Spatial Separation of Molecular Conformers and Clusters
Summary
We present a technique that allows the spatial separation of different conformers or clusters present in a molecular beam. An electrostatic deflector is used to separate species by their mass-to-dipole moment ratio, leading to the production of gas-phase ensembles of a single conformer or cluster stoichiometry.
Abstract
Gas-phase molecular physics and physical chemistry experiments commonly use supersonic expansions through pulsed valves for the production of cold molecular beams. However, these beams often contain multiple conformers and clusters, even at low rotational temperatures. We present an experimental methodology that allows the spatial separation of these constituent parts of a molecular beam expansion. Using an electric deflector the beam is separated by its mass-to-dipole moment ratio, analogous to a bender or an electric sector mass spectrometer spatially dispersing charged molecules on the basis of their mass-to-charge ratio. This deflector exploits the Stark effect in an inhomogeneous electric field and allows the separation of individual species of polar neutral molecules and clusters. It furthermore allows the selection of the coldest part of a molecular beam, as low-energy rotational quantum states generally experience the largest deflection. Different structural isomers (conformers) of a species can be separated due to the different arrangement of functional groups, which leads to distinct dipole moments. These are exploited by the electrostatic deflector for the production of a conformationally pure sample from a molecular beam. Similarly, specific cluster stoichiometries can be selected, as the mass and dipole moment of a given cluster depends on the degree of solvation around the parent molecule. This allows experiments on specific cluster sizes and structures, enabling the systematic study of solvation of neutral molecules.
Introduction
Modern gas-phase molecular physics and physical chemistry experiments often use supersonic expansions of target molecules to produce rotationally cold molecular samples within a molecular beam. However, even at low rotational temperatures of 1 K, which can routinely be achieved using supersonic expansions, large molecules can still remain in multiple conformations within the beam1. Similarly the production of molecular clusters in a beam source does not result in a single species, but rather in the formation of a "cluster soup", containing many different cluster stoichiometries, as well as remaining pure parent molecules. This makes the study of these systems with novel techniques such as imaging of molecular orbitals2, molecular-frame photoelectron angular distributions3-5 or electron6-10 and X-ray diffraction11-13 difficult, as these require pure, consistent, and homogenous samples in the gas-phase.
While several methodologies are now available to separate different conformers of charged species in the gas-phase (e.g. ion mobility drift tubes14,15) and charged clusters are easily separated by their mass-to-charge ratio, these techniques are not applicable to neutral species. We have recently demonstrated that these issues can be overcome with the use of an electrostatic deflection device16,17, allowing the separation of molecular conformers as well as clusters and the production of rotationally cold molecular beams.
The use of electrostatic deflection is a classic molecular beam technique, the origins of which go a long way back18,19. First ideas of utilizing electrostatic deflection for the separation of quantum states were introduced by Stern in 192620. While early experiments were conducted on small molecules at high temperatures, we demonstrate the application of this technique to large polar molecules and clusters at low temperatures16,21.
Polar molecules experience a force inside an inhomogenous electric field (E) due to the spatial differences in potential energy. This force is dependent on the effective dipole moment, μeff, of the molecule and can be evaluated as
is dependent on the effective dipole moment, μeff, of the molecule and can be evaluated as
 (1)
(1)
As different molecular conformers typically posses different dipole moments and differing numbers of solvent molecules within a cluster lead to different cluster masses and dipole moments, these species will experience a different acceleration in the presence of a strong inhomogeneous electric field. The resulting Stark effect force from an inhomogeneous electric field can therefore be used for the separation of conformers and quantum states22. This is indicated in Figure 1, showing the calculated Stark curves for the J = 0,1,2 rotational states of the cis and trans conformers of 3-fluorophenol, respectively. This leads to large differences in μeff, as shown in Figures 1c and 1d, and hence a different acceleration is experienced by the two conformers in inhomogeneous electric fields. Therefore, an electrostatic deflection device can be used as a mass-to-dipole moment ratio (m/μeff) separator, in analogy to a mass spectrometer acting as a mass-to-charge ratio (m/z) filter23.
Furthermore, these techniques allow the separation of rotational quantum states24,25. As the ground rotational states (blue curves in Figures 1a and 1b) exhibit the largest Stark shift, these will be deflected most and can be spatially separated from molecules in higher J states17. The coldest part of a molecular beam can therefore be selected, significantly aiding in many applications, such as alignment and orientation of target molecules17, 26-28.
