使用原子力显微镜测量活细胞的力学性能
Summary
本文演示了一个协议,通过使用原子力显微镜(AFM)的显微表征活细胞的机械性能。
Abstract
细胞及细胞外基质(ECM)的机械性能,在许多生物过程中扮演重要的角色,包括干细胞的分化,肿瘤的形成,和伤口愈合。细胞和ECM刚度的变化往往是在组织细胞的生理或疾病变化的迹象。因此,单元的刚度是一个指数来评估细胞培养的状态。在人群中的用于测量细胞和组织的刚度的方法,使用原子力显微镜(AFM)的显微压痕提供了一种方法来可靠地测量活细胞的刚度。这种方法已被广泛地应用于表征微观尺度刚度,为各种材料,包括金属表面的生物软组织和细胞。这种方法的基本原理是,缩进一个细胞与一个选定的几何形状的针尖,并测量所施加的力从原子力显微镜悬臂梁弯曲。力压痕曲线拟合赫兹模式l为相应的尖端几何能给材料刚度的定量测量。本文演示程序,利用原子力显微镜的活细胞的刚度特性。关键步骤包括的AFM校准的过程中,力曲线采集,数据分析使用MATLAB例程证明。这种方法的局限性进行了讨论。
Introduction
的机械性能,特别是刚性,单个细胞和其周围的细胞外基质(ECM)的许多生物过程,包括细胞生长,运动,分裂,分化,和组织的平衡是至关重要的。1已经证明,主要是由细胞的机械刚度细胞骨架,特别是肌动蛋白和中间纤维和与它们相关联的其他蛋白质的网络2上在体外的网络的肌动蛋白和中间丝的机械性能试验的结果表明,细胞力学很大程度上依赖于细胞骨架结构和预加应力的细胞骨架3-5刚度的活细胞,然后把作为指标来评价细胞骨架结构6,肌球蛋白活性7和许多其他细胞的过程。更重要的是,在细胞的机械特性的变化也经常发现有密切associated与各类疾病,如肿瘤的形成和转移。8-10监控的机械刚度活细胞,因此可以提供一种新的方式来监控细胞生理学检测和诊断疾病,以及药物治疗的有效性进行评估。 12
已经开发了多个方法,包括粒子跟踪微流变学,13-16磁捻流式细胞仪,17微吸管18,19和20-22显微测量细胞的弹性。粒子跟踪微流变学的痕迹注入细胞或细胞内的细胞骨架的基准标记或亚微米的荧光颗粒的热振动23细胞的弹性和粘性性质从测量粒子位移涨落耗散定理计算14,23此方法允许同时测量的地方高空间分辨率的机械性能在不同的地方,在一个单元格。然而,注入到细胞中的荧光发色粒子,可能会导致细胞功能的变化,细胞骨架结构,因此细胞力学。微管吸吮方法适用于微量的直径范围从1到5μm至细胞膜的一小片进入移液管吸负压。细胞刚度的计算是由所施加的负压力和细胞膜的变形18此方法,但是,不能检测刚度整个单元中的非均匀分布。磁性捻流式细胞仪(MTC)施加磁场,超顺磁性珠连接到细胞膜上产生转矩。来自在该方法中的(17)细胞的刚度,从所施加的转矩之间的关系和细胞膜的扭转变形。这是很难控制的位置磁珠在MTC方法,并且它是也是challengi的ng来表征高分辨率的扭曲变形。显微适用于压头具有良好定义几何冲进入细胞。在细胞的缩进力以及由此产生的压痕往往遵循赫兹模型的预测。的杨氏模量,可以计算出从细胞的力压痕曲线拟合赫兹模型。这种方法已被广泛地应用到测试的组织和细胞的机械性能,尽管它的局限性,如在接触点的确定,赫兹模型的适用性,以及潜在的物理损伤,使细胞的不确定性。在众多的设备在microindentaion 20,原子力显微镜(AFM)是市售的,并已被广泛应用来表征力学性能的活细胞和组织中21,24-27。
