Isolation of DRG Neurons and Coculture with Schwann Cell Precursors to Generate Schwann Cells
Abstract
Source: Tsui, Y, et al. Hypoxic Preconditioning of Marrow-derived Progenitor Cells as a Source for the Generation of Mature Schwann Cells. J. Vis. Exp. (2017).
This video demonstrates a procedure for isolating dorsal root ganglion (DRG) neurons from rat embryos and co-culturing them with Schwann cell precursors to generate mature Schwann cells. The isolated DRGs are processed to isolate neurons and glial cells. The isolated cells are cultured to purify the neurons, which are then co-cultured with Schwann cell-like cells (SCLCs) in a co-culture medium. Interaction with the DRG neurons leads to the maturation of SCLCs into Schwann cells.
Protocol
Generation of Fate-committed Schwann Cells via Coculture with DRG Neurons
1. Preparation of purified rat DRG neurons
- Autoclave all dissection tools (i.e., dissection scissors, forceps, two microdissection forceps, and microdissecting scissors) at 180 °C for at least 2 h prior to use.
- Coat 6-well tissue culture plates with poly-D-lysine (PDL, 10 µg/mL in PBS) at 4°C overnight. Remove the PDL and rinse with 1.5 m of PBS per well.
- Proceed with coating the plates with laminin (10 µg/mL in PBS) at 37 °C for 2 h. Rinse the plates with 1.5 mL of PBS per well.
- Prepare DRG neuron maintenance medium, comprised of neurobasal medium supplemented with B27 (2% v/v), L-glutamine (1% v/v), nerve growth factor (NGF, 20 ng/mL), and penicillin/streptomycin (P/S, 1% v/v).
- Prepare DRG neuron purification medium, comprised of neurobasal medium supplemented with B27 (2% v/v), L-glutamine (1%), nerve growth factor (NGF, 20 ng/mL), P/S (1%), fluorodeoxyuridine (FDU, 10 µg/mL), and uridine (10 µg/mL).
- Sacrifice pregnant rats at gestational day 14-15 by pentobarbital overdose (240 mg/kg bodyweight, intraperitoneal).
- Place the sacrificed animals in supine position. Clean their abdomen thoroughly with 70% ethanol.
- Cut the lower abdominal wall of the animal longitudinally using fine dissecting scissors and forceps. Identify and remove the uterus using dissection scissors. Cut the uterine wall to expose and extract the embryos. Transfer the embryos to a sterile, 10 cm culture dish filled with PBS. Place the culture dish on ice.
- Transfer the embryo intended for dissection to a sterile, 10 cm culture dish filled with PBS (room temperature) and position it beneath a dissection microscope. Have the embryo in prone position.
NOTE: The whitish spinal cord and the attached DRGs can be seen over the dorsal aspect of the embryo, through its translucent skin. - Insert microdissecting forceps along either side of the spinal cord and use blunt dissection to begin separating the spinal cord from the surrounding soft tissue. Cut the spinal cord free from the animal using microdissecting forceps along the neck opening and tail stub. Perform further blunt dissection over the ventral aspect of the cord to free it from surrounding soft tissue.
- Use microdissecting forceps to remove residual soft tissue over the dorsal aspect of the freed spinal cord.
NOTE: At this stage, only the spinal cord, nerve roots, and attached DRG should remain. - Detach individual DRGs from their connecting nerve roots using microdissecting forceps. Use a pipette pen attached to a 1 mL tip to transfer the DRGs to a 1.5 mL, sterile centrifuge tube containing PBS.
NOTE: For each 1.5 mL tube, a maximum of 100 DRGs can be accommodated. - Centrifuge the DRGs at 250 x g for 5 min and resuspend them in recombinant enzymatic cell dissociation reagent (200 µL per tube). Incubate (37 °C, 5% CO2) for 10 min. Centrifuge the DRGs at 250 x g for 5 min, remove the supernatant, and resuspend in DRG neuron maintenance medium. Dissociate the pellet by gentle trituration using a 200 µL pipette tip. Quantify the cells within the pellet using a hemocytometer after an appropriate dilution.
- Seed the cells at a density of 5,000 cells/cm2 into PDL/laminin-coated 6-well plates in 1.5 mL of DRG neuron maintenance medium per well. After two days of culture, remove the DRG neuron maintenance medium, rinse with PBS, and replace with DRG neuron purification medium.
NOTE: For each purification cycle, treat the DRG cultures with purification medium for 2 days, followed by 1 day of incubation in maintenance medium. After 3-4 purification cycles, the removal of all endogenous glia is expected. This should take approximately 14 days. Purified cultures test positive for the neuronal marker TUJ1 and are absent for S100β expression (Figure 1).
2. Generation of Schwann cell-like cells
- Prepare glial induction medium comprised of αMEM (Minimum essential medium Eagle (MEM), alpha modifications) supplemented with β-Heregulin (100 ng/mL), basic fibroblast growth factor (bFGF, 10 ng/ml), platelet-derived growth factor (PDGF-AA, 5 ng/mL), fetal bovine serum (FBS, 10%), and P/S (1% v/v).
- Plate the neurospheres prepared in section 4 in PDL/laminin-coated 6-well plates at a density of 5-10 spheres per cm2 in 1.5 mL of glial induction medium per well. Replace glial induction medium every 2 days after rinsing the cells with PBS.
- NOTE: Cells from seeded neurospheres are seen to migrate outwards by day 2. By day 7, migratory cells have a tapered appearance and should demonstrate immunopositivity for the Schwann cell markers p75 neurotrophin receptor (p75) and S100β7. These cells are referred to as SCLCs.
3. Coculture of SCLCs with DRG neurons
- Prepare coculture medium comprised of DRG neuron maintenance medium (step 1.1.4) and glial induction medium (step 1.2.1) at a 1:1 volume-to-volume ratio.
