). Often, more than seven concentration points were used for higher accuracy. For each concentration point, three technical trials (50 µL each) should be made. Therefore, the total volume for each concentration was 160 µL (10 µL excess, considering the pipette error).
The DNM technique presents higher sensitivity for SNVs than other hybridization-based systems. High selectivity to SNV can be relevant for certain objectives, such as identification of point mutations associated with bacterial genotyping22 or the selection of heterozygotes in plants25. Non-specific analytes would require the same experimental procedure. To avoid any incomprehension of SNV to a low concentration of analyte, we suggest comparing the signal of the experimental unknown analyte with that of signal of a known fully complementary analyte of the same concentration.
The method can be successfully applied to ssDNA, miRNA, folded RNA, or dsDNA amplicons. Long RNA or dsDNA fragments, especially the CG-rich, may require DNM equipped with 4-6 analyte-binding arms, while two analyte-binding arms are sufficient for single-stranded analytes with an unstable secondary structure (ΔG folding above -10 kcal/mol). The method can be applied for the detection of PCR, SDA, or LAMP amplicons. DNM can be used for amplification-free detection of rRNA in boiled samples of bacterial culture without isolating total RNA22,34,35. The technique might not work well with concentrations as low as the femtomolar level and with dsDNA fragments, whose CG content in the flanking regions is higher than 65%. The CG content can be estimated, for example, with the Biologics CG content calculator36. We suggest adding hook parts37 to the DNM designs to increase the sensitivity or transform the dsDNA into ssDNA in cases of high CG content. Another limitation of the method is that the DNMs are sensitive to the quality of the samples, so the nucleic acids are to carefully purified. The same applies to the quality of the oligonucleotide sequences of DNMs. It is, therefore, suggested that the stability and purity of every oligonucleotide supply be verified. In comparison to conventional amplification techniques, the DNMs' design requires skills and, by the date of the article release, is almost entirely manual. The DNMs need some preliminary tests on synthetic fragments before subjecting them to actual DNA or RNA samples, thus increasing the overall duration of the test-system development.
The method is versatile and can adopt different types of reporter probes. It can be multiplexed38, and the technique can be transformed into a colorimetric version if the F-sub is replaced with a G-quadruplex-forming substrate39. The presence of polymerase is associated with errors in the strand growth; hence, this method is advantageous over conventional real-time PCR and digital PCR as well as other nuclease-dependent techniques, such as SHERLOCK and DETECTR, due to the lack of perishable protein enzymes and the robust and straightforward detection procedure40,41.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Ekaterina V. Nikitina for kindly providing gDNA of EBV. Muhannad Ateiah, Maria Y. Berezovskaya, and Maria S. Rubel thank the Ministry of Education and Science of the Russian Federation (Grant No FSER-2022-0009) and the Priority 2030 program.
Materials
| 1.5 mL tube | Biofil | CFT011015 | |
| 100 bp+ DNA Ladder | Evrogen | NL002 | |
| 100-1000 µL pipette | Kirgen | KG-Pro1000 | |
| 10-100 µL pipette | Kirgen | KG-Pro100 | |
| 1-10 µL pipette | Kirgen | KG-Pro10 | |
| 20-200 µL pipette | Kirgen | KG-Pro200 | |
| 2-20 µL pipette | Kirgen | KG-Pro20 | |
| 4x Gel Loading Dye | Evrogen | PB020 | |
| Acrylamide 4K | AppliChem | A1090,0500 | |
| Ammonium persulfate | Carl Roth | 2809447 | |
| Biorender | Biorender | https://www.biorender.com | |
| Bisacrylamide | Molekula | 22797959 | |
| Boric Acid | TechSnab | H-0202 | |
| ChemiDoc imaging system | BioRad | 12003153 | |
| Costar 96-Well Black Polystyrene Plate | Corning | COS3915 | |
| DinaMelt | RNA Institute | http://www.unafold.org/Dinamelt/applications/two-state-melting-hybridization.php | |
| EDTA | Amresco | Am-O105 | |
| Ethidium bromide | BioLabMix | EtBr-10 | |
| HEPES | Amresco | Am-O485 | |
| Magnesium chloride | AppliChem | 131396 | |
