Enteric Bacterial Invasion Of Intestinal Epithelial Cells In Vitro Is Dramatically Enhanced Using a Vertical Diffusion Chamber Model
Summary
Performing cell culture assays for investigating bacterial adhesion and invasion under aerobic conditions is usually unrepresentative of the in vivo environment. A Vertical Diffusion Chamber model allows study of interactions of the human pathogen Campylobacter jejuni with intestinal epithelial cells under more in vivo-like conditions, resulting in enhanced bacterial invasion.
Abstract
The interactions of bacterial pathogens with host cells have been investigated extensively using in vitro cell culture methods. However as such cell culture assays are performed under aerobic conditions, these in vitro models may not accurately represent the in vivo environment in which the host-pathogen interactions take place. We have developed an in vitro model of infection that permits the coculture of bacteria and host cells under different medium and gas conditions. The Vertical Diffusion Chamber (VDC) model mimics the conditions in the human intestine where bacteria will be under conditions of very low oxygen whilst tissue will be supplied with oxygen from the blood stream. Placing polarized intestinal epithelial cell (IEC) monolayers grown in Snapwell inserts into a VDC creates separate apical and basolateral compartments. The basolateral compartment is filled with cell culture medium, sealed and perfused with oxygen whilst the apical compartment is filled with broth, kept open and incubated under microaerobic conditions. Both Caco-2 and T84 IECs can be maintained in the VDC under these conditions without any apparent detrimental effects on cell survival or monolayer integrity. Coculturing experiments performed with different C. jejuni wild-type strains and different IEC lines in the VDC model with microaerobic conditions in the apical compartment reproducibly result in an increase in the number of interacting (almost 10-fold) and intracellular (almost 100-fold) bacteria compared to aerobic culture conditions1. The environment created in the VDC model more closely mimics the environment encountered by C. jejuni in the human intestine and highlights the importance of performing in vitro infection assays under conditions that more closely mimic the in vivo reality. We propose that use of the VDC model will allow new interpretations of the interactions between bacterial pathogens and host cells.
Introduction
The interactions of bacterial pathogens with host cells have been investigated extensively using in vitro cell culture methods. Using such cell culture assays, bacterial adhesion to host cells, identification of host cell receptors, host cell signaling pathways and bacterial invasion of host cells have all been studied in detail, resulting in many important observations. However such cell culture assays are performed under aerobic conditions that may not be representative of the in vivo environment. A major limitation of in vitro models used to study gastrointestinal infections is that culture conditions including high oxygen levels generally favor eukaryotic cell survival. However conditions in the intestinal lumen will be almost anaerobic. Enteric pathogens in a very low oxygen environment express virulence genes whose expression changes under aerobic conditions2. As such, data obtained using standard cell culture models may give an inaccurate indication of bacterial interactions with host cells.
Campylobacter jejuni is the leading causative agent of bacterial acute gastroenteritis worldwide, with symptoms that range from mild diarrhea to severe inflammatory enteritis. The majority of C. jejuni infections result in uncomplicated gastroenteritis, however C. jejuni is also the most commonly identified infectious agent in peripheral neuropathies including Guillain-Barré syndrome (GBS). In the UK, it is estimated that there are over 500,000 cases of enteritis caused by C. jejuni infection each year with a predicted cost to the UK economy of £580 million. In the developing world, C. jejuni is a leading cause of mortality among children. Despite the undoubted importance of Campylobacter infection and decades of research, including thorough genomics-based analysis, C. jejuni pathogenesis is still poorly understood, in contrast to other enteric pathogens such as Salmonella, Escherichia coli, Shigella, and Vibrio cholerae. The lack of a convenient small animal model is one major reason for this3. Also the widely used in vitro infection models are more inappropriate for studying microaerophilic C. jejuni than for other enteric pathogens that are facultative anaerobes. Whilst C. jejuni is recognized as an invasive pathogen, the mechanisms of C. jejuni invasion of intestinal epithelial cells (IECs) are still unclear4,5. C. jejuni invasion has been shown to be dependent on either microfilaments, microtubules, a combination of both or neither5. The confusion in this area is most probably due to the use of inappropriate in vitro assay conditions.
