A Protocol to Infect Caenorhabditis elegans with Salmonella typhimurium
Summary
C. elegans has emerged as a new genetic model to study host-pathogen interactions. Here we describe a protocol to infect C. elegans with Salmonella typhimurium coupled with the double-strand RNAi interference technique to examine the role of host genes in defense against Salmonella infection.
Abstract
In the last decade, C. elegans has emerged as an invertebrate organism to study interactions between hosts and pathogens, including the host defense against gram-negative bacterium Salmonella typhimurium. Salmonella establishes persistent infection in the intestine of C. elegans and results in early death of infected animals. A number of immunity mechanisms have been identified in C. elegans to defend against Salmonella infections. Autophagy, an evolutionarily conserved lysosomal degradation pathway, has been shown to limit the Salmonella replication in C. elegans and in mammals. Here, a protocol is described to infect C. elegans with Salmonella typhimurium, in which the worms are exposed to Salmonella for a limited time, similar to Salmonella infection in humans. Salmonella infection significantly shortens the lifespan of C. elegans. Using the essential autophagy gene bec-1 as an example, we combined this infection method with C. elegans RNAi feeding approach and showed this protocol can be used to examine the function of C. elegans host genes in defense against Salmonella infection. Since C. elegans whole genome RNAi libraries are available, this protocol makes it possible to comprehensively screen for C. elegans genes that protect against Salmonella and other intestinal pathogens using genome-wide RNAi libraries.
Introduction
The free-living soil nematode Caenorhabditis elegans is a simple and genetically tractable model organism used to study many biological questions. C. elegans dominantly exists as self-fertilizing hermaphrodites. Males are spontaneously generated by non-disjunction of the X chromosome during gametogenesis1,2. In the presence of abundant food, C. elegans continuously develop through four larval stages to adult. Temperature also influences C. elegans development; faster development is observed at higher temperatures. In the laboratory, C. elegans is cultured at a standard temperature of 20 °C on agar plates with seeded bacterium Escherichia coli (strain OP50) as food1,2.
In the last decade, C. elegans has emerged as an invertebrate organism to study host-pathogen interactions3-5. In nature, C. elegans eats bacteria as its nutrient source1,2. Its normal bacterial laboratory food, OP50, can be easily substituted with other pathogens to examine the interactions between C. elegans and any chosen pathogen. Under these conditions, the intestine is the primary site of the infection. Indeed, a wide range of bacterial pathogens has been shown to lethally infect C. elegans3-5.
The gram-negative bacterium Salmonella is a gastrointestinal pathogen that causes human food-borne illness worldwide6,7. C. elegans is a good model host for Salmonella typhimurium as this bacterium replicates and exhibits persistent intestinal infections8-10. C. elegans has been used to identify both novel and previously known Salmonella virulence factors11. Interestingly, the C. elegans immune system successfully limits Salmonella replication. It has been reported previously that inhibition of autophagy genes renders increased Salmonella replication in C. elegans, resulting in early death of infected worms10. Macroautophagy (herein referred to as autophagy) is a dynamic process involving the rearrangement of subcellular membranes to sequester cytoplasm and organelles for delivery to the lysosome for degradation12. Autophagy has been reported to limit the Salmonella replication in C. elegans and in mammals10,13.
The C. elegans genome was the first multicellular eukaryotic genome sequenced; it is responsive to RNAi treatment14-16. Moreover, RNAi can be administrated effectively by subjecting worms to ingest bacteria containing the double-stranded RNA of the target gene, known as RNAi feeding16,17. Whole genome RNAi feeding libraries have been generated for genome-wide RNAi screening16,18. Herein, a Salmonella infection protocol is coupled with RNAi feeding to allow testing C. elegans genes of interest for their ability to protect against Salmonella infection.
Protocol
1. XLD (Xylose Lysine Desoxycholate) Agar Plates
XLD agar is a selective growth medium for Salmonella, which appears as black colonies on XLD agar plates. However, if there are no concerns of contamination, a regular LB plate can be substituted.
- Weigh out 5.5 g XLD agar and resuspend in 5 ml deionized water.