| Mini Protean Tetra Cell | BioRad | 1658001EDU | |
| pipette 10 µL tips | Kirgen | KG1111-L | |
| pipette 1000 µL tips | Kirgen | KG1636 | |
| pipette 200 µL tips | Kirgen | KG1212-L | |
| Pixelmator Pro | Pixelmator Team | https://www.pixelmator.com/pro/ | |
| Potassium chloride | Carl Roth | 1782751 | |
| PowerPac Basic Power Supply | BioRad | 1645050 | |
| RNAse/DNAse free water | Invitrogen | 10977049 | |
| Sealing film for PCR plates | Sovtech | P-502 | |
| Sodium chloride | Vekton | HCh (0,1) | |
| Spark multimode microplate reader | Tecan | Spark- 10M | |
| T100 amplificator | BioRad | 10014822 | |
| TEMED | Molekula | 68604730 | |
| Tris(hydroxymethyl)aminomethane | Amresco | Am-O497 | |
| UNAFold | RNA Institute | http://www.unafold.org/mfold/applications/dna-folding-form.php | |
| Water bath | LOIP | LB-140 | |
| Oligos used | |||
| Analyte: | Evrogen | direct order, standard desalting purification | |
| GAGCACTTGGTCAGGCACACGG ACAGGGTCAGCGGAGGACGCG TGGCACAGCAGCCCGGGGTAG GTCCCCTGGACCTGCCGCTGG CGGACTACGCCTTCGTTGC |
|||
| Tile-Arm3: | Evrogen | direct order, standard desalting purification | |
| CCG GGCTGCTGTGCCATTTT TTGCTGACTACTGTCACCTCT CTGCTAGTCT |
|||
| Dzb-Tile: AGACTAGCAGAGAGGTGACAG TAGTCAGCTTTTTTCGCGTCCTC CGCTGACCACAACGAGAGGAA ACCTT |
Evrogen | direct order, standard desalting purification | |
| Dza: TGCCCAGGGAGGCTAGCTCT GTCCGTGTGCCTGACCA |
Evrogen | direct order, standard desalting purification | |
| F-sub oligonucleotide: AAGGTT(FAM)TCCTCrGrU CCCTGGGCA-BHQ1 |
DNA-Syntez | direct order, HPLC purification |
References
- Kolpashchikov, D. M. Evolution of hybridization probes to DNA machines and robots. Acc. Chem Res. 52, 1949-1956 (2019).
- Guo, J., Ju, J., Turro, N. J. Fluorescent hybridization probes for nucleic acid detection. Anal. Bioanal Chem. 402, 3115-3125 (2012).
- Demidov, V. V., Frank-Kamenetskii, M. D. Two sides of the coin: affinity and specificity of nucleic acid interactions. Tr Bioche Sc. 29 (2), 62-71 (2004).
- Kolpashchikov, D. M. Binary probes for nucleic acid analysis. Chem Rev. 110, 4709-4723 (2010).
- Marti, A. A., Jockusch, S., Stevens, N., Ju, J., Turro, N. J. Fluorescent hybridization probes for sensitive and selective DNA and RNA detection. Acc Chem Res. 40 (6), 402-409 (2007).
- Gerasimova, Y. V., Kolpashchikov, D. M. Detection of bacterial 16S rRNA using a molecular beacon-based X sensor. Biosens.Bioelectron. 41, 386-390 (2013).
- Dark, P., et al. Accuracy of LightCycler Septi Fast for the detection and identification of pathogens in the blood of patients with suspected sepsis: a systematic review and meta-analysis. Intensive Care Med. 41, 21-33 (2015).
- Maltzeva, Y. I., Gorbenko, D. A., Nikitina, E. V., Rubel, M. S., Kolpashchikov, D. M. Visual Detection of Stem-Loop Primer Amplification (SPA) products without denaturation using peroxidase-like DNA machines (PxDM). Int J Mol Sc. 24 (9), 7812 (2023).
- Gorbenko, D. A., et al. DNA nanomachine for visual detection of structured RNA and double stranded DNA. Chem Comm. 58 (35), 5395-5398 (2022).
- Roembke, B. T., Nakayama, S., Sintim, H. O. Nucleic acid detection using G-quadruplex amplification methodologies. Methods. 64 (3), 185-198 (2013).
- Mokany, E., Bone, S. M., Young, P. E., Doan, T. B., Todd, A. V. MNAzymes, a versatile new class of nucleic acid enzymes that can function as biosensors and molecular switches. JACS. 132 (3), 1051-1059 (2010).
- Gerasimova, Y. V., Cornett, E., Kolpashchikov, D. M. RNA cleaving deoxyribozyme sensor for nucleic acid analysis: the limit of detection. Chem Bio Chem. 11, 811-817 (2010).
- Gerasimova, Y. V., Kolpashchikov, D. M. Folding 16S RNA in a signal-producing structure for detection of bacteria. Angew Chem Int Ed. 52, 10586-10588 (2013).
- Hu, M., et al. Allosteric DNAzyme-based encoder for molecular information transfer. Chin. Chem Let. , 109232 (2023).
- Peeters, B., et al. Solid-phase PCR-amplified DNAzyme activity for real-time FO-SPR detection of the MCR-2 gene. Anal Chem. 92 (15), 10783-10791 (2020).
- Wood, H. N., et al. Species typing of nontuberculous Mycobacteria by use of deoxyribozyme sensors. Clin Chem. 65 (2), 333-341 (2019).
- Yan, T., et al. Ultralow background one-pot detection of Lead (II) using a non-enzymatic double-cycle system mediated by a hairpin-involved DNAzyme. Biosen Bioelectron. 237, 115534 (2023).