- Prepare Schwann cell maintenance medium comprised of Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12)) supplemented with FBS (5%), β-Heregulin (10 ng/mL), and P/S (1% v/v).
- Remove the culture medium from day 7 SCLCs, rinse them with PBS, and incubate them with 0.5 mL/well of recombinant enzymatic cell dissociation reagent at 37 °C for 5 min. Resuspend the SCLCs in coculture medium.
- Quantify the cells using a hemocytometer after an appropriate dilution.
- Seed the SCLCs onto purified DRG neuron cultures at a density of 1,000 cells/cm2. Maintain the cocultures for 14 days, with medium replacement every 2 days.
NOTE: During coculture, SCLCs acquired the spindle-like morphology that is typical of mature Schwann cells (Figure 2). These cells persist in their phenotype after the withdrawal of growth factors and are able to myelinate axons in vitro and in vivo. The positivity of Schwann cell markers (i.e., p75 and S100β) should be monitored by immunofluorescence. - Upon completion of coculture, passage fate-committed Schwann cells. Use 0.5 mL of dissociation reagent per well. Quantify the cells using a hemocytometer after an appropriate dilution.
Representative Results
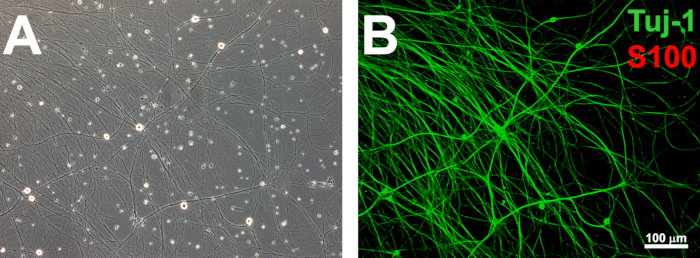
Figure 1: Establishing purified rat DRG networks. Purified DRG networks are established after pulsed treatment with the antimitotic agents FDU and uridine (A). Neurite networks that are devoid of S100β-expressing endogenous glia (B) are ready for coculture with SCLCs. Scale bars = 100 µm.
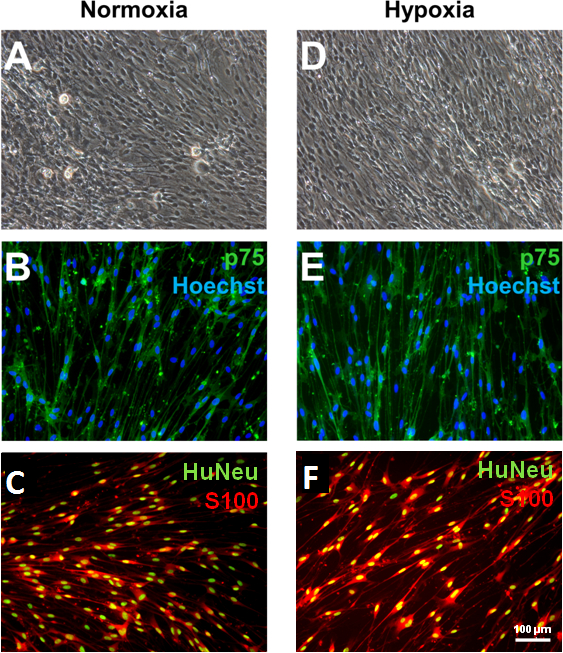
Figure 2: Generation of bone marrow-derived Schwann cells via coculture with DRG neurons. Upon completing 2 weeks of coculture with purified DRG neurons and human SCLCs, spindle-shaped, fate-committed Schwann cells emerge from both normoxia- and hypoxia-treated groups (A and D). These cells express the Schwann cell markers p75 (B and E) and S100β (C and F). The expression of human nuclei antigen (HuNeu) demonstrates that the S100β-positive cells were not contaminating glial cells originating from rat DRGs. Scale bars = 100 µm.
Divulgations
The authors have nothing to disclose.
Materials
| αMEM | Sigmaaldrich | M4526 | |
| DMEM/F12 | Thermofisher scientific | 12400-024 | |
| Neurobasal medium | Thermofisher scientific | 21103-049 | |
| FBS | Biosera | FB-1280/500 | |
| B27 | Thermofisher scientific | 17504-001 | |
| Epidermal growth factor (EGF) | Thermofisher scientific | PHG0313 | |
| Basic fibroblast growth factor (bFGF) | Peprotech | 100-18B/100UG | |
| Nerve growth factor (NGF) | Millipore | NC011 | |
| Platelet-derived growth factor-AA (PDGF-AA) | Peprotech | 100-13A | |
| Heregulin beta-3, EGF domain (β Her) | Millipore | 01-201 | |
| Uridine | Sigmaaldrich | U3003 | |
| 5-Fluro-2' – deoxyuridine (FDU) | Sigmaaldrich | F0503 | |
| Poly-D-lysine (PDL) | Sigmaaldrich | P7886-1G | |
| Laminin | Thermofisher scientific | 23017015 | |
| GlutaMAX | Thermofisher scientific | 35050061 | |
| Penicillin / streptomycin (P/S) | Thermofisher scientific | 15140-122 | |
| TrypLE Express | Thermofisher scientific | 12604-013 | |
| 10 cm plate for adherent culture | TPP | 93100 | Used for selection of MSCs by tissue culture adherence |
| 6-well plate for adherent culture | TPP | 92006 | Used for expansion of MSCs following passaging |
| UltraLow 6-well plate for non adherent culture | Corning | 3471 | Used for neural progenitor enrichment |
| Hypoxia chamber | Billups-Rothenberg | MIC-101 | |
| HEPES buffer | Sigmaaldrich | H4034-100G |