本文演示了使用一个的庇护MFP3D生物AFM表征细胞力学过程。 AFM不上光年提供高分辨率的地形细胞也已被广泛应用到组织细胞的机械性质。 AFM压痕的原则,在图1中示出。原子力显微镜悬臂梁接近从几微米以上的细胞与细胞接触;缩进的单元格,这样的悬臂偏转达到预选的设定点;和拉相差的细胞。在此过程中被记录,作为其位置的函数,如在图1中所示的悬臂偏转。在与细胞接触之前,悬臂移动介质中,没有任何明显的偏转。缩进时对细胞,悬臂弯曲和偏转信号的增加。被建模为悬臂弹性梁,以使它们的偏转是在该室施加的力成比例。通过设置最大悬臂挠度,施加在样品上的力的最大量值被限制到避免d豪悦国际细胞。的力的部分,从点b,点c在图1中 ,进入细胞的前端缩进,适合赫兹模型,提取细胞的刚度曲线。
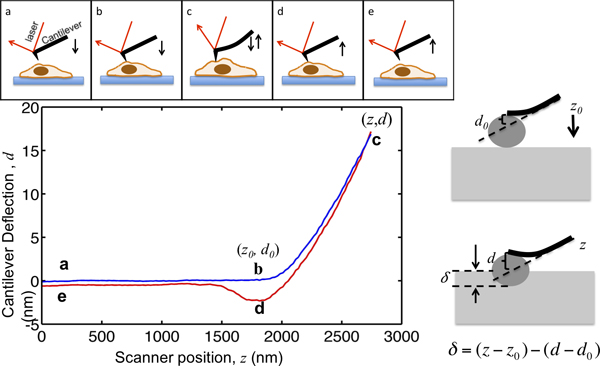
图1。 AFM显微插图和解释力曲线顶部面板显示驱动压电扫描器AFM悬臂的运动。悬臂式z和悬臂偏转信号 d的垂直位置被记录在这个过程中。从点a,几微米的单元格上方的悬臂。在接近该单元格,样品压痕δ保持为零,直到它到达b点的前端开始与细胞接触。 b点的坐标中的情节是临界值对数据进行分析,记为(Z 0,D 0>)。从b到c的悬臂的悬臂偏转进入细胞内的缩进,直到达到设定点时,它被设置为对象的最大的缩进力和悬臂弹簧常数之间的比值。 ,一旦偏转信号达到预先设定的最大值,然后撤回悬臂从点d,它经常被向下拉由于小费样本粘附细胞,从细胞中分离并返回到其初始位置在e。右边的窗格中示出的缩进和记录的z和D信号之间的关系。在左下面板是一块有代表性的力曲线,以悬臂的最大压痕,其中的弹簧常数测定为0.07N /米,被设定为17纳米,以便最大缩进力施加到样品是1.2 NN。在压痕标记的关键位置。
Protocol
Representative Results
Discussion
AFM的压痕法表征力学性能的活细胞的优点。尽管不那么敏感的磁扭仪和光学镊子,它可以测量的力量piconewton 32级,原子力显微镜能检测阻力纳米牛顿微微牛顿从几十到几百不等,从样品比较力范围可以应用到使用微量吸管19的细胞。此范围内的力适合需要建立可测量的变形,在所有类型的细胞19。高空间分辨率,使得它可以组织中,于单电池33上的亚微米级特性的…
Divulgations
The authors have nothing to disclose.
Acknowledgements
作者感谢保罗Janmey博士在宾夕法尼亚大学提供本文中使用的细胞株。 QW JF拜菲尔德和埃文安德森也承认他们独到的讨论AFM技术。
References
- Discher, D. E., Janmey, P., Wang, Y. L. Tissue cells feel and respond to the stiffness of their substrate. Science. 310, 1139-1143 (2005).
