A Method of Nodose Ganglia Injection in Sprague-Dawley Rat
Summary
Afferent vagal signaling transmits important information to central nervous system from receptors located in organs of the abdomen and thorax. The nodose ganglia of vagus nerves contain many types of receptors that modulate vagal activity. This protocol describes a method of local injections of neurochemicals into the nodose ganglia.
Abstract
Afferent signaling via the vagus nerve transmits important general visceral information to the central nervous system from many diverse receptors located in the organs of the abdomen and thorax. The vagus nerve communicates information from stimuli such as heart rate, blood pressure, bronchopulmonary irritation, and gastrointestinal distension to the nucleus of solitary tract of the medulla. The cell bodies of the vagus nerve are located in the nodose and petrosal ganglia, of which the majority are located in the former. The nodose ganglia contain a wealth of receptors for amino acids, monoamines, neuropeptides, and other neurochemicals that can modify afferent vagus nerve activity. Modifying vagal afferents through systemic peripheral drug treatments targeted at the receptors on nodose ganglia has the potential of treating diseases such as sleep apnea, gastroesophageal reflux disease, or chronic cough. The protocol here describes a method of injection neurochemicals directly into the nodose ganglion. Injecting neurochemicals directly into the nodose ganglia allows study of effects solely on cell bodies that modulate afferent nerve activity, and prevents the complication of involving the central nervous system as seen in systemic neurochemical treatment. Using readily available and inexpensive equipment, intranodose ganglia injections are easily done in anesthetized Sprague-Dawley rats.
Introduction
Afferent signaling via the vagus nerve (cranial nerve X) transmits important general visceral information to the central nervous system (CNS) from baro-, chemo-, hepatic osmo-, cardiac, pulmonary, and gastric receptors located in the organs of the abdomen and thorax. The vagus nerve communicates information from stimuli such as heart rate, blood pressure, bronchopulmonary irritation, and gastrointestinal distension to the nucleus of solitary tract (NTS) of the medulla. The cell bodies of the pseudounipolar neurons of the vagus nerve are located in the nodose and petrosal ganglia, of which the majority is found in the former. Nodose ganglion cells contain a wealth of receptors for amino acids, monoamines, neuropeptides, and other neurochemicals that when activated, can modify afferent vagus nerve activity.1 Numerous innervations of the afferent vagus nerves coupled with the diversity of receptors located on the nodose ganglia illustrate the biological importance of this cranial nerve, and systemic drugs that do not cross into the CNS targeted at receptors on nodose ganglia can be used to treat various diseases, such as sleep apnea, gastroesophageal reflux disease, or chronic cough.2-4
The ease of access to the nodose ganglia lends itself to experimental manipulation by midline longitudinal incision made at the neck. The vagus nerve emerges from the posterior lacerated foramen at the base of the skull, and immediately displays a swelling of the nerve that is the nodose ganglion. The nodose ganglion is easily recognizable due to two nerve branches that arise from it: anteriorly the pharyngeal branch; and posteriorly superior laryngeal branch.5 Previous experimental manipulations of the nodose ganglia involved electrophysiological recordings,6 injections of immunohistochemical or immunofluorescent compounds for nerve tracings,7-10 superfusion or injections of neuroexcitotoxins,11-13 injections of adeno-associated virus to knockdown receptors,14,15 and injections of receptor-specific neurochemicals to change the activity of the vagus nerve.16,17
Systemic injections of neurochemicals are problematic in that systemic treatment affects both peripheral and central nervous systems. Thus, systemic treatment does not isolate the effect of neurochemicals on afferent vagal nerve activity. This protocol describes a method using readily available equipment of intranodose injections in the Sprague-Dawley rat that modulates vagus nerve activity without affecting the central nervous system. Stimulation of serotonin type 3 (5-HT3) receptors on nodose ganglia by intravenous (IV) infusion of serotonin (5-HT) induces the Bezold-Jarisch reflex, a vagal response trifecta of bradycardia, hypotension, and apnea, which can be abolished by supranodose vagatomy.11,17-19 Apnea is easily measured by placing a respiratory transducer around the abdomen of the rat.17,18 Cannabinoids decrease 5-HT-induced current in nodose ganglia cells,20 and intranodose ganglia injections of dronabinol attenuate 5-HT-induced apnea.