In this contribution we show how an electrostatic deflection device can be used to spatially separate different species of large polar molecules and clusters. Example data is presented for the production of a pure beam of an individual conformer and of a solute-solvent cluster of well-defined size and ratio. Specifically we present data on 3-fluorophenol, where a pure beam containing only the trans conformer is produced, and on indole-water clusters, where the indole(H2O)1 cluster can be spatially separated from water, indole, indole(H2O)2 , etc.
Protocol
1. Description of the Experimental Setup
A schematic of the gas-phase molecular beam setup and deflector is shown in Figure 221. It consists of
- A pulsed Even-Lavie valve29 containing the molecular sample. Other pulsed molecular beam valves can be used equally well as long as a cold molecular beam (O(1 K)) is formed. The following parameters are specific for the employed Even-Lavie valve. In the experiments presented here the valve is operated at 20 Hz repetition rate with high backing pressures (helium at ~50 bar) and expanded into a vacuum chamber evacuated to <10-6 mbar.
- A molecular beam skimmer (2 mm diameter) is placed 22 cm downstream from the valve, collimating the molecular beam and leading to differential pumping conditions between the pulsed valve and the rest of the vacuum system.
- Immediately after the skimmer the molecules enter the electrostatic deflection device. This consists of a rod (radius 3.0 mm) and a trough (radius of curvature 3.2 mm), each 24 cm long. The vertical gap between the electrodes in the center of the device is 2.3 mm. A potential difference between 0-26 kV is applied between the rod and trough, producing a strong inhomogeneous electric field with a nearly constant field gradient30, as indicated in the inset of Figure 2.
- Directly after the deflector molecules enter the interaction region through a second skimmer, providing a further differential pumping stage.
- The interaction region (evacuated to pressures <10-9 mbar) contains a standard Wiley-McLaren time-of-flight (TOF) setup. Molecules are ionized by focused laser pulses in the center of the extraction region, between the repeller and extractor electrodes. Produced ions are accelerated towards a multichannel plate (MCP) detector, where a mass spectrum is recorded.
- Laser pulses are derived from an Nd:YAG pumped dye laser, providing typical output wavelengths around 283 nm (indole experiments) or 272 nm (3-fluorophenol experiments) and pulse energies of a few mJ. Pulse durations are on the order of 10 nsec and pulses are focused with a f = 750 mm lens to a spot size of ~100 μm in the interaction region.
- The timing sequence is controlled by a digital delay generator providing the master clock. This triggers the Nd:YAG laser (flash lamps and Q-switch), the pulsed valve, and the digitizer card used to record mass spectra.
- Mass spectra are recorded on a digitizer card, triggered at the same time as the laser Q-switch. Molecular beam densities are extracted from appropriate mass gates in the recorded time-of-flight spectra.
2. Production and Characterization of a Conformer Selected Molecular Beam
- A cold molecular beam of the target molecules is created via supersonic expansion and characterized using spatial (x, y directions) and temporal (z direction) profiling.
- Load the sample reservoir of the pulsed valve with the chemical sample. Dissolve solid samples in an appropriate solvent and place a few drops on a small piece of filter paper which is inserted into the sample cartridge. Place liquid samples directly on the filter paper.
- Produce the supersonic expansion, using a high-purity high-pressure backing gas. Adjust the temperature of the sample reservoir within the valve such that the partial pressure of the sample is approximately 10 mbar.
Note: For liquid samples typically no heating is necessary. The valve opening time depends on the exact model of pulsed valve used, for the experiments presented here the Even-Lavie valve is operated with an electric pulse duration of 10 μsec. - Characterize the produced molecular beam with the electrostatic deflector turned off. Set the ionization laser to a known wavelength for resonance-enhanced multiphoton ionization (REMPI) of a particular conformer of the sample. Record a temporal profile of the molecular beam pulse by monitoring the total parent ion yield on the MCP detector as a function of valve-laser delay.
- Fix the valve-laser delay at the position of maximum intensity for all subsequent measurements.
- Record a transverse spatial profile of the molecular beam by monitoring the total parent ion yield as a function of the y position of the laser focus. Do this by moving the focusing lens perpendicular to the laser propagation direction, such that the focus moves in the y direction relative to the molecular beam.