A number of different cell culture assays have been used to investigate the interactions of C. jejuni with host cells. Caco-26, INT 4077, and T848 cell lines have all been used to study the adhesion and invasion capabilities of different C. jejuni strains. However, the levels of bacterial adhesion and invasion for C. jejuni with IECs are dramatically lower than for other enteric pathogens9. The coculturing of C. jejuni with IECs is normally performed in a CO2 incubator under conditions close to atmospheric oxygen levels, required for the survival of IECs. C. jejuni gene expression will be very different in the low oxygen environment of the intestinal lumen compared to atmospheric oxygen conditions.
The use of a Vertical Diffusion Chamber (VDC) system has been developed which permits the coculture of bacteria and host cells under different medium and gas conditions1,10,11. This system mimics the conditions in the human intestine where bacteria will be under conditions of very low oxygen whilst tissue will be supplied with oxygen from the blood stream. Polarized IEC monolayers grown in special 0.4 μm filters were placed into a VDC creating an apical and basolateral compartment, which were individually filled with bacterial broth and cell culture medium respectively (Figure 1). The VDC was placed into the variable atmosphere incubator containing 85% N2, 5% O2, and 10% CO2 at 37 °C, representing optimum conditions for C. jejuni. The apical compartment was left open and exposed to the microaerobic atmosphere within the variable atmosphere incubator, whilst the sealed basolateral compartment was supplied with oxygen by constant administration of 5% CO2/95% O2 gas mixture with an outlet tube preventing accumulation of pressure. Caco-2 cell survival and monolayer integrity under these conditions were confirmed by monitoring the transepithelial electrical resistance (TEER) across the monolayer over 24 hr to demonstrate survival of the Caco-2 cell monolayer and physical separation of the apical and basolateral compartments under low oxygen conditions in the apical compartment. The TEER of monolayers in VDCs maintained in the variable atmosphere incubator (microaerobic conditions) and in a standard cell culture CO2 incubator (aerobic conditions) showed no significant differences, indicating integrity of the cell monolayer under different atmospheric conditions1. Under microaerobic conditions, tight junctions remained present and evenly distributed between the cell borders with an occludin staining pattern similar to cells maintained under aerobic conditions1.
The interactions of C. jejuni with Caco-2 and T84 cells in the VDC were investigated by assessing bacterial interaction (adhesion and invasion) and invasion. Two different C. jejuni wild-type strains were used1. C. jejuni 11168H is a hypermotile derivative of the original sequence strain NCTC11168. The 11168H strain shows much higher colonization levels in a chick colonization model and is thus considered a suitable strain to use for host-pathogen interaction studies. 81-176 is a human isolate and is one of the most invasive widely studied laboratory strains. C. jejuni strains were added to the apical compartment of a VDC under either microaerobic or aerobic conditions. We observed higher rates for both interaction and invasion were recorded for C. jejuni under microaerobic conditions1. The increased C. jejuni interactions were not due to an increase in bacterial numbers under microaerobic conditions1. This data supports our hypothesis that the low oxygen environment in the apical compartment of the VDC enhances bacteria-host interactions and indicates that the invasive properties of C. jejuni are increased under these conditions. This was the first report of the use of the VDC model to study an invasive bacterial pathogen and highlights the significance of performing in vitro infection assays under conditions that more closely mimic the in vivo situation. The VDC model could be used to study the host-pathogen interactions for many different bacterial species.
Protocol
1. Growth of IEC Monolayers on Special 0.4 μm Filters
- Culture Caco-2 cells in Dulbecco's modified essential media (DMEM) supplemented with 10% (v/v) fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin and 1% (v/v) nonessential amino acids in a standard tissue culture incubator at 37 °C in 5% CO2 and 95% air. Seed 4 x 105 Caco-2 IECs per 0.4 μm filter and grow to polarization over 21 days, changing the media every 2-3 days.
- Measure the TEER to confirm polarization status of the monolayer using a Volt-Ohm resistance meter. In our studies, the TEER of 21 day Caco-2 cells grown in these 0.4 μm filters is 700–800 Ωcm2.
2. Preparation of C. jejuni and the Bacterial Medium for the Coculturing Assay
- 24 hr prior to desired starting point of the VDC coculturing assay, prepare a fresh blood agar plate of C. jejuni and incubate at 37 °C under microaerobic conditions (85% N2, 5% O2, and 10% CO2) in the variable atmosphere incubator.
- At the same time, preincubate 30 ml of brucella broth at 37 °C under microaerobic conditions in a variable atmosphere incubator.
