Advancements in Bovine Organoid Technology Using Small and Large Intestinal Monolayer Interfaces
Summary
This study presents a protocol for generating bovine intestinal 2D monolayers from organoids, offering improved access for studying host-pathogen interactions. It includes methods for assessing membrane integrity and functionality, advancing in vitro models that mimic cattle’s gastrointestinal physiology. This approach promises significant biomedical and agricultural benefits, including enhanced treatment strategies.
Abstract
Advancing knowledge of gastrointestinal physiology and its diseases critically depends on the development of precise, species-specific in vitro models that faithfully mimic in vivo intestinal tissues. This is particularly vital for investigating host-pathogen interactions in bovines, which are significant reservoirs for pathogens that pose serious public health risks. Traditional 3D organoids offer limited access to the intestinal epithelium’s apical surface, a hurdle overcome by the advent of 2D monolayer cultures. These cultures, derived from organoid cells, provide an exposed luminal surface for more accessible study. In this research, a detailed protocol is introduced for creating and sustaining 2D monolayer cultures from cells of bovine small and large intestinal organoids. This method includes protocols for assessing membrane integrity through transepithelial electrical resistance and paracellular permeability alongside immunocytochemistry staining techniques. These protocols lay the groundwork for establishing and characterizing a 2D bovine monolayer culture system, pushing the boundaries of these method applications in biomedical and translational research of public health importance. Employing this innovative approach enables the development of physiologically pertinent in vitro models for exploring both normal and diseased states of cattle intestinal physiology. The implications for biomedical and agricultural advancements are profound, paving the way for more effective treatments for intestinal ailments in cattle, thereby enhancing both animal welfare and food safety.
Introduction
The culture of intestinal epithelial stem cells in three-dimensional (3D) cultures, known as intestinal organoids, marks a significant advancement in in vitro technology for investigating intestinal functions, nutrition, and interactions with pathogens1,2. These organoids mimic the complex structure of the in vivo intestinal epithelium by self-replicating and organizing into 3D formations that encompass various intestinal cell lineages3. This feature highlights their considerable potential to propel the understanding of intestinal biology forward.
The rising interest in applying intestinal organoid technology to farm animals necessitates the refinement of culture and maintenance techniques4,5. This technology's relevance is underscored by its potential impact on studying the gut health of farm animals, which plays a critical role in their productivity and, consequently, the economics of the food-animal industry by influencing animal welfare and operational costs6,7. Specifically, employing intestinal organoid cultures to explore the gut function of cattle is of paramount importance, given their role as reservoirs for zoonotic enteric pathogens, such as Salmonella spp. and Escherichia coli (E. coli) O157:H78. These pathogens are localized in particular segments of the gut, making it essential to differentiate intestinal organoid culture methods by gut segment to enhance precision in studies9.
A significant obstacle in the study of intestinal organoids is the restricted access to the epithelial cell's apical surface10. When cultured within an extracellular matrix (ECM), the cells naturally orient themselves so the basal surface faces outward, and the apical surface is directed inward10. To address this challenge, methods are presented that involve dissociating 3D organoids into single cells and seeding them into semi-permeable cell culture inserts. This setup establishes an interface between the apical surface and a basolateral compartment. This protocol demonstrates that cells derived from bovine intestinal organoids can form a coherent 2D monolayer, as evidenced by transepithelial electrical resistance (TEER) measurements and paracellular permeability assays. Additionally, the development of cellular polarity with brush borders and tight junctions in the organoid-derived 2D monolayer cells is confirmed through immunofluorescence and electron microscopy, reflecting the in vivo gut epithelium's properties.
In this study, the ileum represents the small intestinal tract, and the rectum signifies the large intestinal tract. These selections are based on relevant enteric pathogens such as Salmonella spp., which can translocate the ileum11, and E. coli O157:H7 known to primarily colonize the rectum9 in cattle. The selection of these specific intestinal segments highlights the necessity of tailoring intestinal organoid culture methods to the gut region for precision in research. These methods detail the procedure for effectively culturing an organoid-derived 2D monolayer interface from these intestinal segments, providing a robust model for exploring cattle gut health, pathogen infections, and interactions between the gut microbiome and host.
Protocol
Intestinal crypts were procured from surplus intestinal specimens sourced at a local slaughterhouse, and signalment of donors is provided in Supplementary Table 1. Organoids were generated using tissues derived from animals that were humanely euthanized at a slaughterhouse, and animals were not procured solely for this research; therefore, this study is exempt from IACUC review, and an ethics statement is not applicable.
1. ECM coating on cell culture inserts for organoid-derived 2D monolayer culture
NOTE: All procedures are carried out using sterilized materials and aseptic techniques in a biosafety cabinet. All reagents are kept on ice throughout the procedure unless otherwise stated.
- Prepare 100 µL of ECM for coating each 0.33 cm2 cell culture insert by mixing basal medium with 2% (v/v) ECM-based hydrogel in a microtube thoroughly.
- Remove individual cell culture insert from packaging with sterilized forceps and place individually into the wells of a 24-well clear, flat bottom cell culture plate.
- Apply 100 µL of ECM coating prepared in step 1.1 to the apical chamber of each cell culture insert prepared in step 1.2.
- Replace the lid and incubate the cell culture plate containing coated cell culture insert(s) in a humidified incubator at 37 °C and 5% CO2 for 1 h.
- For ileal organoid cells, the insert is ready for use following 1 h incubation. For rectal organoid cells, following 1 h incubation, replace ECM coating with rectal monolayer culture medium and incubate overnight (Table 1). Prepare extra cell culture inserts for blank controls if intended to perform TEER measurements.
| Ileum | Rectum | |
| ECM coating incubation time | 1 h | 1 h followed by overnight in monolayer culture media |
| Supplementations to organoid culture medium |
||
| CHIR99021 | ||
| LY2157299 | LY2157299 | |
| Y-27632 | Y-27632 | |
| Fetal bovine serum | Fetal bovine serum | |
| Cell seeding density (cells/well) | 5 x 105 | 3 x 105 |
Table 1: Summary of the optimized protocol for creating 2D monolayers derived from adult bovine ileal and rectal organoids.