Protocol
All procedures and protocols were approved by the Institutional Animal Care and Use Committee of the University of Illinois at Chicago. Experiments described here are acute non-survival experiments, and there was no use of eye ointment. Maintenance of sterile conditions only occurs when surgical instruments are washed with 70% ethanol in DiH2O. Sacrifice of rats at the end of the experiment occurred via overdose of IV ketamine/xylazine.
1. Preparation of Instruments and Chemicals
- Prepare stock solution of 0.05 M of 5-HT HCl in PBS. Then dilute stock with PBS to a final 5-HT concentration of 0.203 mM.
- Dilute dronabinol in sesame oil at a concentration of 20 µg/µl.
- Cut 20 cm length of polyethylene (PE)-50 tubing (I.D. 0.58 mm, O.D. 0.965 mm). At one end of the tubing, cut a bevel tip using scissors, and at the other square end, insert a 23 g needle. Connect this 23 g needle to a 1 ml syringe, and fill it up with 0.3 ml of 50 u/ml heparin.
- Cut four pieces of 4-0 braided silk thread and set to the side.
- Sterilize surgical scissors, 2 curved Graefe forceps, and 3 micro clamps with 70% ethanol in diH2O.
2. Catheterization of Femoral Vein
Protocol for catheterization is modified from Jespersen et al.21
- Anesthetize a Sprague-Dawley rat with intraperitoneal (IP) ketamine/xylazine (100 mg/kg:10 mg/kg). Pinch the toe of the rat and observe any movement to confirm a proper level of anesthesia. Shave the ventral aspect of the left thigh, and the ventral aspect of the neck. If necessary by the local Institutional Animal Care and Use Committee, apply eye ointment to prevent dryness.
- Secure rat in a supine position on a surgical board. Use surgical scissors to cut away skin in the left hind thigh.
- Using forceps, conduct blunt dissection of the superficial muscle to expose the femoral vein. Use the forceps to separate the vein from the femoral artery, and place 2 threads around the femoral vein.
- Use the curved Graefe forceps to pull-up the vein to stop blood flow. Using a 22 G syringe needle, puncture the femoral vein and then insert the beveled end of the PE-50 tubing into the vein.
- Check if the PE-50 tubing was inserted correctly by retracting the plunger of the syringe. Blood should be seen entering the PE-50 tubing. Tie 2 knots around the vein and PE-50 tubing using the 2 threads.
3. 5-HT-induced Apnea via IV Infusion
- Place a piezoelectric strain gauge around the rat to measure respiration.
- Change settings for amplification level of the electronic amplifier via amplifier software on the computer: to amplify (100x) and band-pass filter (1-10 Hz) the respiratory signals obtained from the strain gauge, set “highpas filter” at “AC @ 1 HZ” and “Lowpass Filter” at “10 Hz,” and set the amplification by inputting “10” for the “Initial Gain” and “100” for “Total Gain.”.
- Change settings for sampling rate by opening “Analog Input” via recording software on the computer. To digitize (500 Hz sampling rate) using an analog-to-digital converter, “sample” at “1,000 Hz” and “skip” every other recording point by “1” (effective sampling rate 500 Hz), and then record the signal using recording software.
- Remove the 1 ml syringe from the catheter and insert a 500 µl precision glass syringe filled with 0.203 mM of 5-HT into the catheter.
- Place the 500 µl precision glass syringe into an infusion pump. Infuse 12.5 µg/kg per 350 µl/kg of 5-HT solution at a rate of 63 ml/hr. Perform multiple infusions and observe apnea in rat, which is seen as a pause in breathing (≥ 2.5 sec) in the recorded respiratory signals on the computer monitor.
- Before proceeding with neck surgery, monitor breathing pattern and check for pain reflex from toe pinch in the rat. If breathing is irregular, or if there is a pain reflex from toe pinch, administer IP ketamine/xylazine (100 mg/kg:5 mg/kg) and then re-confirm a proper level of anesthesia.
4. Neck Surgery to Expose Nodose Ganglia
- Secure rat in a supine position on a surgical board. Make a midline longitudinal cut using surgical scissors at the neck.
- Using blunt dissection on the platysma muscle (using 2 micro clamps to keep this muscle clear of the surgical site), expose the sternohyoideus and omohyoideus muscles. Separate these muscles to expose the internal carotid artery and one of its branches, the pterygopalatine artery.