3. Preparation and Sterilization of the VDC Half Chambers Prior to the Assay
- Completely immerse VDC half chambers as well as the O-rings and the plugs in the sterilization solution.
- Using a sterile 20 ml syringe, flush the sterilization solution through the gas inlets in both half chambers. Failure to do so may result in contamination of the samples during coculturing.
- Leave all components immersed in the sterilization solution for >1 hr.
- Rinse all components with fresh sterile water, using another sterile 20 ml syringe to flush the gas inlets.
4. Preparation of the Bacterial Inoculum
- Harvest half a plate of C. jejuni growth from the blood agar plate prepared the previous day into 1 ml of the preincubated brucella broth. This should yield ~1010 cfu/ml.
- Measure the optical density of the bacterial suspension at 600 nm to semiquantify bacterial colony forming units (CFUs).
- Adjust the bacterial suspension to the desired inoculum level in 4 ml of the preincubated brucella broth.
- Perform a serial dilution from this final bacterial suspension followed by plating of the appropriate dilutions on blood agar plates in triplicate, then incubate at 37 °C in the variable atmosphere incubator for 48 hr to quantify the number of bacterial CFUs present in the inoculum.
5. Setting up the VDC
- Place the lower half chamber of a VDC flat onto the bench. Fit an O-ring onto the lower half chamber.
- Detach one filter carrying the IECs from the carrier and wash three times with 400 μl of sterile PBS. Place the filter onto the lower half chamber, making sure the O-ring remains in place.
- Gently lower the upper half chamber into place. Once the two half-chambers are assembled, clamp together using the ring-clamps. Place the VDC horizontally on the bench top, with the two openings facing upwards.
- Add the 4 ml of bacterial inoculum into the apical half chamber.
- Add 4 ml of cell culture medium into the basolateral half chamber.
- Place the end pieces into the openings on both half chambers.
6. Attaching the VDC to the Gas Manifold within the Variable Atmosphere Incubator
- Place the VDC into the variable atmosphere incubator in close proximity to the gas manifold.
- Open the gas supply regulator connected to the gas manifold. Attach the tube from the manifold into the gas inlet on the basolateral half chamber of the VDC. Attach the gas outlet pipe leading out of the variable atmosphere incubator to one of the outlets in the end-piece on the basolateral half chamber.
- Close off the other outlet in the end-piece on the basolateral half chamber. Open the gas flow on the gas manifold, taking care to open it very slowly to avoid excess gas pressure, as this can lead to expulsion of the basolateral medium.
- The aim is a gas flow of 1 bubble every 2-5 sec. Periodically check on the gas flow during the course of the experiment and adjust if necessary.
7. Disassembling the VDC after Coculture
- After the appropriate coculture period, close the gas supply regulator connected to the gas manifold. Close off the gas flow into the VDC at the manifold. Disconnect the gas inlet, the gas outlet and the stopper from the VDC.
- Remove the VDC from a variable atmosphere incubator. Remove the apical and basolateral supernatants and store at -80 °C for subsequent analysis.
- Remove the ring clamps and gently take apart the VDC. Remove the filter from the half chamber and transfer to a sterile 6-well cell culture dish. Wash the IECs three times with 400 μl of sterile PBS.
- For enumeration of the total number of interacting bacteria, add 400 μl of sterile PBS containing 0.1% (v/v) Triton X-100 to the IECs and incubate for 20 min at room temperature to lyse the IEC. Perform a serial dilution from this cell lysate followed by plating of the appropriate dilutions on blood agar plates in triplicate, then incubate at 37 °C in the variable atmosphere incubator for 48 hr to quantify the number of interacting bacterial CFUs.
- For enumeration of the total number of invading bacteria, add 400 μl of DMEM cell culture medium containing 150 μg/ml gentamicin to the IECs and incubate for 2 hr in a standard tissue culture incubator at 37 °C in 5% CO2 and 95% air to kill extracellular bacteria, then follow as for step 7.4 above.
8. Cleaning the VDC after Coculture
Wash the VDC with sterile water and store for the next round of experiments.
Representative Results
Coculturing experiments performed with a C. jejuni wild-type strain and IECs in the VDC model with microaerobic conditions in the apical compartment have demonstrated an increase in the number of interacting (almost 10-fold) and intracellular (almost 100-fold) bacteria compared to aerobic culture conditions in a time-dependent manner1. This observation was reproducible using two different C. jejuni wild-type strains (11168H and 81-176) and two different IEC lines (Caco-2 and T84), highlighting the validity of the VDC model1.