- Wagner, O. I., et al. Softness, strength and self-repair in intermediate filament networks. Exp. Cell Res. 313, 2228-2235 (2007).
- Wang, N., et al. Mechanical behavior in living cells consistent with the tensegrity model. Proceedings of the National Academy of Sciences of the United States of America. 98, 7765-7770 (2001).
- Wang, N., et al. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. American Journal of Physiology. Cell Physiology. 282, 606-616 (2002).
- Kasza, K. E., et al. Filamin A is essential for active cell stiffening but not passive stiffening under external force. Biophysical Journal. 96, 4326-4335 (2009).
- Elson, E. L. Cellular mechanics as an indicator of cytoskeletal structure and function. Annual Review of Biophysics and Biophysical Chemistry. 17, 397-430 (1988).
- Schafer, A., Radmacher, M. Influence of myosin II activity on stiffness of fibroblast cells. Acta Biomaterialia. 1, 273-280 (2005).
- Guck, J., et al. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophysical Journal. 88, 3689-3698 (2005).
- Cross, S. E., Jin, Y. S., Rao, J., Gimzewski, J. K. Nanomechanical analysis of cells from cancer patients. Nature Nanotechnology. 2, 780-783 (2007).
- Plodinec, M., et al. The nanomechanical signature of breast cancer. Nature Nanotechnology. 7, 757-765 (2012).
- Rotsch, C., Radmacher, M. Drug-induced changes of cytoskeletal structure and mechanics in fibroblasts: an atomic force microscopy study. Biophysical Journal. 78 (00), 520-535 (2000).
- Cross, S. E., Jin, Y. S., Lu, Q. Y., Rao, J., Gimzewski, J. K. Green tea extract selectively targets nanomechanics of live metastatic cancer cells. Nanotechnology. 22, 215101 (2011).
- Hoffman, B. D., Crocker, J. C. Cell mechanics: dissecting the physical responses of cells to force. Annual Review of Biomedical Engineering. 11, 259-288 (2009).
- Hoffman, B. D., Massiera, G., Van Citters, K. M., Crocker, J. C. The consensus mechanics of cultured mammalian cells. Proceedings of the National Academy of Sciences of the United States of America. 103, 10259-10264 (2006).
- Lau, A. W., Hoffman, B. D., Davies, A., Crocker, J. C., Lubensky, T. C. Microrheology, stress fluctuations, and active behavior of living cells. Physical Review Letters. 91, 198101 (1981).
- Liu, J., et al. Microrheology probes length scale dependent rheology. Physical Review Letters. 96, 118104 (2006).
- Deng, L., et al. Fast and slow dynamics of the cytoskeleton. Nature Materials. 5, 636-640 (2006).
- Oh, M. J., Kuhr, F., Byfield, F., Levitan, I. Micropipette Aspiration of Substrate-attached Cells to Estimate Cell Stiffness. J. Vis. Exp. (67), e3886 (2012).
- Hochmuth, R. M. Micropipette aspiration of living cells. Journal of Biomechanics. 33, 15-22 (2000).
- Levental, I., et al. A simple indentation device for measuring micrometer-scale tissue stiffness. J. Phys-Condens. Mat. 22, (2010).
- Mahaffy, R. E., Park, S., Gerde, E., Kas, J., Shih, C. K. Quantitative analysis of the viscoelastic properties of thin regions of fibroblasts using atomic force microscopy. Biophysical Journal. 86, 1777-1793 (2004).
- Radmacher, M. Measuring the elastic properties of biological samples with the AFM. IEEE Engineering in Medicine and Biology Magazine: The Quarterly Magazine of the Engineering in Medicine & Biology Society. 16, 47-57 (1997).
- Crocker, J. C., Hoffman, B. D. Multiple-particle tracking and two-point microrheology in cells. Methods in Cell Biology. 83, 141-178 (2007).