- Repeat the temporal and spatial profiling for all conformers of interest in the beam.
Note: These typically have distinct REMPI resonances, such that each conformer can be probed separately. In the absence of a deflection field however, the temporal and spatial profiles are identical for all conformers. - Characterization of the deflected beam. Turn on the high voltage supply to the deflector and record spatial profiles for all isomers. These should now be deflected according to mass-to-dipole moment ratio.
Note: For species undergoing large deflections it may be necessary to move the skimmer immediately following the deflector to ensure good transmission of the deflected beam into the detection region. - Conduct experiments on the conformer or size-selected part of the molecular beam by ensuring the interaction (e.g. a crossing laser beam) takes place within the part of the molecular beam containing only the species of interest.
Representative Results
The electrostatic deflection technique has been successfully applied to the separation of structural isomers16 and neutral clusters21, as well as the production of rotational quantum state selected molecular samples31. We demonstrate this with representative results for the separation of cis and trans conformers of 3-fluorophenol, and size selected indole(H2O)n clusters.
3-Fluorophenol conformers were separated in a molecular beam from the supersonic expansion of 50 bars of helium. The individual species were probed via their distinctive REMPI resonances around 272 nm32. Due to its significantly larger dipole moment (see Figure 1), the trans conformer experiences a larger deflection following passage through the deflector and is spatially separated from the cis conformer and the carrier gas of the beam.
To characterize the molecular beam formed during supersonic expansion, a temporal profile is collected with the electrostatic deflector turned off, as shown in Figure 3. For comparison, a temporal profile of a neon seeded beam is also shown. For helium carrier gas we observe a temporal width of approximately 12 μsec full-width at half maximum (FWHM), typical for an expansion from an Even-Lavie valve under these operating conditions.
The spatial distribution of the molecular beam is monitored by translation of the REMPI laser relative to the molecular beam direction, and spatial profiles are shown in Figure 4. This shows the spatial extent of the cis (red trace) and trans (blue trace) conformers at two different deflection fields, created by applying a potential difference of 14 kV or 28 kV across the deflector. For comparison field-free profiles are shown in both plots by the magenta (cis) and cyan (trans) curves. These yield a spatial width of the molecular beam of about 2 mm and show that, without the deflector, both species are mixed within the beam. In the presence of a deflection field the trans conformer undergoes a significantly larger deflection than the cis conformer and can effectively be separated from the other species present in the beam, such that at a position of y = 3 mm a pure trans sample is created and can be utilized for further experiments.
Cluster separation is demonstrated by supersonic expansion of indole in a "wet" carrier beam of helium containing trace amounts of water, leading to the formation of molecular clusters of the type indolem(H2O)n. According to the literature and to ab initio calculations, the indole(H2O)1 cluster has a significantly larger dipole moment (4.4 D) than pure indole (1.96 D), water (1.86 D) or the indole(H2O)2 cluster, and should, therefore, be deflected the most21,33. All indole containing species can be selectively probed via REMPI around 283 nm24,35, utilizing the lowest energy allowed electronic-excitation transition of indole. As this resonant excitation step involves different frequencies depending on the solvation of indole, the detection is fully species selective. Spatial profiles of the molecular beam are shown in Figure 5, these have been recorded with a potential difference of 26 kV between the rod and trough electrode and are fully species selective for indole (blue), indole(H2O)1 (red) and indole(H2O)2 (green). The lines indicate simulated values; details of numerical simulations methods can be found in the literature17,21. For comparison a field-free (deflector grounded) spatial profile is shown by the black curve. As expected the 1:1 cluster of indole and water experiences the largest deflection and at a position of y = 2-3 mm a pure beam of indole(H2O)1 is created. To highlight the effect of the deflector on the spatial molecular beam profile, the inset in Figure 5 shows the molecular beam density of indole(H2O)1 as a function of potential difference applied across the deflector. It indicates that as the field strength is increased, the coldest part of the molecular beam experiences an increasing deflection, while the warmer constituents experience a significantly smaller spatial separation and some density remains at the original position. This furthermore highlights the selection of the coldest part of the molecular beam.