The representative results presented here are a comparison between the number of C. jejuni interacting with or invading Caco-2 cells after 3, 6, or 24 hr of coculture under either standard cell culture assay conditions in a CO2 incubator (Figures 2A and B) or in the VDC model with microaerobic conditions in the apical compartment (Figures 2C and D). Data for two different C. jejuni wild-type strains (11168H and 81-176) are presented. Under the standard cell culture conditions used in the vast majority of studies in the scientific literature, <107 cfu were observed interacting with Caco-2 cells (Figure 2A) compared to ~108 cfu observed interacting with Caco-2 cells in the VDC model (Figure 2C) after 24 hr. After coculture in the VDC model, ~107 intracellular cfu were isolated from Caco-2 cells (Figure 2D), compared to only ~105 intracellular cfu isolated from Caco-2 cells (Figure 2B) after coculture under standard cell culture conditions for 24 hr.
A direct comparison between the number of C. jejuni interacting with or invading IECs after coculture in the VDC model with either microaerobic or aerobic conditions in the apical compartment has been performed previously1. Use of the VDC model results in an increase in interacting C. jejuni of almost 10-fold and an increase in intracellular C. jejuni of almost 100-fold after 24 hr coculture1.
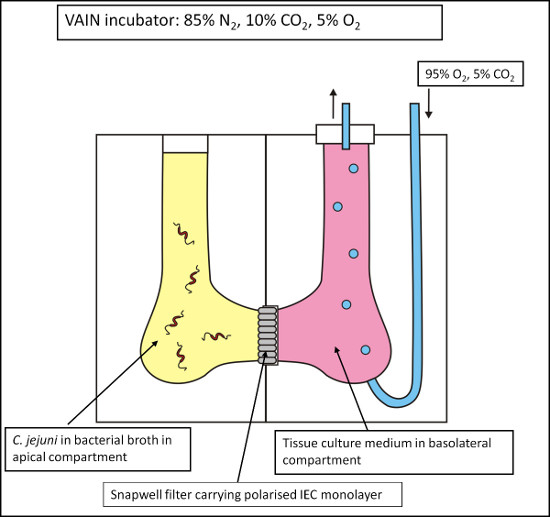
Figure 1. Schematic of the Vertical Diffusion Chamber (VDC) model. Intestinal epithelial cells (IECs) are grown on permeable 0.4 μm filter supports and inserted into a VDC, thus creating an apical and a basolateral compartment. These compartments can then be individually adjusted with respect to medium and gas composition to allow the coculturing of the IECs with C. jejuni with microaerobic conditions at the apical surface of the IECs and aerobic conditions at the basolateral surface of the IECs.
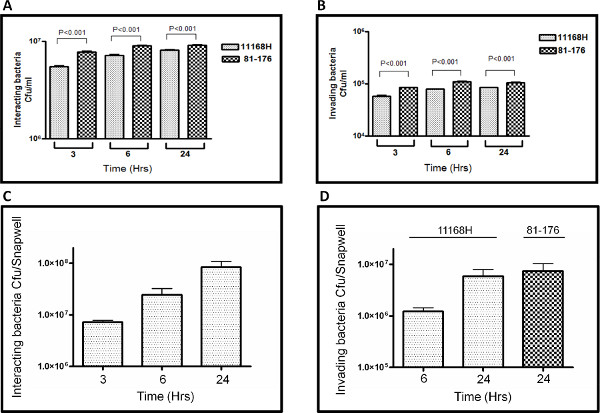
Figure 2. Representative results comparing numbers of C. jejuni 11168H or 81-176 wild-type strains interacting with (A and C) or invading (B and D) Caco-2 cells after 3, 6 or 24 hr when the coculture assay was performed under either standard cell culture conditions in a CO2 incubator (A and B) or in the VDC model with microaerobic conditions in the apical compartment (C and D). All experiments represent at least three biological replicates performed in duplicate in each experiment. Click here to view larger figure.