- Mix thoroughly until all agar is wet. Add 95 ml deionized water until all lumps are gone and the medium is completely resuspended.
- Boil the medium to dissolve completely (do not autoclave).
- Cool the medium at room temperature to 50 °C.
- Pour 25 ml agar in each 95 x 15 mm (diameter x height) plate (plates sealed with Parafilm can be stored at 4 °C for up to 1 month).
2. Nematode Growth Medium (NGM) RNAi Feeding Plates
Preparation of C. elegans NGM plates has been described previously19. Here a procedure is briefly described to add the antibiotic ampicillin and the RNAi chemical inducer isopropyl β-D-1-thiogalactopyranoside (IPTG) into the NGM media to make the RNAi feeding plates.
- Dissolve 3 g NaCl and 2.5 g Bacto peptone in 1 L deionized water.
- Add 17 g Bacto agar into the media.
- Autoclave the media for 45 min and cool the media to 50 °C in a water bath.
- Add the following solutions: 1 ml cholesterol (5 mg/ml in 95% ethanol), 1 ml 1 M CaCl2, 1 ml 1 M MgSO4, and 25 ml 1 M potassium phosphate buffer (pH 6.0). Mix well.
- Add 1 ml 1 M IPTG and 500 µl ampicillin (100 mg/ml in sterile water).
- Mix the solution well and pour into 60 x 15 mm (diameter x height) petri plates using a Pipet Aid and 25 ml serological pipette following sterile procedures. Fill each plate with 12 ml agar. Plates can be stored at 4 °C for up to 1 month.
3. Prepare RNAi-treated Animals for Infection
The essential autophagy gene bec-1 is used as an example to examine the function of a host gene in defense against Salmonella infection. The experimental procedures are illustrated in Figure 1 and Table 1. The protocol for preparing RNAi-treated animals for infection follows, with the day of each experimental step given in parentheses.
- Inoculate bec-1 RNAi feeding and control empty vector L4440 RNAi feeding bacteria by placing a flake of -80 °C frozen bacteria into 2 ml LB medium supplemented with 100 mg/ml ampicillin (Day 1). Repeat this step once a week during the entire experiment to have fresh RNAi bacteria. Store the culture in the 4 °C refrigerator when not used.
- Seed 100 ml of overnight RNAi bacterial culture on RNAi plates. Prepare three bec-1 RNAi and three control empty vector RNAi plates. Incubate the plates at 37 °C overnight (Day 2).
- Remove the RNAi plates from the 37 °C incubator and allow them to cool down to room temperature on the bench. Pick up well-fed L4 wild type N2 hermaphrodites and transfer them to bec-1 RNAi and control empty vector RNAi plates. Place three worms per plate, on triplicate plates. On the same day, prepare RNAi plates as described in step 3.2 (Day 3).
- Incubate the RNAi plates with worms in the 20 °C incubator for 36-40 hr and transfer worms to fresh corresponding RNAi plates prepared in step 3.3. After worms are transferred, incubate the plates in the 20 °C incubator for 64 hr (Day 4).
4. Prepare Salmonella for Infection
- Streak Salmonella -80 °C frozen stock on 1 XLD agar plate and incubate the plate at 37 °C overnight (Day 5).
- Pick a well-isolated black Salmonella colony and inoculate it in 2 ml LB medium at 37 °C with shaking overnight (Day 6).
- Seed 80 ml Salmonella overnight culture on 1 C. elegans 60 x 15 mm (diameter x height) NGM agar plate and prepare 6 plates in total. Incubate the plates at room temperature for 6 hr. The bacterial culture should dry and form a lawn on the plate (Day 7).
5. Infect RNAi-treated Worms with Salmonella
- Transfer bec-1 RNAi-treated and control empty vector RNAi-treated L4 N2 hermaphrodites (progeny of worms set up in Step 3) to Salmonella plates. Place 40 worms per plate on 3 plates for each group. Incubate the worm plates at 20 °C for 48 hr (Day 7).
- Prepare one set of fresh RNAi plates as described in steps 3.1 and 3.2 (Day 7 and Day 8).
- After 48 hr infection, transfer Salmonella-infected worms to the corresponding RNAi plates prepared in step 5.2 and incubate at 20 °C (Day 9).