- Observe the vagus nerve as it runs along the internal carotid artery and then along the pterygopalatine artery. Observe how the vagus nerve and pterygopalatine artery, along with the glossopharyngeal nerve (cranial nerve IX) and the spinal accessory nerve (cranial nerve XI), enter the posterior lacerated foramen at the base of the skull.
- Notice that the nodose ganglion is displayed as a swelling of the vagus nerve right before it enters the posterior lacerated foramen. Also notice that the pharyngeal and laryngeal nerve branches come off the anterior and posterior aspects, respectively, of the nodose ganglion.5
- Using the Graefe forceps, separate the vagus nerve from the arteries, and place a piece of thread around the vagus nerve. Place a clamp on the thread to apply slight tension on the vagus nerve, and clean the nodose ganglion of any connective tissue to provide less resistance when injecting.
- Repeat 5-HT-induced apnea as stated in section 3 to confirm that no damage was done to the nodose ganglia.
5. Intranodose Ganglia Injection of Dronabinol
- Fill a 10 µl gastight precision glass syringe affixed with a custom-made 28 g half-inch syringe needle with a 35° beveled tip with 5 µl of dronabinol in sesame oil.
- Place a micro clamp on the thread to apply slight tension on the vagus nerve, and puncture the nodose ganglion taking care not to puncture through it. Depress the syringe slowly (≥ 60 sec) to inject the entire contents of the syringe. Note that some of the syringe contents will leak out of the ganglion.
- Repeat 5-HT-induced apnea as stated in section 3.
Representative Results
Figure 1 represents sample breathing recording in rats that had infusion of 5-HT to induce apnea before and after intranodose ganglia injections of dronabinol. 5-HT activates 5-HT3 receptors on the nodose ganglia that contribute to the Bezold-Jarisch reflex of bradycardia, hypotension, and apnea.11,17-19 Intranodose ganglia injections of dronabinol activate inhibitory CB receptors, or allosterically modulates 5-HT3 receptors that inhibit the 5-HT-induced excitation of the vagus nerve.16,17,20,22 Before (Figure 1, top panel) and after (Figure 1, middle panel) neck surgery infusion of 5-HT (Figure 1, indicted by red line) elicits an apneic response seen as a lack of abdomen movement measured by the piezoelectric strain gauge. After intranodose ganglia injections of dronabinol (Figure 1, bottom panel), IV 5-HT infusion did not elicit an apneic response. After sesame oil injections (data not shown) into nodose ganglia of vehicle control rats, IV 5-HT infusion elicited an apneic response comparable to baselines.17
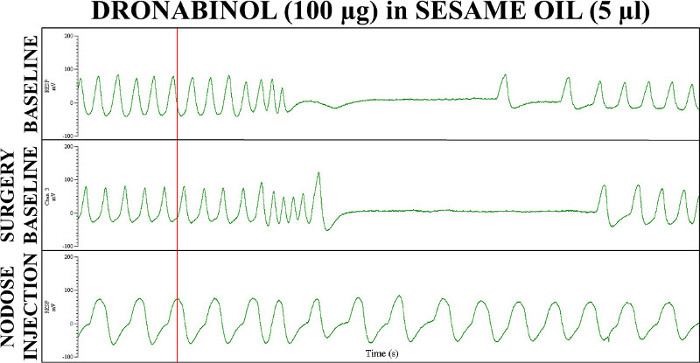
Figure 1: Sample recordings from acute 5-HT-indudced apnea experiments before and after 100 µg dronabinol in 5 µl sesame oil injections into the nodose ganglia of the vagus nerves.17 Respiratory recordings were taken before surgery (top panel), after surgery (middle panel), and after nodose ganglia injections (bottom panel). Red line signifies IV 5-HT infusion to induce apnea. After sesame oil injections (data not shown) into nodose ganglia of vehicle control rats, IV 5-HT infusion elicited an apneic response comparable to baselines.17 This figure has been modified from Calik et al.17 A.U. = arbitrary units; RESP = respiratory.
Discussion
The critical steps for successful injection of neurochemicals into the nodose ganglia are: 1) identifying and cleaning the connective tissue off the nodose ganglia; 2) confirming the integrity of the nodose ganglia before injection; 3) and using a small gauge needle to delicately inject into, but not completely puncturing through, the nodose ganglia.