Discussion
The use of in vitro cell culture methods to study the interactions of bacterial pathogens with host cells is a technique widely employed in many research laboratories. However as such cell culture assays are performed under aerobic conditions, these in vitro models may not accurately represent the in vivo environment in which the host-pathogen interactions take place. The widely used in vitro infection models are especially inappropriate for studying microaerophilic C. jejuni compared to other enteric pathogens that are facultative anaerobes. There is considerable confusion in the literature regarding the mechanisms of invasion of human IECs by C. jejuni4,5. We suggest that the reason the mechanisms of invasion are so poorly understood is because the in vitro models used in these studies do not accurately reflect the in vivo situation. Using standard cell culture assays, the level of C. jejuni invasion of IECs is always very low9. The development of the VDC model was our response to address this issue.
We have established that two different IEC lines can be maintained in the VDC with microaerobic conditions at the apical surface without any observed disadvantageous effects over a 24 hr period1. A time-dependent increase of the numbers of both interacting and intracellular C. jejuni 11168H wild-type strain bacteria was observed after coculturing with Caco-2 IECs in the VDC with microaerobic conditions in the apical compartment1. These observations were further confirmed using a second IEC line (T84) as well as a second C. jejuni wild-type strain (81-176), indicating no cell line or bacterial strain specific effects1. These increased levels of bacterial interaction and invasion resulted in an increased, polarized innate immune response from the IECs1. Higher levels of IL-8 were detected after microaerobic coculturing, indicating that the increased bacterial challenge is matched by an increased host response. Higher levels of IL-8 were detected in the basolateral supernatants compared to the apical supernatants, indicating that the IL-8 secretion by the IECs occurs predominantly from the basolateral IEC surface. IL-8 is as a neutrophil attractant, which would be of limited use in the intestinal lumen. This data demonstrates that the two-compartmental setup of the VDC also allows for efficient identification of a polarized host response.
Several features regarding the technical/setup side of the VDC model must be considered prior to applying the model to study any pathogenic organism. Firstly, the IECs must be grown to form an impermeable monolayer on special 0.4 μm filters. This takes between 14-21 days depending on the cell line used and makes the experiments fairly lengthy compared to classical coculturing experiments performed in cell culture plates and using IECs grown for between 1-7 days. On the other hand, only after such a long period of growth have the IECs been demonstrated to form a fully polarized monolayer. Such polarized monolayers mimic the situation in vivo in the human intestinal epithelium much more closely than the nonpolarized, nonconfluent IEC lines used in some studies and as such provide another advantage of the VDC model. Secondly, the special 0.4 μm filters are significantly more expensive than a standard 24-well cell culture plate. The main limitation of the VDC is the relatively low throughput. This is mainly due to the availability of VDC chambers and the setup requiring the gas manifold. Only relatively low numbers of parallel replicates can be performed at one time, in contrast to the classical cell culture method. The VDC model is therefore less suitable for large-scale screening experiments or experiments requiring a high number of replicates. Another factor to consider is that the gravity effect of performing coculture assays in the VDC model means that over time bacteria will start to aggregate at the bottom of the apical compartment resulting in reduced opportunity for interactions with the IEC monolayer. However using the VDC model, we have shown that the interactions of a nonmotile, nonaggregating 11168H rpoN mutant are dramatically reduced compared to the motile, aggregating 11168H wild-type strain after 6 hr1. We are currently working on modifications to the VDC model that would allow some mixing in the apical compartment that would more closely mimic peristalsis in the gut lumen. Therefore, whilst the VDC model is better than the classical, aerobic cell culture methods due to more closely mimicking the in vivo situation, especially for bacteria such as C. jejuni that have a stringent atmospheric requirements, the VDC model still has certain limitations that need to be taken into account when designing experiments.
Our data supports the findings reported for a similar VDC model used to study the human gastric pathogen Helicobacter pylori, which showed increased bacterial adhesion to host cells, increased synthesis of bacterial virulence factors as well as an increased host innate immune response when H. pylori was cocultured with epithelial cells under microaerobic compared to aerobic conditions11. As both H. pylori and C. jejuni require microaerobic conditions for growth, the finding that microaerobic conditions promote the interaction of the organisms with host cells is unsurprising. However, another study using a VDC system to analyze the interactions of the facultative anaerobe enterohemorrhagic Escherichia coli with IECs demonstrated increased levels of bacterial interaction under anaerobic/microaerobic coculture conditions in the VDC apical compartment10. This indicates that the behavior of bacteria that are capable of proliferating under atmospheric oxygen conditions is also changed when cocultured with IECs under anaerobic/microaerobic conditions. This suggests that the VDC model is an improved and valuable model for the analysis of the host-pathogen interactions of many different pathogenic bacteria under conditions that more faithfully resemble the in vivo situation in the human intestinal lumen.