2. Bovine ileal and/or rectal organoid cell seeding and 2D monolayer culture
NOTE: The protocol described in this section uses bovine ileal and rectal organoids, which were cultured and maintained on 48-well plates using the described techniques5. For optimal results, it is advised to use stably maintained organoids that have been passaged more than 3x after initial establishment and have been cultured for greater than 3 days after the most recent passage.
- Without disturbing the ECM-based hydrogel dome containing mature organoids, remove organoid culture media using a disposable glass Pasteur pipet attached to a vacuum system and add 300 µL of ice-cold ECM depolymerization solution per well. Incubate for at least 1 h at 4 °C.
NOTE: Alternatively, mechanically disrupt the organoid containing ECM-based hydrogel dome after the addition of ECM depolymerization solution and collect suspension in a 15 mL conical tube prior to incubating at 4 °C. This method is recommended when organoids that are not intended to be used for 2D monolayer culture are cultured simultaneously on the same plate. The density of organoid cultures significantly influences the number of required organoid culture wells for optimal seeding to cell culture inserts.- For high-density organoid cultures (Supplementary Figure 1A), rectal cell culture inserts use a seeding ratio in the range of 1:1 to 1:2, meaning one culture well can seed one to two wells. For ileum, keep the ratio at 1:1. In contrast, lower-density cultures (Supplementary Figure 1B) require more culture wells; use 3-4 rectal organoid culture wells for one rectal cell culture insert (3-4:1 ratio), and 4-5 ileal organoid culture wells for one ileal cell culture insert (4-5:1 ratio).
- Visually inspect for complete ECM-based hydrogel dissolution and collect organoid suspension to a 15 mL conical tube.
- Pellet organoids by centrifugation at 200 x g and 4 °C for 5 min. Discard supernatant. Resuspend pellet in 1 mL of recombinant cell dissociation enzyme solution supplemented with 10 µM Y-27632.
- Incubate organoid suspension in a 37 °C water bath for 10 min with intermittent shaking of 3-5 s with a vortex every 2-3 min to facilitate effective organoid dissociation.
- Following enzymatic digestion, add 5 mL of basal medium and pipette aggressively with a P1000 micropipette to further disrupt organoids to single cells. Inspect suspension for organoid clumps, and if still visually appreciable, repeat pipetting to enhance single-cell dissociation.
- Prepare a 50 mL conical tube with a 70 µm cell strainer. Prewet the strainer by applying 1-2 mL of basal medium.
- Filter cell suspension through the strainer to remove residual ECM-based hydrogel debris and large cell clumps. Rinse the original 15 mL tube and 70 µm cell strainer with an additional 10 mL of basal medium.
NOTE: If cell suspension does not easily pass through a strainer, it may be an indication of incomplete organoid dissociation. Repassing the collected cell suspension through the same 70 µm cell strainer can be attempted. Additional enzymatic or mechanical disruption may be necessary. - Pellet single-cell suspension by centrifugation at 200 x g and 4 °C for 5 min. Remove supernatant and resuspend cells with an appropriate volume of basal medium to perform cell count.
- Count viable cells with a hemocytometer following trypan blue staining to calculate the total number of cells collected.
- Pellet single-cell suspension by centrifugation at 200 x g and 4 °C for 5 min. Remove supernatant and resuspend cells to appropriate seeding density in respective monolayer culture medium (Table 1).
- For ileal organoid cells, resuspend cells to the concentration of 2.5 x 106 cells/mL to achieve a seeding density of 5 x 105 cells per cell culture insert in 200 µL of ileal monolayer culture medium, which is the organoid culture media supplemented with 500 nM LY2157299, 10 µM Y-27632, and 20% fetal bovine serum (FBS).
- For rectal organoid cells, resuspend cells to the concentration of 1.5 x 106 cells/mL to achieve a seeding density of 3 x 105 cells per cell culture insert in 200 µL of rectal monolayer culture medium, which is the organoid culture media supplemented with 100 nM CHIR99021, 500 nM LY2157299, 10 µM Y-27632, and 20% FBS.
- Retrieve the 24-well cell culture plate with ECM-coated cell culture insert(s) and empty the apical chamber of the cell culture insert with careful vacuum suction to not disrupt the coating.
- Gently apply 200 µL of single-cell suspension prepared in step 2.10 to the apical chamber of the cell culture insert. For the blank control, add 200 µL of culture media without cells in the apical chamber. For the blank control, add 200 µL of culture media without cells in the apical chamber.
- Apply 500 µL of appropriately supplemented monolayer culture medium (depending on ileal vs rectal cells) to the basolateral chamber of each well.
- Incubate in a humidified incubator at 37 °C and 5% CO2 to facilitate cell adhesion and growth to form a confluent 2D monolayer on the cell culture insert.
- Change culture media in both apical and basolateral chambers every other day starting 48 h after cell seeding. Ensure equal incubation time for the blank control and the cell-containing insert.
3. TEER measurement
NOTE: The method described here uses a commercially available manual TEER measurement system known as an epithelial voltohmmeter with a pair of Ag/AgCl electrodes (Figure 1A). Determination of TEER across a 2D monolayer requires the measurement of blank wells. Ensure that the blank reading is taken in the same manner as the sample.
- Retrieve plate containing cell culture inserts with organoid-derived 2D monolayer(s) and blank from the incubator. Allow the plate to come to room temperature for approximately 10 min.
- Disinfect the electrodes with 70% ethanol and allow them to dry completely.
- Carefully introduce the electrodes with the short end in the apical chamber and the long end in the basolateral chamber (Figure 1A).
NOTE: Extra caution is warranted so as not to disrupt the cell monolayer. - Allow the reading to equilibrate and record the value in ohm when it stabilizes.