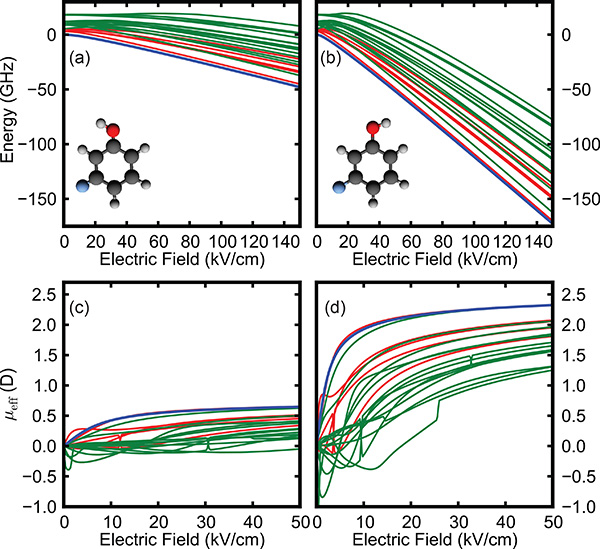
Figure 1. Calculated Stark energies E (top) and effective dipole moments μeff (bottom) for the cis and trans conformers of 3-fluorophenol. The blue line corresponds to the J = 0 rotational ground state, the red lines to J = 1 and green to J = 2 states. The deflection experienced is proportional to μeff/m (Equation 1). Hence, lower rotational quantum states, which exhibit larger μeff, experience a larger deflection and, therefore, can be separated. Equally, the significantly larger μeff for the trans conformer leads to a greater spatial deflection following passage through the electrostatic deflector.
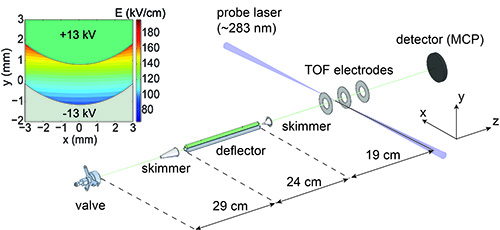
Figure 2. The experimental setup, consisting of a pulsed valve creating a supersonic expansion of target molecules, an electrostatic deflector and a detection region with time-of-flight mass spectrometer. The inset shows the inhomogeneous electric field created inside the deflector for voltages of ±13 kV applied to the rod and trough, respectively.
Click here to view larger image.
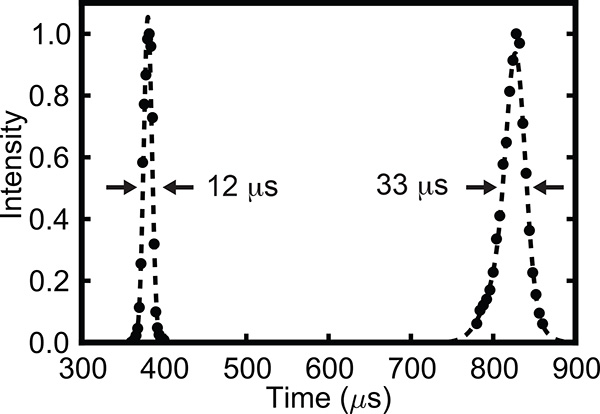
Figure 3. Temporal profile of the molecular beam for helium (at 380 μsec) and neon (at 826 μsec) carrier gas. The temporal width of the beam is approximately 3% and 4% of the total flight time for helium and neon, respectively.
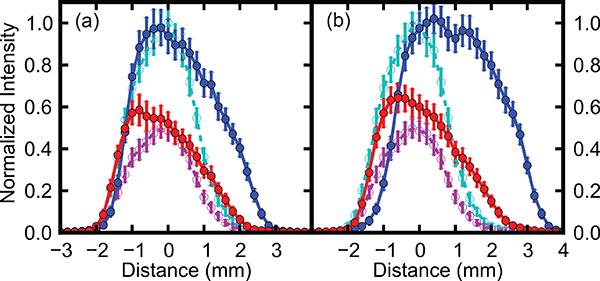
Figure 4. Spatial profiles of the molecular beam containing 3-fluorophenol, probed selectively for cis (red) and trans (blue) conformers, with the deflector at potential differences of (a) 14 kV and (b) 28 kV. For comparison the field free profile (deflector at 0 kV) is shown in both plots by magenta and cyan traces (cis and trans respectively).