6. Survival Assay
- Score the survival of worms daily and transfer worms to fresh corresponding RNAi plates during the egg-laying time. Prepare a set of fresh RNAi plates prior to each worm transfer as described in steps 3.1 and 3.2. Touch the worm body (head, middle part and tail) gently with an end-flattened platinum wire. A worm is scored as dead if no movement of the worm body is observed.
- Score the survival of worms daily or every other day, and transfer worms to fresh corresponding RNAi plates twice a week after worms stop laying eggs.
- After all worms die, pool the survival data from triplicate plates as one data set. Input the survival data of each group into appropriate statistical software such as GraphPad Prism to generate survival curves and to perform Kaplan-Meier survival analysis. The entire experiment is repeated at least once to confirm the conclusion.
Representative Results
At 20 °C, the median lifespan of wild type N2 worms is 17 days (Figure 2A and Table 2). Salmonella infection significantly decreases the median lifespan of N2 worms to 10.5 days (p = 0.0002, log-rank test) (Figure 2A).
If a C. elegans gene plays an important role in defense against Salmonella infection, it is predicted that its inhibition will impart susceptibility to Salmonella infection. In fact, compared to Salmonella-infected control RNAi-treated N2 animals, the median lifespan of Salmonella-infected bec-1 RNAi-treated N2 worms is decreased from 10.5 days to 9 days (p < 0.0001, log-rank test) (Figure 2B and Table 2). The maximum lifespan is dramatically shortened by 14 days (from 24 days to 10 days, Figure 2B and Table 2). Moreover, the bec-1 RNAi has no obvious effect on the lifespan of N2 worms that are not infected by Salmonella (p = 0.2593, log-rank test) (Figure 2C and Table 2), indicating that Salmonella infection, not bec-1 RNAi treatment, decreases the lifespan of Salmonella-infected bec-1 RNAi-treated worms. Also, bec-1 is an essential gene in C. elegans defense against Salmonella infection.

Figure 1. Flow chart of the experimental procedures.
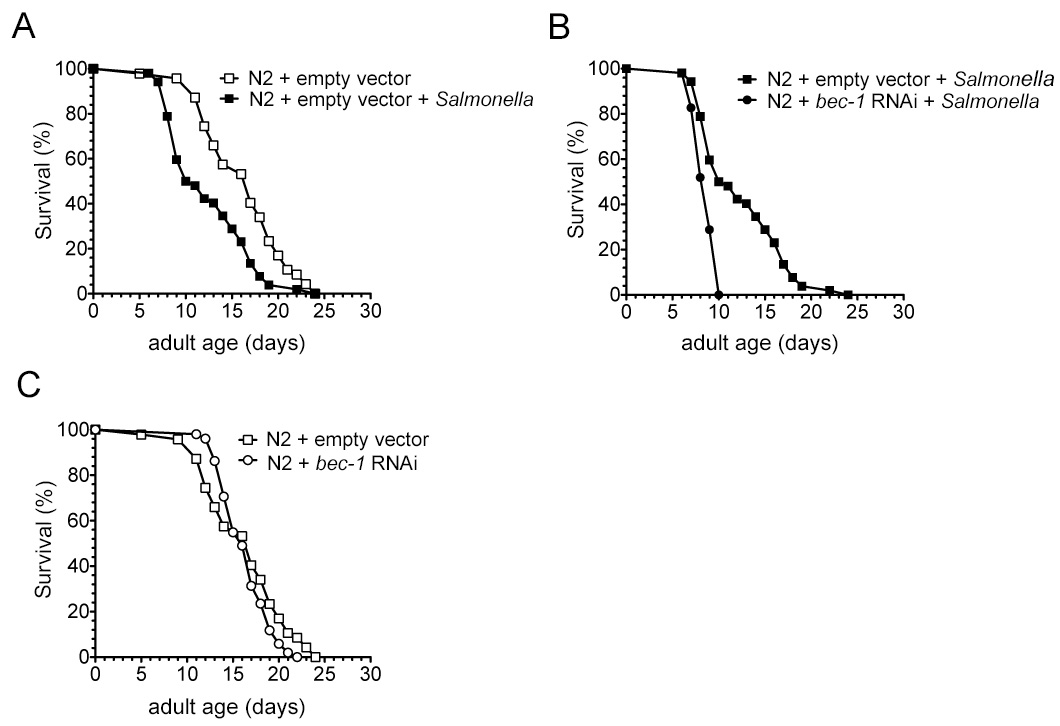
Figure 2. Inhibition of bec-1 gene by RNAi confers susceptibility to Salmonella infection in C. elegans. A-C) Survival curves of wild type N2 animals treated with either control empty vector RNAi or bec-1 gene RNAi following a 2 day exposure to Salmonella typhimurium or nonpathogenic Escherichia coli at 20 °C. Please click here to view a larger version of this figure.