NOTE: The sensitivity of the voltohmmeter is such that fluctuations of the ohm value will occur while taking the electrical resistance measurement. A reliable reading is obtained when the measurements stabilize and consistently hover around a plateau value. - Determine TEER of 2D monolayer with the following formula:
TEER (Ω x cm2) = surface area of cell culture inserts (cm2) x Net electrical resistance
Where Net electrical resistance is equal to the measured resistance of the cell monolayer insert minus the measured resistance of the blank insert. Cell culture inserts for 24-well plates have a surface area of 0.33 cm2.
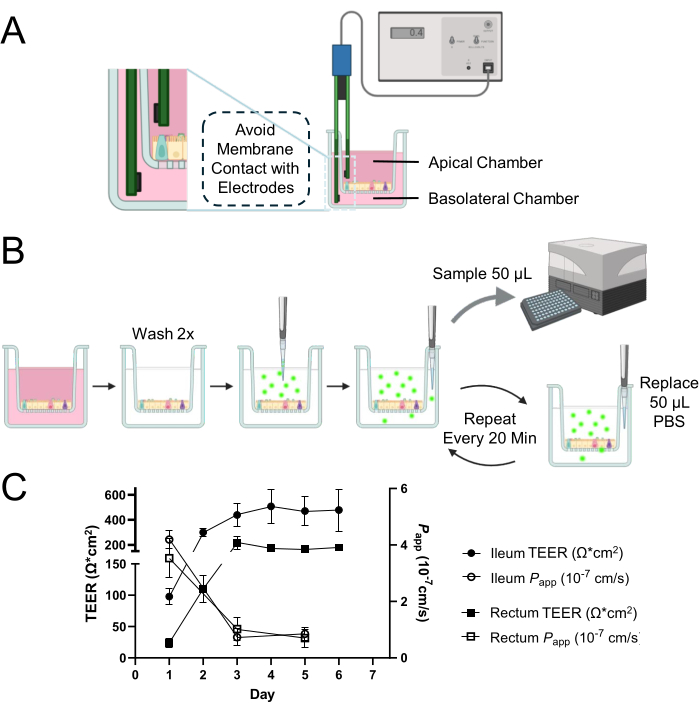
Figure 1: Epithelial barrier integrity evaluation of bovine intestinal organoid-derived 2D monolayer. (A) Schematic of appropriate positioning of electrodes within the apical and basolateral chambers of a cell culture insert for TEER measurements. The short electrode is inserted into the apical chamber, and the long electrode is placed in the basolateral chamber with care to avoid contact with the membrane. (B) Schematic of the permeability assay process. Cell culture chambers are washed 2x with warmed PBS, and 0.5 mg/mL 4 kDa FITC-dextran tracer dissolved in PBS is applied to the apical chamber. Repeated 50 µL aliquots from the basolateral chamber are sampled with the replacement of an equal volume of PBS to maintain the total volume in the basolateral chamber throughout the duration of the incubation period. The fluorescence intensity of the aliquot is measured using a microplate reader to quantify the diffusion of 4 kDa FITC-dextran tracer across the cell monolayer. (C) The dynamic development of barrier integrity within ileal and rectal monolayers over time was evaluated utilizing TEER measurements (represented by closed circles for the ileum and closed squares for the rectum) and permeability assays (denoted by open circles for the ileum and open squares for the rectum) with a 4 kDa FITC-dextran tracer. By day 3 of culture, both types of monolayers exhibited the establishment of stable and functional epithelial barriers, as evidenced by their respective TEER and permeability profiles. The results are the mean of at least two independent experiments with two technical replicates. Error bars represent the SEM of the measurements. Please click here to view a larger version of this figure.
4. Paracellular permeability assay
NOTE: This assay involves the determination of fluorescence intensity resulting from diffusion of fluorescein isothiocyanate (FITC)-dextran from the apical chamber to the basolateral chamber across 2D monolayers over 120 min (Figure 1B). For optimum results, it is advised to minimize exposure to light during the assay and take measurements in a microplate reader immediately after every sampling to prevent any decrease or quenching of fluorescence. Each well can only be used once and cannot be reused in subsequent assays. Prepare at least 2 wells to serve as technical replicates for each assay. For instance, a total of 6 wells are needed to obtain the results presented in Figure 1C, where assays were run in duplicate at 3 different time points (Days 1, 3, and 5 of culture).
- Prepare standard curve dilution series with 4 kDa FITC-dextran in phosphate-buffered saline (PBS). For each dilution, pipette 50 µL to a 96-well plate in triplicate.
NOTE: It is recommended to create a series of 5-7 dilutions ranging from 0 to 0.5 mg/mL. - Determine the fluorescence intensity of the standards in a pre-calibrated microplate reader at an excitation wavelength of 495 nm and emission wavelength of 535 nm.
- Calculate the linear regression with fluorescence intensity results to create a standard curve.
- Retrieve plate containing cell culture inserts with organoid-derived 2D monolayer(s) from the incubator. Remove monolayer culture media from the apical and basolateral chamber of the cell culture insert containing the organoid-derived 2D monolayer to be assessed.
- Gently wash each chamber 2x with 200 µL (apical chamber) and 500 µL (basolateral chamber) of pre-warmed PBS, respectively.
- Remove the wash solution from the apical chamber and apply 200 µL of 0.5 mg/mL 4 kDa FITC-dextran tracer in PBS to the apical chamber of the cell culture insert.
- Incubate in a humidified incubator at 37 °C and 5% CO2 for 20 min.
- Sample 50 µL from the basolateral chamber of the incubated 24-well plate and transfer to a microplate reader-compatible 96-well plate.
- Replace 50 µL of fresh PBS in the basolateral chamber of the sampled well.
- Immediately take fluorescence intensity measurement in a pre-calibrated microplate reader at an excitation wavelength of 495 nm and emission wavelength of 535 nm.
- Repeat steps 4.6-4.10 every 20 min until 120 min. At the end of the assay, if preservation of the 2D monolayer is desired, rinse both the apical and basolateral chamber with fresh PBS 2x, replace with fresh monolayer culture medium, and incubate.