The vagus nerve innervates many organs in the neck and abdomen, and relays important information such as heart rate, blood pressure, bronchopulmonary irritation, and gastrointestinal distension to the CNS. The nodose ganglia of the vagus nerve contain a diverse array of receptors for amino acids, monoamines, neuropeptides, and other neurochemicals.1 Intranodose ganglia injections of neurochemicals for those receptors to modify the activity of the vagus nerve have been done before,16,17 and represent an excellent experimental model to elucidate peripheral pharmacological effects on the vagus nerve that can modify diseases such as sleep apnea, gastroesophageal reflux disease, or chronic cough.2-4
To expose the nodose ganglia, neck surgery is conducted and muscles of the neck are separated to expose the internal carotid. The vagus nerves are seen running along the internal carotid and the pterygopalatine arteries. The cell bodies of the unipolar vagus nerves are located in the nodose and petrosal ganglia, which are seen as a swelling of the vagus nerves before entering the skull at the posterior lacerated foramens.5
Manipulations of the nodose ganglia are important insofar that the nodose ganglia and vagus nerves are not damaged during surgery. Improper and crude surgical technique can damage the nodose ganglia and its nerve branches, along with the arteries and the other cranial nerves. In this protocol, to stabilize the vagus nerve, we provide a slight tension to the vagus nerve via silk thread attached to a micro clip. This enables us to adequately clean any connective tissue that might make it more difficult to inject into the nodose ganglion. Moreover, we confirm that the nodose ganglia are intact before proceeding with the injection by inducing apnea by 5-HT infusion. To inject into the nodose ganglion, we used inexpensive small gauge (28 G, O.D. 362 µm) needles attached to a precision glass syringe. Since injection site depth to prevent puncturing through the nodose ganglia was a concern, the beveled tips of the syringes were custom made with a more vertical angle of 35°. This differs from other microinjection techniques in which glass micropipettes are used.8-10,14-16 The advantage of glass micropipettes is a smaller tip diameter; however, there is risk of breaking the tip during the procedure due to the fragility of glass. Moreover, expensive equipment is needed to pull the glass micropipette, which not every lab may possess. The custom-made needles have no risk of breaking, and though they were of bigger diameter (362 µm)17 compared to glass micropipettes (20-100 µl),8,9,14-16 the needles were still small enough for nodose ganglia injections. Moreover, these needles were reusable for many experiments and were considerable less expense than equipment used to pull micropipettes.
Due to the size of the custom-needles used for injection into nodose ganglia, it is important to assess damage, if any, to the nodose ganglia. One method of assessing damage to the nodose ganglia is through histological processing and microscopy of the nodose ganglia.6-9,13,15 A second method, which is used in this protocol, to assess vagal afferent function is comparing electrophysiological or behavioral outcomes between experimental and control injection groups.14-17 Dronabinol injections have been shown to attenuate 5-HT-induced apnea compared to control injections,17 signifying that if there was some damage from the larger diameter custom needles, there were still enough functional nodose ganglia cells that were inhibited by dronabinol. Similarly, quantifying the extent of diffusion of the 5 µl of dronabinol in oil is not necessary since functional outcomes (i.e., apnea) were the important metric of this protocol. The volume used in this protocol was chosen to guarantee wide diffusion of the solution injected into the nodose ganglia. In other similar protocols, injected volumes were not excessively different and ranged from 0.1 to 3 µl.8,9,14-16 The extent of diffusion of dronabinol of oil was sufficient to attenuate 5-HT-induced apnea.17
Modifications to this protocol are possible. First, if damage prevention to the nodose ganglia is a necessity, smaller gauge needles (smallest 33 G) can be used in this protocol. The drawback to using a smaller gauge needles is: 1) they can be easily broken or bent; 2) they the inner diameter is too small to allow for injection of viscous fluids like sesame oil. Moreover, if depth site injection is important, more vertical angle needles (up to 45°) can be purchased. However, a more vertical angle will make it more difficult to puncture the nodose ganglia. During injection of the nodose ganglia, modifying tension of the vagus nerve can be changed by placing micro clamps of various sizes. If damage to the vagus nerves/nodose ganglia needs to be prevented, then using smaller micro clamps is ideal. Lastly, injection of fluids containing neurochemicals can be modified via volume and concentration of fluid injected. In this protocol, 5 µl was more than enough to saturate the nodose ganglia.