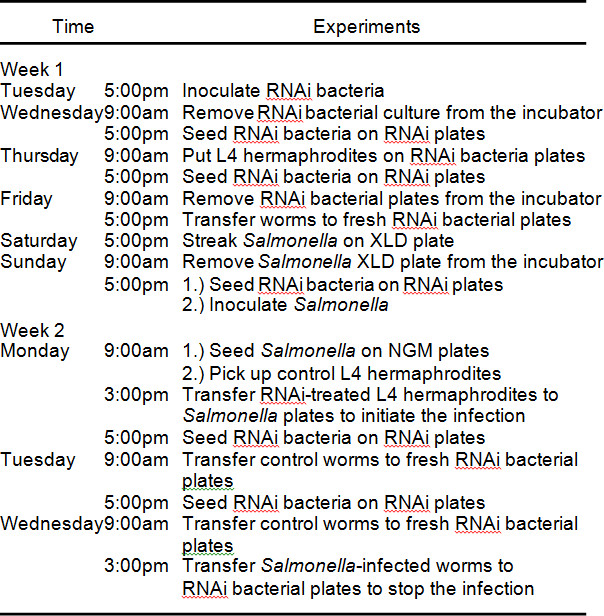
Table 1. Salmonella infection protocol timeframe.
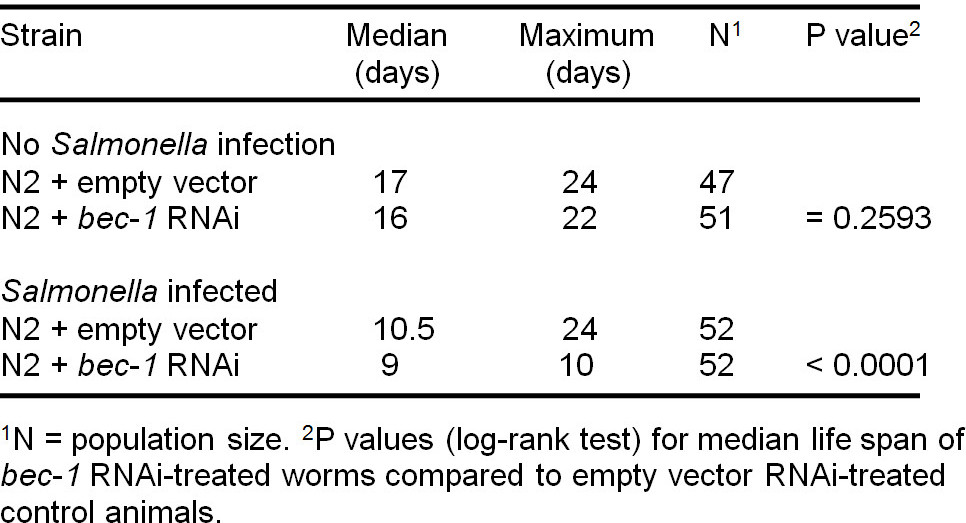
Table 2. Statistical analysis of lifespan data in Figure 2.