- Hasick, N. J., Radhika, R., Todd, A. V. Subzymes: Regulating DNAzymes for point of care nucleic acid sensing. Sens. Act. B: Chem. 297, 126704 (2019).
- Safdar, S., et al. DNA-only, microwell-based bioassay for multiplex nucleic acid detection with single base-pair resolution using MNAzymes. Biosen Bioelectron. 152, 112017 (2020).
- Cox, A. J., Bengtson, H. N., Gerasimova, Y. V., Rohde, K. H., Kolpashchikov, D. M. DNA antenna tile-associated deoxyribozyme sensor with improved sensitivity. Chem Bio Chem. 17 (21), 2038-2041 (2016).
- Lyalina, T. A., Goncharova, E. A., Prokofeva, N. Y., Voroshilina, E. S., Kolpashchikov, D. M. A DNA minimachine for selective and sensitive detection of DNA. Analyst. 144 (2), 416-420 (2019).
- Ateiah, M., Gandalipov, E. R., Rubel, A. A., Rubel, M. S., Kolpashchikov, D. M. DNA Nanomachine (DNM) Biplex Assay for Differentiating Bacillus cereus Species. Int J Mol Sc. 24 (5), 4473 (2023).
- El-Deeb, A. A., et al. Toward a home test for COVID-19 diagnosis: DNA machine for amplification-free SARS-CoV-2 detection in clinical samples. Chem Med Chem. 17 (20), 202200382 (2022).
- Kirichenko, A., Bryushkova, E., Dedkov, V., Dolgova, A. A Novel DNAzyme-based fluorescent biosensor for detection of RNA-containing Nipah henipavirus. Biosensors. 13 (2), 252 (2023).
- Akhmetova, M. M., Rubel, M. S., Afanasenko, O. S., Kolpashchikov, D. M. Barley haplotyping using biplex deoxyribozyme nanomachine. Sens Act Rep. 4, 100132 (2022).
- Okonechnikov, K., et al. Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics. 28, 1166-1167 (2012).
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31 (13), 3406-3415 (2003).
- Markham, N. R., Zuker, M. DINAMelt web server for nucleic acid melting prediction. Nucleic Acids Res. 33, W577-W581 (2005).
- Stancescu, M., Fedotova, T. A., Hooyberghs, J., Balaeff, A., Kolpashchikov, D. M. Nonequilibrium hybridization enables discrimination of a point mutation within 5-40 C. JACS. 138 (41), 13465-13468 (2016).
- Dong, J., Ouyang, Y., Wang, J., O’Hagan, M. P., Willner, I. Assembly of dynamic gated and cascaded transient DNAzyme networks. ACS Nano. 16 (4), 6153-6164 (2022).
- Montserrat Pagès, A., Hertog, M., Nicolaï, B., Spasic, D., Lammertyn, J. Unraveling the kinetics of the 10-23 RNA-cleaving DNAzyme. Int J Mol Sc. 24 (18), 13686 (2023).
- Nguyen, C., Grimes, J., Gerasimova, Y. V., Kolpashchikov, D. M. Molecular-beacon-based tricomponent probe for SNP analysis in folded nucleic acids. Chem-eur J. 17 (46), 13052-13058 (2011).
- MacDougall, D., Crummett, W. B. Guidelines for data acquisition and data quality evaluation in environmental chemistry. Anal Chem. 52 (14), 2242-2249 (1980).
- Gerasimova, Y. V., Yakovchuk, P., Dedkova, L. M., Hecht, S. M., Kolpashchikov, D. M. Expedited quantification of genetically modified ribosomal RNA by binary deoxyribozyme sensors. RNA. 10, 1834-1843 (2015).
- Gerasimova, Y. V., Kolpashchikov, D. M. Folding 16S RNA in a signal-producing structure for detection of bacteria. Angew Chem Int Ed. 52, 10586-10588 (2013).
- . GC Content Calculator Available from: https://www.biologicscorp.com/tools/GCContent/#.ZEfxPiyOHYU (2023)
- Hussein, Z., et al. DNAzyme nanomachine with fluorogenic substrate delivery function: advancing sensitivity in nucleic acid detection. Anal Chem. 95 (51), 18667-18672 (2023).
- Safdar, S., et al. DNA-only, microwell-based bioassay for multiplex nucleic acid detection with single base-pair resolution using MNAzymes. Biosen Bioelectron. 152, 112017 (2020).
- Gerasimova, Y. V., et al. Deoxyribozyme cascade for visual detection of bacterial RNA. Chem Bio Chem. 14, 2087-2090 (2013).
- Eckert, K. A., Kunkel, T. A. DNA polymerase fidelity and the polymerase chain reaction. Gen Res. 1 (1), 17-24 (1991).
- Pandya, K., Jagani, D., Singh, N. CRISPR-Cas systems: Programmable nuclease revolutionizing the molecular diagnosis. Mol Biotech. , (2023).

.