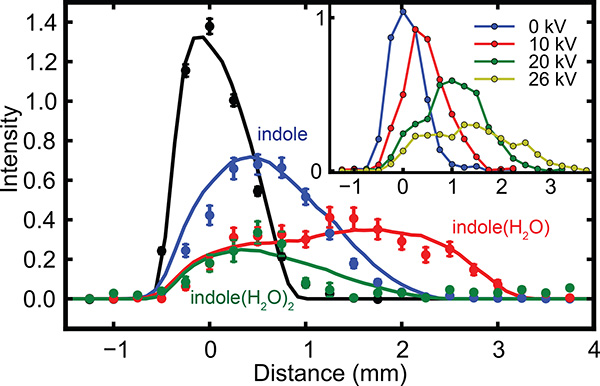
Figure 5. Spatial profiles of indole (blue), indole(H2O)1 (red) and indole(H2O)2 (green) for a deflector potential of 28 kV. Shown for comparison is the field-free profile of indole (black). Solid lines in the main panel indicate simulations. Shown inset are the measured spatial profiles for indole(H2O)1 at various potential differences applied across the deflector.
Discussion
Throughout this manuscript, familiarity with ultra-high vacuum components, pulsed molecular beam valves and laser sources is assumed and the associated safety procedures should always be adhered to. Special care needs to be taken when handling the high voltage electrodes for the deflector. Their surfaces need to be polished to a high standard and must be absolutely clean to avoid arcing inside the vacuum chamber. Before first use the electrodes should be conditioned under vacuum. The voltage applied is slowly increased and the current through the electrodes measured. The electrodes should draw no current (on the order of a few nA at most), independent of the applied voltage. A typical conditioning schedule for stepwise increasing the applied voltage is the following: 0-6 kV in 3 kV steps, 6-10 kV in 1 kV steps, 10-15 kV in 0.5 kV steps. At each step the voltage should be left constant for at least 15 min and the current monitored.
Of crucial importance when setting up a deflection experiment is the alignment of the molecular beam components. Initial alignment of the setup should be conducted with an alignment laser, ensuring line-of-sight from the pulsed valve, through the skimmers and the deflector into the center of the detection region. It is furthermore advisable to place the molecular beam skimmers on xy-translation (radial directions as defined in Figure 2) mounts, to optimize the observed signal. This furthermore circumvents a problem encountered for species of large dipole moments, or when using very strong deflection fields. If the spatial separation is too large, molecules will not travel through the last skimmer anymore. A moveable skimmer allows one to optimize transmission of the species of interest while discriminating against others.
It should be noted that it is the molecules in low energy rotational quantum states that have the largest Stark interaction (as indicated in Figure 1) and therefore experience the largest deflection. As the deflection technique does not change the population distribution, but merely disperses the existing beam, it is essential that the low J states are initially populated. This requires a good supersonic expansion from the nozzle, producing a cold molecular beam with typical temperatures of around 1 K or less 27.
The general applicability of the electrostatic deflection technique for the separation of conformers or clusters is dependent on the differences in mass-to-dipole moment ratio of the species to be separated. In the case of different conformers one typically encounters different dipole moments due to differing orientations of functional groups within a species, whilst for clusters mass selection is highly desirable to enable the separation of specific cluster stoichiometries, as well as cluster isomers of a given size. Several options are available to increase the spatial separation. One approach is to increase the interaction time of the molecules with the electrostatic field. This can be achieved by using a slower molecular beam, for example through the use of heavier (e.g., neon, argon or krypton) backing gas. Alternatively, the fabrication of a longer deflector would similarly increase the interaction time. A different approach to increase the spatial separation achieved is the use of higher deflection fields, either by increasing the applied voltage or by decreasing the gap between the two electrodes. The difficulty for both these methods is the danger of arcing between the two electrodes, which could potentially damage the electrodes beyond repair. The maximum potential difference that can safely be applied (under ultra-high vacuum conditions) crucially depends on the materials used and the quality of the surface finish.
While different conformers of molecules could previously be studied using high-resolution spectroscopic methods, utilizing their distinct microwave36, IR, or UV-Vis1,37 spectra, the electrostatic deflection method presented here allows the production of a pure beam of a single molecular species. Other electrode geometries can be used to select neutral molecules or clusters, e.g., the electric quadrupole filter or the alternating gradient decelerator22,38-40. These devices, however, are significantly larger (>1 m) and much more complex to manufacture and install. Furthermore they are extremely sensitive to mechanic missalignment24. The presented static two-wire field deflector consists of a simple geometry that can be incorporated into existing molecular beam setups30,41-44.