Discussion
C. elegans is a simple genetic model organism that eats bacteria as its nutrient source. Thus, it is easy to substitute its normal bacterial food with an intestinal pathogen to investigate the interactions between C. elegans and the chosen pathogen. Herein a protocol is described to combine Salmonella infection and C. elegans RNAi feeding treatment to examine the role of host genes in defense against Salmonella infection. Previous infection protocols expose C. elegans worms to pathogenic bacteria including Salmonella during their lifetime20. In the present protocol, Salmonella infects the worms in a two-day period. After that, the worms are no longer exposed to Salmonella. This Salmonella infection significantly decreases the lifespan of C. elegans wild type animals. Thus, invaded Salmonella replicate inside worms and kill the animals10. This short period of exposure to Salmonella mimics human Salmonella infection, which should help to uncover useful information to understand human food-borne illness caused by Salmonella infection. Moreover, this protocol combines the RNAi feeding treatment with Salmonella infection, making it possible to test any candidate genes that might be involved in host defense against Salmonella infection, especially when the genetic mutants are not available. The autophagy gene bec-1 known to be involved in defense against Salmonella infection is used as an example in the present study. bec-1 mutations are lethal21, which prevents testing its role in defense against Salmonella infection in adults. Using the current protocol, it was shown that inhibition of bec-1 by RNAi yields susceptibility to Salmonella infection in C. elegans. In the present study, N2 wild type worms fed with L4440 bacteria have a similar lifespan as animals fed with OP50. The animals start to die around day 6 and the maximum lifespan is around four weeks. N2 worms infected by Salmonella live a few days shorter. By contrast, bec-1 RNAi-treated worms infected with Salmonella die about two times faster than control animals although the beginning date for animals to die in both groups is only a few days apart (Figure 2). The entire experiment lasts about 1 month.
In this protocol, the coordination of RNAi feeding and Salmonella preparation is required so that RNAi-treated L4 stage hermaphrodites are subjected to Salmonella infection. A typical timeframe of the protocol used in the authors’ lab is described in Table 1. RNAi feeding bacteria are prepared weekly and the bacterial culture is stored at 4 °C when not used. Of note, on day 7, the infection will start 6 hr after Salmonella overnight cultures are placed on NGM plates. During this 6 hr period, RNAi-treated L4 N2 hermaphrodites are picked from corresponding bec-1 and control empty vector RNAi plates. The non-infected worms are used as controls to ascertain if Salmonella infection shortens the lifespan of infected worms and if bec-1 RNAi treatment has any influence on worm lifespan.
Currently, survival of C. elegans after infection is commonly used to measure the pathogen virulence3-5. However, RNAi inhibition of certain C. elegans genes result in decreased lifespan. Therefore, one should be careful when interpreting the data. When this situation is encountered, different concentrations of the RNAi inducer, IPTG, can be tested to identify the desired concentration that only influences the host response to the pathogen infections without impact on the animal lifespan. As reported previously10, the 1 nM IPTG concentration was successfully used to examine the role of autophagy in IGF signaling-mediated pathogen resistance in C. elegans.
Given that the C. elegans genome has been sequenced and C. elegans RNAi feeding libraries have been generated16,18, it is possible to revise the described protocol to perform a genome-wide RNAi screening to identify all host genes involved in defense against Salmonella infection. For example, instead of using the median survival to measure the virulence of Salmonella, the maximum survival is used. Moreover, infected worms can be sterilized by supplementing plates with fluorodeoxyuridine, a DNA synthesis inhibitor. Thus, transferring of infected worms is unnecessary as long as food is supplied to prevent worms from starvation. These modifications will reduce the workload for a high-throughput screen tremendously. This type of large-scale study may shed light on understanding the human response to Salmonella infection as many biological pathways in C. elegans are evolutionarily conserved in humans.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We thank Dr. Diane Baronas-Lowell for critical reading of the manuscript. This work was supported by an FAU Charles E. Schmidt College of Science Seed Grant and an Aging Scholarship from the Ellison Medical Foundation to K.J.