- Liu, F., Tschumperlin, D. J. Micro-mechanical characterization of lung tissue using atomic force microscopy. J. Vis. Exp. (54), e2911 (2011).
- Solon, J., Levental, I., Sengupta, K., Georges, P. C., Janmey, P. A. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophysical Journal. 93, 4453-4461 (2007).
- Wu, H. W., Kuhn, T., Moy, V. T. Mechanical properties of l929 cells measured by atomic force microscopy: Effects of anticytoskeletal drugs and membrane crosslinking. Scanning. 20, 389-397 (1998).
- Radmacher, M., Fritz, M., Kacher, C. M., Cleveland, J. P., Hansma, P. K. Measuring the viscoelastic properties of human platelets with the atomic force microscope. Biophysical Journal. 70, 556-567 (1996).
- Levy, R., Maaloum, M. Measuring the spring constant of atomic force microscope cantilevers: thermal fluctuations and other methods. Nanotechnology. 13, 33-37 (2002).
- Lin, D. C., Dimitriadis, E. K., Horkay, F. Robust strategies for automated AFM force curve analysis–I. Non-adhesive indentation of soft, inhomogeneous materials. Journal of Biomechanical Engineering. 129, 430-440 (2007).
- Davis, J. T., Wen, Q., Janmey, P. A., Otteson, D. C., Foster, W. J. Muller cell expression of genes implicated in proliferative vitreoretinopathy is influenced by substrate elastic modulus. Investigative Ophthalmology & Visual Science. 53, 3014-3019 (2012).
- Engler, A. J., Rehfeldt, F., Sen, S., Discher, D. E. Microtissue elasticity: Measurements by atomic force microscopy and its influence on cell differentiation. Method Cell Biol. 83, 521-545 (2007).
- Lele, T. P., et al. Tools to study cell mechanics and mechanotransduction. Methods in Cell Biology. 83 (07), 443-472 (2007).
- A-Hassan, E., et al. Relative microelastic mapping of living cells by atomic force microscopy. Biophysical Journal. 74, 1564-1578 (1998).
- Costa, K. D., Sim, A. J., Yin, F. C. Non-Hertzian approach to analyzing mechanical properties of endothelial cells probed by atomic force microscopy. Journal of Biomechanical Engineering. 128, 176-184 (2006).
- Xiong, Y., Lee, A. C., Suter, D. M., Lee, G. U. Topography and nanomechanics of live neuronal growth cones analyzed by atomic force microscopy. Biophysical Journal. 96, 5060-5072 (2009).
- Byfield, F. J., et al. Absence of filamin A prevents cells from responding to stiffness gradients on gels coated with collagen but not fibronectin. Biophysical Journal. 96, 5095-5102 (2009).
- Cross, S. E., et al. AFM-based analysis of human metastatic cancer cells. Nanotechnology. 19, 384003 (2008).
- Storm, C., Pastore, J. J., MacKintosh, F. C., Lubensky, T. C., Janmey, P. A. Nonlinear elasticity in biological gels. Nature. 435, 191-194 (2005).
- Wen, Q., Janmey, P. A. Polymer physics of the cytoskeleton. Current Opinion in Solid State & Materials Science. 15, 177-182 (2011).
- Dimitriadis, E. K., Horkay, F., Maresca, J., Kachar, B., Chadwick, R. S. Determination of elastic moduli of thin layers of soft material using the atomic force microscope. Biophysical Journal. 82 (02), 2798-2810 (2002).
- Trache, A., Lim, S. M. Live cell response to mechanical stimulation studied by integrated optical and atomic force microscopy. J. Vis. Exp. (44), e2072 (2010).
- Lim, S. M., Kreipe, B. A., Trzeciakowski, J., Dangott, L., Trache, A. Extracellular matrix effect on RhoA signaling modulation in vascular smooth muscle cells. Exp. Cell Res. 316, 2833-2848 (2010).