NOTE: Further evaluation of 2D monolayers can be performed, i.e., TEER measurements, immunofluorescence staining, etc.; however, it is not recommended as residual fluorescent tracer is likely and may impact analysis. - Determine the apparent permeability coefficient (Papp) with the following formula:
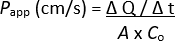
ΔQ / Δt = concentration of fluorescent tracer that passed the monolayer to the basolateral chamber of the cell culture insert over the specific duration of time, measured by fluorescence intensity and extrapolated to µg/mL via the standard curve
A = surface area of the cell culture inserts
Co = concentration of fluorescent tracer added to the apical chamber of the cell culture insert in µg/mL
5. Immunofluorescence staining of organoid-derived 2D monolayer
- Remove monolayer culture media from cell culture insert with vacuum suction and add 200 µL of 4% paraformaldehyde (PFA). Incubate at room temperature for 15-30 min for cell fixation.
- Remove PFA with vacuum suction and wash with 100 µL of PBS 2x.
- Permeabilize cells with 100 µL of 0.3% Triton X-100 in 2% bovine serum albumin (BSA) in PBS by incubating at room temperature for 10 min.
- Remove supernatant with vacuum suction and wash with 100 µL of PBS 2x.
- Remove supernatant and replace with 2% BSA in PBS and incubate at room temperature for 1 h for blocking.
- Remove supernatant, apply 100 µL of primary antibody diluted in 2% BSA in PBS and incubate at room temperature for 1 h, or overnight at 4 °C.
NOTE: Concentrations of primary antibodies used are in the Table of Materials. Unless specified, the manufacturer's recommendations are followed. - Remove supernatant and wash with 100 µL of PBS 3x.
- Apply 100 µL of secondary antibody diluted in 2% BSA in PBS and incubate at room temperature for 1 h, or overnight at 4 °C.
NOTE: This step can be skipped if the primary antibody used is conjugated with a fluorescence probe. Concentrations of secondary antibodies used are in the Table of Materials. Unless specified, the manufacturer's recommendations are followed. - Remove supernatant and wash with 100 µL of PBS 3x.
- Optional: For counter staining of F-actin and nuclei (DAPI), prepare by mixing both probes at appropriate dilution (per manufacturer's recommendation) in PBS, apply 100 µL, and incubate at room temperature for 30 min. Remove supernatant and wash with 100 µL of PBS 3x.
- Carefully cut out cell culture insert membrane with a scalpel blade, and mount on a glass slide with a mounting solution. Place a cover slip and observe.
Representative Results
This protocol reliably generates robust bovine intestinal organoid-derived 2D monolayers from the small and large intestinal tract, emulating the complexity of the in vivo intestinal epithelium. This method utilizes mature organoids developed from intestinal crypt specimens of healthy cattle cultured with optimized conditions. Interestingly, the successful and repeatable conditions for the organoid-derived 2D monolayers are unique to the segment of the gut (Table 1). This reinforces the importance of having optimized culture techniques for the gut segment of interest.
At 1 day after seeding dissociated mature organoids onto a cell culture insert, a 2D monolayer appeared to form (Figure 2A). However, despite this initial appearance, TEER measurements for both ileal and rectal monolayers remained low at this stage (Figure 1C). Moreover, a paracellular permeability assay revealed that the monolayer, after just 1 day of culture, allowed the passage of a 4 kDa FITC-dextran tracer across the cell layer (Figure 1C). By the 3rd day of culture, both types of organoid-derived 2D monolayers showed significant maturation, evidenced by increased TEER values and resistance to the 4 kDa FITC-dextran tracer, a trend that continued until day 5 of culture.
Particularly notable is the interspecies variability, where despite lower TEER values of bovine organoid-derived 2D monolayer cultures relative to human and canine counterparts under similar conditions12,13,14,15, the integrity of the membrane remains intact. This conclusion is drawn from the monolayers' appropriate response in permeability assays, suggesting that low TEER values in bovine samples do not necessarily reflect a lack of barrier function. This integrity is crucial for a functional epithelial barrier and is effectively demonstrated through the careful interpretation of permeability assay results alongside TEER measurements.
Visualization of cellular demarcation and well-developed microvilli on the apical surface of the 2D monolayers with scanning electron microscopy, displaying specialized microanatomical structures, further reinforced maturation of both ileal and rectal organoid-derived 2D monolayers (Figure 2B). Additionally, immunofluorescence staining of the 2D monolayers confirmed the presence of apical brush border, basolateral adherence junctions, and mucus-producing goblet cells in both ileal (Figure 3A) and rectal (Figure 3B) organoid-derived 2D monolayers. These results reinforce that the developed 2D monolayer is complex in composition and formation, not only expressing key features of an intact intestinal epithelium but being comprised of a multilineage cellular population.
Successful 2D monolayer development relies on the adherence of cells to ECM and growth to confluence to create an intact epithelial layer. Notably, an uneven ECM distribution or suboptimal conditions during incubation on the cell culture insert can result in the partial detachment of the cell layer, particularly noticeable along its edges (Figure 4Ai). This issue is further compounded if the cells are seeded at higher than optimal density or the seeding cells are not evenly distributed across the culture surface, a scenario often stemming from the incomplete dissociation of organoids into a single cell suspension. Such uneven distribution can lead to the formation of gaps within the monolayer and/or 3D morphogenesis on a cell culture insert (Figure 4Aii). In contrast, under-seeding of the cells can also result in unsuccessful or delayed monolayer development over the expected culture period, which inadvertently impacts the efficiency of subsequent studies utilizing the 2D monolayer system (Figure 4Aiii). Furthermore, contamination of the culture system can also lead to the formation of gaps within the monolayer, disrupting the once-formed confluent monolayer at a later stage of culture (Figure 4Aiv). Sustained TEER values and paracellular permeability response can be impacted by disturbances in the cell layer from aggressive washing or handling techniques, even when the aforementioned potential causes of failure had not been encountered prior to these assays (Figure 4B). Thus, careful cell handling and evaluation of monolayer formation or disruptions are paramount for the successful development of organoid-derived 2D monolayers through the effective application of troubleshooting strategies.