From the model perspective, the VDC model has proved to possess some clear advantages over aerobic in vitro C. jejuni-IEC coculturing models. The environment created in the VDC model more closely mimics the environment in the human intestine during C. jejuni infection, leading to a highly significant increase in numbers of bacteria interacting with and invading the IECs. The VDC model in this current format is ideal to study the interactions of enteric bacteria with gastric or intestinal epithelial cells, but there is the potential to adapt the VDC model for the study of strict anaerobes from stool samples or for oral microbiological samples, as just two examples. The important principle should always to be to aim to perform these coculture experiments under conditions that more closely mimic the in vivo situation. The VDC model will be an important tool for further studies of C. jejuni host-pathogen interactions and should be applicable to the study of the pathogenic mechanisms of many different bacteria.
Divulgations
The authors have nothing to disclose.
Acknowledgements
Dominic Mills was supported by a Bloomsbury Colleges PhD Studentship (2007-2010). The authors would like to thank both Ozan Gundogdu and Abdi Elmi for their assistance in developing the VDC model.
Materials
| Material Name | Company | Catalogue Number | Comments (optional) |
| Speciality vertical diffusion system for use with Snapwell inserts | Harvard Apparatus | 66-0001 | Manifold & six Snapwell chambers |
| Caps | Harvard Apparatus | 66-0020 | |
| O-rings | Harvard Apparatus | 66-0007 | |
| Clamps | Harvard Apparatus | 66-0012 | |
| Snapwell filters (pore size 0.4 μm) | Corning Costar | 3407 | |
| Millicell ERS-2 Volt-Ohm resistance meter | Millipore | MERS00002 | |
| WPA Lightwave II spectrophotometer | Biochrom | 80-3003-72 |
References
- Mills, D. C., et al. Increase in Campylobacter jejuni invasion of intestinal epithelial cells under low-oxygen coculture conditions that reflect the in vivo environment. Infect. Immun. 80, 1690-1698 (2012).
- Marteyn, B., et al. Modulation of Shigella virulence in response to available oxygen in vivo. Nature. 465, 355-358 (2010).
- Dorrell, N., Wren, B. W. The second century of Campylobacter research: recent advances, new opportunities and old problems. Curr. Opin. Infect. Dis. 20, 514-518 (2007).
- Young, K. T., Davis, L. M., Dirita, V. J. Campylobacter jejuni: molecular biology and pathogenesis. Nat. Rev. Microbiol. 5, 665-679 (2007).
- Hu, L., Kopecko, D. J., Nachamkin, I., Szymanski, C. M., Blaser, M. J. Chapter 17. Campylobacter. , 297-313 (2008).
- Everest, P. H., et al. Differentiated Caco-2 cells as a model for enteric invasion by Campylobacter jejuni and C. coli. J. Med. Microbiol. 37, 319-325 (1992).
- Konkel, M. E., Hayes, S. F., Joens, L. A., Cieplak, W. Characteristics of the internalization and intracellular survival of Campylobacter jejuni in human epithelial cell cultures. Microb. Pathog. 13, 357-370 (1992).
- Monteville, M. R., Konkel, M. E. Fibronectin-facilitated invasion of T84 eukaryotic cells by Campylobacter jejuni occurs preferentially at the basolateral cell surface. Infect. Immun. 70, 6665-6671 (2002).
- Friis, L. M., Pin, C., Pearson, B. M., Wells, J. M. In vitro cell culture methods for investigating Campylobacter invasion mechanisms. J. Microbiol. Methods. 61, 145-160 (2005).
- Schuller, S., Phillips, A. D. Microaerobic conditions enhance type III secretion and adherence of enterohaemorrhagic Escherichia coli to polarized human intestinal epithelial cells. Environ. Microbiol. 12, 2426-2435 (2010).
- Cottet, S., Corthesy-Theulaz, I., Spertini, F., Corthesy, B. Microaerophilic conditions permit to mimic in vitro events occurring during in vivo Helicobacter pylori infection and to identify Rho/Ras-associated proteins in cellular signaling. J. Biol. Chem. 277, 33978-33986 (2002).