We see the future applications of this technique in three main areas. Firstly, the study of conformer specific reactivity. Using the electrostatic deflector a conformerically pure sample beam can be created, which can subsequently be used to study chemical properties and reactivities of a single structural isomer and cluster size.
Secondly, systematic studies of solvation of neutral molecules. Using the m/μ-selector allows for the creation of molecular clusters with a well defined stoichiometry. Systematically studying molecular clusters of increasing size allows the study of solvation effects and tries to bridge the gap between the condensed phase, in which most chemistry happens, and the gas-phase, that allows for high-resolution studies. This technique is well-established for molecular ions45-48, but the lack of size selectivity for neutrals has so far limited the study of neutral molecule solvation, e.g., using ultrafast imaging experiments.
Thirdly, the electrostatic deflector allows for the selection of the coldest part of the molecular beam, due to the larger Stark effect for low-energy rotational quantum states. This significantly aids in 1D and 3D alignment and orientation experiments17,26,27,49. This is a crucial prerequisite for the next generation of molecular physics experiments extracting molecular frame information from complex molecules, such as molecular orbital imaging2 or diffraction50 experiments.
The presented electrostatic deflection method is novel, yet based on conceptually simple and well-established ideas, and leads to the separation of species within a molecular beam by their mass-to-dipole moment ratio, utilizing the Stark effect. It enables the creation of cold, conformer and mass-selected molecular beams, allowing for numerous applications in molecular physics and physical chemistry.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work has been supported by the excellence cluster "The Hamburg Center for Ultrafast Imaging – Structure, Dynamics and Control of Matter at the Atomic Scale" of the Deutsche Forschungsgemeinschaft and by the Helmholtz Virtual Institute "Dynamic Pathways in Multidimensional Landscapes".
Materials
| Vacuum system | various, e.g. Pfeiffer Vacuum, Varian, Edwards, Leybold | ||
| Dye laser system | various, e.g. Coherent, Spectra Physics, Syrah, LIOP-TEC, Radiant Dyes… | ||
| Pulsed valve | Even-Lavie | ||
| High voltage power supply | eg. FUG | HCP 14-20000 | |
| Deflector | Custom made | ||
| Time-of-flight spectrometer | Jordan TOF | C-677 | |
| TOF power supply | Jordan TOF | D-603 | |
| Focusing lens | e.g. Thorlabs | LA4745 | |
| Translation stage | e.g. Vision Lasertechnik | 8MT167-25 | |
| Digitizer | e.g. Agilent | Acquiris DC440 | |
| Digital delay generator | e.g. Stanford Systems | SRS DG645 | |
| Molecular beam skimmer | Beam Dynamics Inc. | http://www.beamdynamicsinc.com/ |
References
- Rizzo, T. R., Park, Y. D., Peteanu, L., Levy, D. H. Electronic spectrum of the amino acid tryptophan cooled in a supersonic molecular beam. J. Chem. Phys. 83, 4819-4820 (1985).
- Itatani, J., et al. Tomographic imaging of molecular orbitals. Nature. 432, 867-871 (2004).
- Kumarappan, V., et al. Multiphoton electron angular distributions from laser-aligned CS2 molecules. Phys. Rev. Lett. 100 (9), 093006-0910 (2008).
- Bisgaard, C. Z., et al. Time-resolved molecular frame dynamics of fixed-in-space CS2 molecules. Science. 323 (5920), 1464-1468 (2009).
- Holmegaard, L., et al. Photoelectron angular distributions from strong-field ionization of oriented molecules. Nat. Phys. 6, 428-4210 (2010).
- Ihee, H., et al. Direct imaging of transient molecular structures with ultrafast diffraction. Science. 291 (5503), 458-462 (2001).
- Chergui, M., Zewail, A. H. Electron and x-ray methods of ultrafast structural dynamics: Advances and applications. Chem. Phys. Chem. 10 (1), 28-43 (2009).
- Siwick, B. J., Dwyer, J. R., Jordan, R. E., Miller, R. J. D. An atomic-level view of melting using femtosecond electron diffraction. Science. 302 (5649), 1382-1385 (2003).
- Sciaini, G., Miller, R. J. D. Femtosecond electron diffraction: heralding the era of atomically resolved dynamics. Rep. Prog. Phys. 74 (9), 096101-0910 (2011).