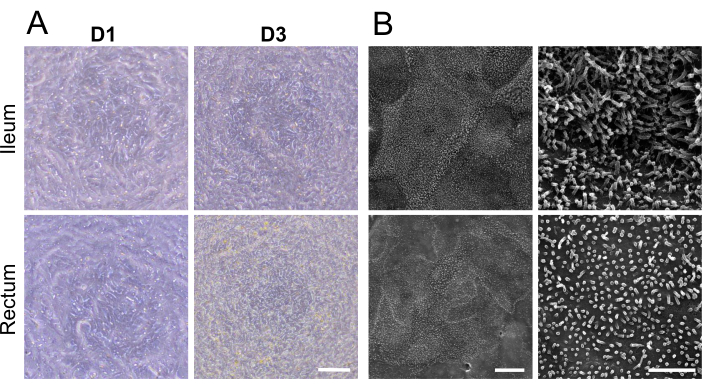
Figure 2: Microscopic characterization of the bovine ileal and rectal organoid-derived 2D monolayers. (A) Representative phase contrast microscopy images of 2D monolayers at day 1 and day 3 (D1 and D3) of culture on a cell culture insert. Scale bar = 100 µm. (B) Representative scanning electron microscopy images of 2D monolayers with lower (left) and higher (right) magnifications. Detailed cell surface structure, including microvilli, can be appreciated in both ileal (top) and rectal (bottom) monolayers. Left scale bar = 10 µm, right scale bar = 2 µm. Please click here to view a larger version of this figure.
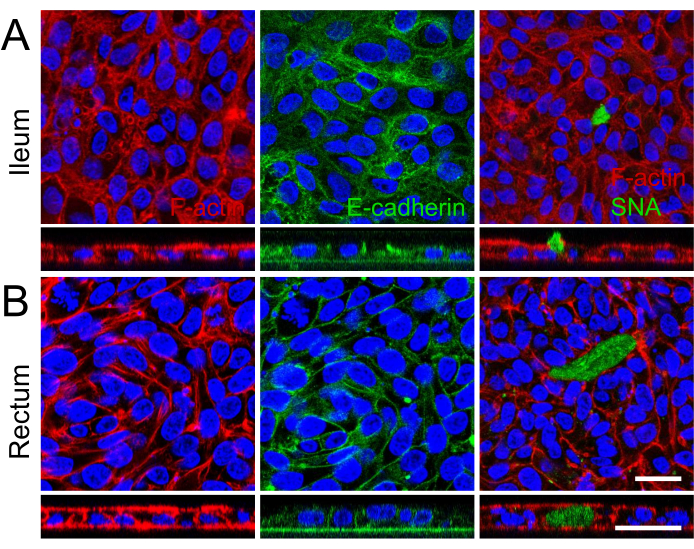
Figure 3: Immunofluorescence characterization of 2D monolayers derived from ileal and rectal organoids. (A, B) The panel (A) shows ileal, and the panel (B) shows rectal organoids. On the left, F-actin fibers are highlighted with Phalloidin (red), illustrating the cytoskeletal architecture and apical brush border formation. The middle image captures the basolateral localization of adherens junctions, marked by E-cadherin (green), indicative of cell-cell adhesion and monolayer integrity. On the right, the presence of mucin-producing goblet cells is identified by SNA (green), with a z-stack image depicting the apical secretion of mucin in the ileal monolayer. Nuclei across all images were counterstained with DAPI (blue). Additionally, z-stack imaging across all images further demonstrates the formation of a single cell layer within the culture insert. Scale bar = 25 µm. Please click here to view a larger version of this figure.
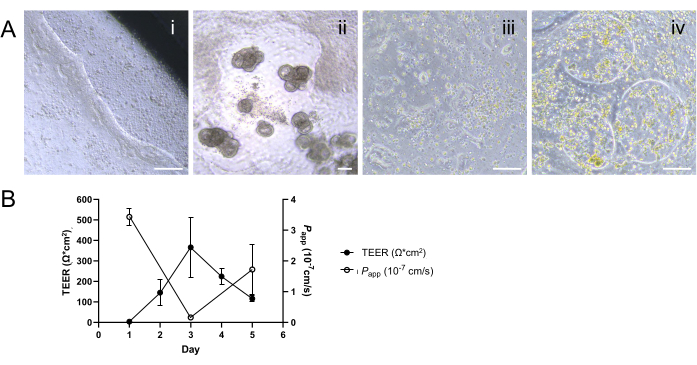
Figure 4: Characterization of suboptimal 2D monolayer formation. (A) Representative phase contrast images demonstrating (i) the partial detachment of the monolayer along the edge of cell culture insert; (ii) the development of 3D outgrowth and the formation of gaps within the monolayer; (iii) incomplete or delayed monolayer formation due to lower than optimal seeding density noted as patchy adherence of cells; and (iv) formation of gaps within the once-formed 2D monolayer at later stage, likely resulting from suspected contamination. Scale bars = 100 µm. (B) Declining TEER measurements coupled with rising permeability profiles after day 3 indicate a failure to establish stable and functional epithelial barriers. The results are presented as mean ± standard error of the mean (SEM) from a single experiment with two technical replicates. Please click here to view a larger version of this figure.
Supplementary Figure 1: Density variations in bovine intestinal organoid cultures in ECM-based hydrogel. Bovine intestinal organoids cultured in an ECM-based hydrogel; (A) high density and (B) low density. Scale bar = 100 µm. Please click here to download this File.
Supplementary Table 1: Summarized tissue donor signalments. Please click here to download this File.