Materials
| LB Broth | Fisher | BP9723-500 | |
| XLD agar | EMD Chemicals | 1.05287.0500 | |
| Bacto Agar | Fisher | DF0140-01-0 | |
| Peptone | Fisher | BP1420-500 | |
| Sodium Chloride | Fisher | S671-500 | |
| Calcium Chloride | Fisher | C69-500 | |
| Magnesium Sulfate | Fisher | M65-500 | |
| IPTG | Gold Biotechnology | 12481C50 | |
| Cholesterol | Sigma | C8667-25G | |
| Ampicillin | Fisher | BP1760-25 | |
| Salmonella typhimurium | ATCC | ATCC14028 | |
| Petri Dish 95 x 15mm | Fisher | FB0875714G | |
| Petri Dish 60 x 15mm | Fisher | 08-757-13A | |
| Falcon Serological pipet | Fisher | 13-668-2 | |
| Falcon Express Pipet-Aid | Fisher | 13-675-42 | |
| MaxQ6000 shaking incubator | Thermo Scientific | SHKE6000-7 | |
| Incubator | Percival | I-36DL |
References
- Riddle, D. L., Blumenthal, T., Meyer, B. J., Priess, J. R. . C. elegans II. , (1997).
- Brenner, S. The Genetics of Caenorhabditis elegans. Génétique. 77, 71-94 (1974).
- Aballay, A., Ausubel, F. M. Caenorhabditis elegans as a host for the study of host-pathogen interactions. Curr Opin Microbiol. 5, 97-101 (2002).
- Kurz, C. L., Ewbank, J. J. Caenorhabditis elegans: an emerging genetic model for the study of innate immunity. Nat Rev Genet. 4, 380-390 (2003).
- Mylonakis, E., Aballay, A. Worms and flies as genetically tractable animal models to study host-pathogen interactions. Infection and Immunity. 73, 3833-3841 (2005).
- Ford, M. W., et al. A descriptive study of human Salmonella serotype typhimurium infections reported in Ontario from 1990 to 1997. Can J Infect Dis. 14, 267-273 (2003).
- Voetsch, A. C., et al. FoodNet estimate of the burden of illness caused by nontyphoidal Salmonella infections in the United States. Clin Infect Dis. 38 Suppl 3, (2004).
- Aballay, A., Yorgey, P., Ausubel, F. M. Salmonella typhimurium proliferates and establishes a persistent infection in the intestine of Caenorhabditis elegans. Curr Biol. 10, 1539-1542 (2000).
- Alegado, R. A., Tan, M. W. Resistance to antimicrobial peptides contributes to persistence of Salmonella typhimurium in the C. elegans intestine. Cell Microbiol. 10, 1259-1273 (2008).
- Jia, K., et al. Autophagy genes protect against Salmonella typhimurium infection and mediate insulin signaling-regulated pathogen resistance. Proceedings of the National Academy of Sciences of the United States of America. 106, 14564-14569 (2009).
- Tenor, J. L., McCormick, B. A., Ausubel, F. M., Aballay, A. Caenorhabditis elegans-based screen identifies Salmonella virulence factors required for conserved host-pathogen interactions. Curr Biol. 14, 1018-1024 (2004).
- Levine, B., Klionsky, D. J. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Developmental Cell. 6, 463-477 (2004).
- Birmingham, C. L., Smith, A. C., Bakowski, M. A., Yoshimori, T., Brumell, J. H. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J Biol Chem. 281, 11374-11383 (2006).
- . The C. elegans Sequencing Consortium. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science. 282, 2012-2018 (1998).
- Fire, A., et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 391, 806-811 (1998).
- Kamath, R. S., Martinez-Campos, M., Zipperlen, P., Fraser, A. G., Ahringer, J. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2, 1-10 (2001).
- Liang, J., Xiong, S., Savage-Dunn, C. Using RNA-mediated interference feeding strategy to screen for genes involved in body size regulation in the nematode C elegans. J. Vis. Exp. (72), (2013).
- Fraser, A. G., et al. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature. 408, 325-330 (2000).
- Stiernagle, T. Maintenance of C. elegans. WormBook: the online review of C elegans biology. , 1-11 (2006).
- Aballay, A., Ausubel, F. M. Programmed cell death mediated by ced-3 and ced-4 protects Caenorhabditis elegans from Salmonella typhimurium-mediated killing. Proceedings of the National Academy of Sciences of the United States of America. 98, 2735-2739 (2001).
- Melendez, A., et al. Autophagy genes are essential for dauer development and lifespan extension in C. elegans. Science. 301, 1387-1391 (2003).