- Hedberg, K., et al. Bond lengths in free molecules of buckminsterfullerene, C60, from gas-phase electron diffraction. Science. 254 (5030), 410-412 (1991).
- Filsinger, F., Meijer, G., Stapelfeldt, H., Chapman, H., Küpper, J. S. t. a. t. e. -. and conformerselected beams of aligned and oriented molecules for ultrafast diffraction studies. Phys. Chem. Chem. Phys. 13 (6), 2076-2087 (2011).
- Seibert, M. M., et al. Single mimivirus particles intercepted and imaged with an x-ray laser. Nature. 470 (7332), (2011).
- Chapman, H. N., et al. Femtosecond x-ray protein nanocrystallography. Nature. 470 (7332), (2011).
- von Helden, G., Wyttenbach, T., Bowers, M. T. Conformation of macromolecules in the gasphase – use of matrix-assisted laser-desorption methods in ion chromatography. Science. 267, 1483-1485 (1995).
- Jarrold, M. Helices and sheets in vacuo. Phys. Chem. Chem. Phys. 9, 1659-1671 (2007).
- Filsinger, F., et al. Pure samples of individual conformers: the separation of stereo-isomers of complex molecules using electric fields. Angew. Chem. Int. Ed. 48, (2009).
- Filsinger, F., et al. Quantum-state selection, alignment, and orientation of large molecules using static electric and laser fields. J. Chem. Phys. 131, 10-1063 (2009).
- Kallmann, H., Reiche, F. Über den Durchgang bewegter Moleküle durch inhomogene Kraftfelder. Z. Phys. 6, 352-375 (1921).
- Wrede, E. Über die Ablenkung von Molekularstrahlen elektrischer Dipolmoleküle im inhomogenen elektrischen Feld. Z. Phys. 44 (4-5), 4-5 (1927).
- Gerlach, W., Der Stern, O. experimentelle Nachweis der Richtungsquantelung im Magnetfeld. Z. Phys. 9, 349-352 (1922).
- Trippel, S., Chang, Y. -. P., Stern, S., Mullins, T., Holmegaard, L., Küpper, J. Spatial separation of state- and size-selected neutral clusters. Phys. Rev. A. 86, 10-1103 (2012).
- Filsinger, F., Erlekam, U., von Helden, G., Küpper, J., Meijer, G. Selector for structural isomers of neutral molecules. Phys. Rev. Lett. 100, 10-1103 (2008).
- Filsinger, F., Putzke, S., Haak, H., Meijer, G., Küpper, J. Tuning the resolution of the m=_- selector. Phys. Rev. A. 82, 052513-0510 (2010).
- Putzke, S., Filsinger, F., Haak, H., Küpper, J., Meijer, G. Rotational-state-specific guiding of large molecules. Phys. Chem. Chem. Phys. 13, (2011).
- Nielsen, J. H., et al. Stark-selected beam of ground-state OCS molecules characterized by revivals of impulsive alignment. Phys. Chem. Chem. Phys. 13, 18971-18975 (2011).
- Stapelfeldt, H., Seideman, T. Colloquium: Aligning molecules with strong laser pulses. Rev. Mod. Phys. 75 (2), 543-557 (2003).
- Holmegaard, L., et al. Laser-induced alignment and orientation of quantum-state-selected large molecules. Phys. Rev. Lett. 102, 10-1103 (2009).
- Ghafur, O., Rouzee, A., Gijsbertsen, A., Siu, W. K., Stolte, S., Vrakking, M. J. J. Impulsive orientation and alignment of quantum-state-selected NO molecules. Nat. Phys. 5, 289-293 (2009).
- Hillenkamp, M., Keinan, S., Even, U. Condensation limited cooling in supersonic expansions. J. Chem. Phys. 118 (19), 8699-8705 (2003).
- Ramsey, N. F. . Molecular Beams. The International Series of Monographs on Physics. , (1956).
- Nielsen, J. H., Stapelfeldt, H., Küpper, J., Friedrich, B., Omiste, J. J., González-Férez, R. Making the best of mixed-field orientation of polar molecules: A recipe for achieving adiabatic dynamics in an electrostatic field combined with laser pulses. Phys. Rev. Lett. 108 (19), 10-1103 (2012).