Discussion
The health of the intestinal tract is paramount to both the productivity and overall well-being of cattle16. Leveraging organoid-derived 2D monolayer technology, scientists can now more accurately mimic the complex structure of the bovine gut epithelium within an in vitro setting5. This innovative approach not only reproduces the diverse cellular composition of the intestinal lining, including its multicellular lineages but also captures key functional characteristics, such as mucus secretion and the presence of microvilli, essential for understanding gut physiology and pathology3. The development of tailored culture protocols for segments of the ileum and rectum has given rise to an advanced platform that significantly enhances the capacity to study bovine gut health. This sophisticated approach enables detailed investigations into the interactions between zoonotic pathogens and the bovine intestinal environment. The ability to closely replicate and study the unique aspects of the bovine intestinal ecosystem in vitro is a significant stride towards developing targeted strategies for improving livestock health and mitigating the spread of zoonotic diseases.
Nevertheless, to ensure successful 2D monolayer development using bovine intestinal organoids, it is critical to maintain the health and vitality of both the organoids and their dissociated single cells. Careful handling and the minimization of stress are paramount in preserving cell integrity and functionality, which are essential for the effective growth of organoids and the subsequent creation of a functioning monolayer. Furthermore, achieving a uniform monolayer relies on the successful dissociation of organoids to single cells without forming large clumps. Such clumps can disrupt cell distribution and compromise the monolayer's structure. Therefore, employing precise techniques for smooth dissociation is crucial, resulting in a consistent single-cell suspension. Additionally, minimizing disturbances during cell adhesion and when washing away excess non-adherent cells becomes beneficial. This approach is particularly important for addressing potential issues with 3D morphogenesis, thus enhancing the overall quality of the monolayer.
A noted challenge with ECM-based hydrogels that are of biological origin is batch-to-batch variation in composition17. While this was not observed using the described protocols and materials, batch-to-batch variations in ECM composition could pose challenges to successful monolayer development. If monolayer formation is compromised when ECM products, brands, or lot numbers change, optimization steps may be necessary to determine the appropriate ECM concentration required for coating the cell culture inserts.
Moreover, adjusting the culture medium to room temperature before making any changes is a critical step that helps mitigate thermal shock, protect cell health, and maintain the quality of both the organoid and monolayer cultures. Gentle washing practices are also paramount in maintaining the monolayer's integrity during its formation and subsequent assays, and avoiding disruptions can prevent inaccuracies in results. Substituting PBS with Hank's Balanced Salt Solution (HBSS) appeared helpful in minimizing monolayer detachment when it became an issue during repeat washing or prolonged exposure to PBS, such as in the paracellular permeability assays. Finally, tailoring the culture medium to meet the specific needs of cells from different segments of the intestinal tract, such as the ileum and rectum, is essential for accurately replicating in vivo conditions. This specificity ensures optimal cell health and functionality, facilitating precise modeling of cattle gut physiology and interactions with pathogens, thereby highlighting these critical steps in organoid research.
Besides employing a gentle cell handling practice, building good technical competency associated with cell counting and TEER measurements is crucial for the successful development of a functioning 2D monolayer. Since both too-low and too-high seeding densities resulting from over- or under-counting of the cells, respectively, can lead to compromised monolayer growth. It is encouraged to carefully review cell counts and ensure appropriate seeding density in occasions where inaccurate seeding densities are suspected. Additionally, inadequate TEER measurement techniques can result in disruption of the monolayer by inadvertent scratches with the electrodes. Carefully introducing the electrodes to the apical chamber and paying particular attention to maintaining their vertical orientation relative to the membrane surface could help mitigate the risk of accidental damage to the monolayers.
The methods of paracellular permeability assay described here have been adapted from a previous protocol18. Modifications to the reported protocol, which include multiple samplings over 120 min and replacement of the sampled aliquot with equal amounts of PBS, are made to improve the accuracy and reliability of the results. Maintaining the total volume within the chamber is critical for several reasons: it preserves osmotic balance, ensures cell integrity, maintains the concentration gradient essential for accurate permeability assessment, and prevents alterations in hydrostatic pressure that could affect transport rates. This practice of replenishing the basolateral chamber with fresh PBS equivalent to the volume of the fluorescent tracer-containing PBS sampled is pivotal to preserving these conditions, enabling accurate and meaningful evaluations of monolayer permeability. The paracellular permeability assay serves as a complement to the TEER measurement by assessing the movement of tracer molecules through the monolayer directly. Furthermore, comparing TEER values across various laboratories may not yield relevant insights, as these values can be affected by numerous variables, such as temperature and the specific conditions under which cells are cultured, including cell types, passage numbers, and the composition of the culture medium19. The paracellular permeability assay provides a functional in vitro assessment of the effective expression of adherens and tight junctions within an epithelial barrier20.
While the development of 2D monolayers from 3D organoids represents a significant advance in culture technology, it is important to acknowledge the limitations associated with 2D monolayers. One major drawback is that this remains a static culture system, lacking the dynamic stimulation found in the in vivo environment. Additionally, modifying the oxygen content within the culture system presents challenges due to its open setup involving culture plates with lids, making it less suitable for long-term co-culture with anaerobic bacteria. These limitations could potentially be addressed by adopting more dynamic culture platforms, such as microfluidic systems21, which offers a more controlled and physiologically relevant environment. Furthermore, it is crucial to recognize that while current culture conditions are rich in nutrients beneficial for maintaining stem cell growth, they may not be optimal for inducing physiological differentiation of the epithelial cells. This discrepancy highlights a need for optimization in future research to closely mimic the in vivo conditions and support the differentiation process. By addressing these limitations and refining these approaches, the utility and applicability of organoid culture technologies are enhanced, moving closer to replicating the complex dynamics and interactions of the gastrointestinal tract in vitro.