- Fujimaki, E., Fujii, A., Ebata, T., Mikami, N. Autoionization-detected infrared spectroscopy of intramolecular hydrogen bonds in aromatic cations. I. principle and application to fluorophenol and methoxyphenol. J. Chem. Phys. 110, 4238-4247 (1999).
- Kang, C., Korter, T. M., Pratt, D. W. Experimental measurement of the induced dipole moment of an isolated molecule in its ground and electronically excited states: Indole and indole–H2O. J. Chem. Phys. 122 (17), 174301-17 (2005).
- Korter, T. M., Pratt, D. W., Küpper, J. Indole-H2O in the gas phase. structures, barriers to internal motion, and S1 S0 transition moment orientation. solvent reorganization in the electronically excited state. J. Phys. Chem. A. 102 (37), 7211-7216 (1998).
- Küpper, J., Pratt, D. W., Meerts, L., Brand, C., Tatchen, J., Schmitt, M. Vibronic coupling in indole: II. experimental investigation of the 1La–1Lb interaction using rotationally resolved electronic spectroscopy. Phys. Chem. Chem. Phys. 12, 4980-4988 (2010).
- Suenram, R. D., Lovas, F. J. Millimeter wave spectrum of glycine – a new conformer. J. Am. Chem. Soc. 102, 7180-7184 (1980).
- Nir, E., Kleinermanns, K., de Vries, M. S. Pairing of isolated nucleic-acid bases in the absence of the DNA backbone. Nature. 408, 949-951 (2000).
- Bethlem, H. L., van Roij, A. J. A., Jongma, R. T., Meijer, G. Alternate gradient focusing and deceleration of a molecular beam. Phys. Rev. Lett. 88 (13), 10-1103 (2002).
- Wohlfart, K., Grätz, F., Filsinger, F., Haak, H., Meijer, G., Küpper, J. Alternating-gradient focusing and deceleration of large molecules. Phys. Rev. A. 77, 10-1103 (2008).
- Putzke, S., Filsinger, F., Küpper, J., Meijer, G. Alternating-gradient focusing of the benzonitrile-argon van der waals complex. J. Chem. Phys. 137 (10), 10-1063 (2012).
- Knight, W. D., Clemenger, K., de Heer, W. A., Saunders, W. A. Polarizability of alkali clusters. Phys. Rev. B. 31 (4), 2539-2540 (1985).
- Tarnovsky, V., Bunimovicz, M., Vuškovic, L., Stumpf, B., Bederson, B. Measurements of the DC electric-dipole polarizabilities of the alkali dimer molecules, homonuclear and heteronuclear. J. Chem. Phys. 98 (5), 3894-3904 (1993).
- Schäfer, R., Schlecht, S., Woenckhaus, J., Becker, J. . Polarizabilities of Isolated Semiconductor Clusters. Phys. Rev. Lett. 76 (3), 471-474 (1996).
- Antoine, R., et al. Electric dipole moments and conformations of isolated peptides. Eur. Phys. J. D. 20, 583-587 (2002).
- Cosby, P. C., Smith, G. P., Moseley, J. T. Photodissociation and photodetachment of molecular negative ions. IV. Hydrates of O. J. Chem. Phys. 69, 2779-2781 (1978).
- Hunton, D. E., Hofmann, M., Lindeman, T. G., Albertoni, C. R., Castleman Jr, ., W, A. Photodissociation spectroscopy and dynamics of negative ion clusters. II. CO. (H2O)1;2;3. J. Chem. Phys. 82, 2884-2895 (1985).
- Castleman, A. W., Bowen J, K. H., J, Clusters: Structure, energetics, and dynamics of intermediate states of matter. J. Phys. Chem. 100, 12911-12944 (1996).
- Verlet, J. R. R. Femtosecond spectroscopy of cluster anions: insights into condensed-phase phenomena from the gas-phase. Chem. Soc. Rev. 37, 505-517 (2008).
- Nevo, I., et al. Laser-induced 3D alignment and orientation of quantum state-selected molecules. Phys. Chem. Chem. Phys. 11, 9912-9918 (2009).
- Reckenthaeler, P., Centurion, M., Fuss, W., Trushin, S. A., Krausz, F., Fill, E. E. Time-resolved electron diffraction from selectively aligned molecules. Phys. Rev. Lett. 102 (21), 213001-2110 (2009).