The protocol for generating 2D monolayers from bovine ileal and rectal tissues offers researchers a valuable in vitro model of the luminal interface of both small and large intestinal epithelium. This model opens up vast possibilities for application in fundamental animal nutrition studies, particularly in examining how nutrients are absorbed under various conditions. A notable area of interest is the investigation of the leaky-gut syndrome, characterized by an abnormal increase in gastrointestinal permeability, often triggered by dietary shifts and extreme environmental temperatures22,23. Moreover, this model serves as an essential tool for exploring the complex interactions between the gut microbiome and its host. It allows for the study of how commensal microorganisms may affect the health of the host organism, addressing a crucial aspect of veterinary and medical science1,24. Additionally, human food-borne pathogens are frequently found as commensals in different segments of cattle gut8,9,25, this protocol enables detailed studies of the specific conditions that allow these zoonotic agents to thrive in their respective niches.
Throughout this study, it was observed that rectal and ileal organoid-derived monolayers require different conditions for successful development. Specifically, when rectal organoid-derived monolayers were initially seeded onto cell culture inserts prepared with 2% ECM-based hydrogel in basal media for 1 h, large holes and cell sloughing were noted. This issue was resolved by switching to a specialized rectal monolayer culture media and extending the incubation period to overnight before seeding, whereas ileal organoid-derived monolayers were successfully developed using a shorter preparation protocol. Furthermore, the addition of CHIR99021 to the culture medium consistently improved the establishment of rectal monolayers26 but was not necessary for ileal monolayers27. Additionally, ileal monolayers required a higher cell density for successful development compared to rectal organoids27. These optimized conditions (Table 1) have repeatedly developed monolayers that maintain resistant barrier integrity, underscoring the importance of tailoring culture conditions to the specific gut segment.
Access to a model that accurately reflects the multicellular lineage complexity of the in vivo gut is critical for these investigations. It allows researchers to closely mimic the natural conditions of the gut environment, providing a more reliable basis for experiments. With this protocol, investigators are equipped with a robust model that enhances their research capabilities, potentially leading to groundbreaking discoveries in their fields of study. This approach not only contributes to understanding gut health and disease but also aids in the development of strategies to improve livestock management and food safety.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This study was supported in part by the Office of the Director National Institutes of Health (K01OD030515 and R21OD031903 to YMA) and WSU VCS Resident and Graduate Student Research Grant (to GDD). The authors would like to thank the participating slaughterhouse for supplying donor cattle.
Materials
| Basal Medium | |||
| Advanced DMEM/F12 (1X) | Gibco | 12634-010 | n/a |
| GlutaMAX-I (100X) | Gibco | 35050-061 | 2 mM |
| HEPES (1M) | Gibco | 15630-080 | 10 mM |
| Pen Strep Glutamine (100X) | Gibco | 10378-016 | 1X |
| Organoid Culture Medium (Supplements to Basal Medium) | |||
| A-83-01 | Sigma-Aldrich | SML0788-5MG | 500 nM |
| B27 Supplement (50X) | Gibco | 17504-001 | 1X |
| [Leu15]-Gastrin I human | Sigma-Aldrich | G9145-.5MG | 10 nM |
| Murine EGF | PeproTech | 315-09-500UG | 50 ng/mL |
| Murine Wnt-3a | PeproTech | 315-20-10UG | 100 ng/mL |
| N-Acetyl-L-cysteine | MP Biomedicals | 194603 | 1 mM |
| N-2 MAX Media Supplement (100X) | R&D Systems | AR009 | 1X |
| Nicotinamide | Sigma-Aldrich | N0636-100G | 10 mM |
| Noggin Conditioned Medium | n/a | n/a | 10 vol/vol % |
| Primocin | InvivoGen | ant-pm-2 | 100 µg/mL |
| R-Spondin-1 Conditioned Medium | n/a | n/a | 20 vol/vol % |
| SB202190 | Sigma-Aldrich | S7067-25MG | 10 µM |
| Monolayer Culture Medium (Supplements to Organoid Culture Medium) | |||
| CHIR99021 | Sigma-Aldrich | SML1046-5MG | 2.5 µM |
| HI FBS | Gibco | 10438-034 | 20 vol/vol % |
| LY2157299 | Sigma-Aldrich | SML2851-5MG | 500 nM |
| Y-27632 | StemCellTechnologies | 72308 | 10 µM |
| Reagents | |||
| Alexa Fluor 488 Mouse anti-E-cadherin | BD Biosciences | 560061 | 1:200 dilution |
| Alexa Fluor 647 Phalloidin | Invitrogen | A22287 | 1:400 dilution |
| BSA | Cytiva | SH30574.02 | 2 w/vol % |
| Cell Recovery Solution | Corning | 354253 | n/a |
| DAPI Solution (1 mg/mL) | Thermo Scientific | 62248 | 1:1000 dilution |
| DPBS (1X) | Gibco | 14190-144 | n/a |
| Fluorescein Isothiocyanate–Dextran | Sigma-Aldrich | FD4-100MG | 0.5 mg/mL |
| Matrigel Matrix | Corning | 354234 | n/a |
| Paraformaldehyde Solution (4%) | Thermo Scientific | J19943K2 | n/a |
| ProLong Gold antifade reagent | Invitrogen | P36930 | n/a |
| SNA, EBL, Fluorescein | Vector Laboratories | FL-1301 | 1:100 dilution |
| Triton X-100 | Thermo Scientific | A16046.AE | 0.3 vol/vol % |
| TrypLE Express | Gibco | 12605-028 | n/a |
| Trypan Blue Solution, 0.4% | VWR Life Science | K940-100ML | n/a |
| Materials and Equipment | |||
| 0.4 µm Cell Culture Insert | Falcon | 353095 | |
| 24-well Cell Culture Plate | Corning | 3524 | |
| 48-well Cell Culture Plate | Thermo Scientific | 150687 | |
| 70 µm Sterile Cell Strainer | Fisher Scientific | 22-363-548 | |
| 96-well Cell Culture Plate | Greiner Bio-One | 655086 | |
| Centrifuge | Eppendorf | 5910Ri | |
| CO2 Incubator | Thermo Scientific | 370 | |
| Epithelial Volt-Ohm Meter | Millipore | Millicell ERS-2 | |
| Hemocytometer | LW Scientific | CTL-HEMM-GLDR | |
| Inverted Confocal Microscope | Leica Microsystems | SP8-X | |
| Inverted Phase-Contrast Microscope | Leica Microsystems | DMi1 | |
| Microscope Cover Glass | Fisher Scientific | 12-540-B | |
| Microplate Reader | Molecular Devices | SpecrtraMax i3x | |
| Microscope Slides | Fisher Scientific | 22-034-486 | |
| Pasteur Pipets | Fisher Scientific | 13-678-20C | |
| Scalpel Blade | iMed Scientific | – | #11 carbon steel |
| Vortex Mixer | Scientific Industries | SI-0236 | |
| Software | |||
| LAS X imaging software | Leica Microsystems | LAS X 3.7.6.25997 | |
| Microplate Reader software | Molecular Devces | SoftMax Pro 7.1.2 |
References
- Min, S., Kim, S., Cho, S. W. Gastrointestinal tract modeling using organoids engineered with cellular and microbiota niches. Exp Mol Med. 52 (2), 227-237 (2020).
- Fitzgerald, S. F., et al. Shiga toxin sub-type 2a increases the efficiency of Escherichia coli O157 transmission between animals and restricts epithelial regeneration in bovine enteroids. PLoS Pathogens. 15 (10), e1008003 (2019).
- Dutta, D., Heo, I., Clevers, H. Disease modeling in stem cell-derived 3D organoid systems. Trend Mol Med. 23 (5), 393-410 (2017).
- Beaumont, M., et al. Intestinal organoids in farm animals. Vet Res. 52 (1), 33 (2021).
- Kawasaki, M., Dykstra, G. D., McConnel, C. S., Burbick, C. R., Ambrosini, Y. M. Adult bovine-derived small and large intestinal organoids: In vitro development and maintenance. J Tissue Eng Regene Med. 2023, e3095002 (2023).
- Kvidera, S. K., et al. Intentionally induced intestinal barrier dysfunction causes inflammation, affects metabolism, and reduces productivity in lactating Holstein cows. J Dairy Sci. 100 (5), 4113-4127 (2017).
- Crawford, C. K., et al. Inflammatory cytokines directly disrupt the bovine intestinal epithelial barrier. Sci Rep. 12 (1), 14578 (2022).
- Heredia, N., García, S. Animals as sources of food-borne pathogens: A review. Animal Nutri. 4 (3), 250-255 (2018).
- Naylor, S. W., et al. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect Immun. 71 (3), 1505-1512 (2003).
- Co, J. Y., et al. Controlling epithelial polarity: A human enteroid model for host-pathogen interactions. Cell Rep. 26 (9), 2509-2520.e4 (2019).
- Pullinger, G. D., et al. Systemic translocation of Salmonella enterica Serovar Dublin in cattle occurs predominantly via efferent lymphatics in a cell-free niche and requires type III secretion system 1 (T3SS-1) but not T3SS-2. Infect Immun. 75 (11), 5191-5199 (2007).
- Nickerson, K. P., et al. A versatile human intestinal organoid-derived epithelial monolayer model for the study of enteric pathogens. Microbiol Spectr. 9 (1), e0000321 (2021).
- Varani, J., McClintock, S. D., Aslam, M. N. Cell-matrix interactions contribute to barrier function in human colon organoids. Front Med. 9, 838975 (2022).
- Freire, R., et al. Human gut derived-organoids provide model to study gluten response and effects of microbiota-derived molecules in celiac disease. Sci Rep. 9, 7029 (2019).
- Ambrosini, Y. M., et al. Recapitulation of the accessible interface of biopsy-derived canine intestinal organoids to study epithelial-luminal interactions. PLoS ONE. 15 (4), e0231423 (2020).
- Kogut, M. H., Arsenault, R. J. Editorial: Gut health: The new paradigm in food animal production. Front Vet Sci. 3, 71 (2016).
- Lingard, E., et al. Optimising a self-assembling peptide hydrogel as a Matrigel alternative for 3-dimensional mammary epithelial cell culture. Biomater Adv. 160, 213847 (2024).
- Turksen, K. . Permeability Barrier: Methods and Protocols. , (2011).
- Srinivasan, B., et al. TEER measurement techniques for in vitro barrier model systems. J Lab Autom. 20 (2), 107-126 (2015).
- Frost, T. S., Jiang, L., Lynch, R. M., Zohar, Y. Permeability of epithelial/endothelial barriers in Transwells and microfluidic bilayer devices. Micromachines. 10 (8), 533 (2019).
- Bein, A., et al. Microfluidic organ-on-a-chip models of human intestine. Cell Mol Gastroenterol Hepatol. 5 (4), 659 (2018).
- Sanz-Fernandez, M. V., et al. Targeting the hindgut to improve health and performance in cattle. Animals. 10 (10), 1817 (2020).
- Gressley, T. F., Hall, M. B., Armentano, L. E. Ruminant nutrition symposium: Productivity, digestion, and health responses to hindgut acidosis in ruminants. J Anim Sci. 89 (4), 1120-1130 (2011).
- O’Hara, E., Neves, A. L. A., Song, Y., Guan, L. L. The role of the gut microbiome in cattle production and health: Driver or passenger. Ann Rev Animal Biosci. 8 (2020), 199-220 (2020).
- Beach, J. C., Murano, E. A., Acuff, G. R. Prevalence of Salmonella and Campylobacter in beef cattle from transport to slaughter. J Food Protect. 65 (11), 1687-1693 (2002).
- Kawasaki, M., Ambrosini, Y. M. Accessible luminal interface of bovine rectal organoids generated from cryopreserved biopsy tissues. PLoS One. 19 (3), e0301079 (2024).
- Kawasaki, M., McConnel, C. S., Burbick, C. R., Ambrosini, Y. M. Pathogen-epithelium interactions and inflammatory responses in Salmonella Dublin infections using ileal monolayer models derived from adult bovine organoids. Scientific Reports. 14 (1), 11479 (2024).