A major limitation of this protocol is the possibility of puncturing through the nodose ganglia. If this occurs, then re-confirmation of the integrity of the nodose ganglia should be done. If the integrity of the nodose ganglia is confirmed, then nodose ganglia injection may be repeated. However, if there is doubt about the integrity of the nodose ganglia, then an experiment with a new rat should be completed.17
The diversity of receptors on the nodose ganglia can increase or inhibit afferent nerve activity. Increasing evidence suggests that afferent never activity plays an important role in human diseases.2-4,23 This protocol provides an inexpensive method of local administration of neurochemicals to the nodose ganglia to study their effect on afferent nerve activity.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This study was supported by National Institutes of Health (Grant 1UM1HL112856).
Materials
| Name of Material/ Equipment | Company | Catalog Number | Comments/Description |
| 5-HT HCl | MP Biomedicals | 215376591 | 12.5 µg/kg per 350 µl/kg |
| Dronabinol (Marinol) 10 mg Capsules (80 µg/µl) | AbbVie | NDC 0051-0023-21 | Dilute with sesame oil to 20 µg/µl |
| Sesame Oil | Sigma-Aldrich | S3547 | |
| Intramedic Polyethylene-50 | BD | 427411 | Ordered from VWR (Cat. # 63019-047) |
| Graefe Forceps | Roboz | RS-5138 | Two are needed |
| Johns Hopkins Bulldog Clamp | Roboz | RS-7441 | Three are needed |
| Piezoelectric Strain Gauge | Ambu | 813255-100 | |
| Data Acquisition USB Subsystems | DataWave Technologies | NA | |
| Sciworks Experimenter Software | NA | ||
| CyberAmp | Axon Instruments | NA | |
| Syringe, 500 µL, Model 1750 TLL | Hamilton Company | 81220 | |
| Syringe, 10 µL, Model 1801 RN | 7659-01 | ||
| Needle, 28 gauge, Small Hub RN | 7803-02 | Point Style 4, Angle 35, Length 0.5 in |
References
- Zhuo, H., Ichikawa, H., Helke, C. J. Neurochemistry of the nodose ganglion. Prog. Neurobiol. 52 (2), 79-107 (1997).
- Carley, D. W., Radulovacki, M. Pharmacology of vagal afferent influences on disordered breathing during sleep. Respir. Physiol. Neurobiol. 164 (1-2), 197-203 (2008).
- Kuo, P., Holloway, R. H. Beyond acid suppression: new pharmacologic approaches for treatment of GERD. Curr. Gastroenterol. Rep. 12 (3), 175-180 (2010).
- Maher, S. A., Dubuis, E. D., Belvisi, M. G. G-protein coupled receptors regulating cough. Curr. Opin. Pharmacol. 11 (3), 248-253 (2011).
- Greene, E. C. . Anatomy of the rat. , (1963).
- Li, Y., Wu, X., Zhou, S., Owyang, C. Low-affinity CCK-A receptors are coexpressed with leptin receptors in rat nodose ganglia: implications for leptin as a regulator of short-term satiety. Am. J. Physiol. Gastrointest. Liver Physiol. 300 (2), 217-227 (1152).
- Powley, T. L., et al. Vagal afferent innervation of the lower esophageal sphincter. Auton. Neurosci. 177 (2), 129-142 (2013).
- Neuhuber, W. L. Sensory vagal innervation of the rat esophagus and cardia: a light and electron microscopic anterograde tracing study. J. Auton. Nerv. Syst. 20, 243-255 (1987).
- Rogers, R. C., Nasse, J. S., Hermann, G. E. Live-cell imaging methods for the study of vagal afferents within the nucleus of the solitary tract. J. Neurosci. Methods. 150 (1), 47-58 (2006).
- Wan, S., et al. Presynaptic melanocortin-4 receptors on vagal afferent fibers modulate the excitability of rat nucleus tractus solitarius neurons. J. Neurosci. 28 (19), 4957-4966 (2008).
- Verberne, A. J., Lewis, S. J., Jarrott, B., Louis, W. J. Bezold-Jarisch reflex is inhibited by excitotoxin-induced destruction of vagal primary afferent neurons. Eur. J. Pharmacol. 139 (3), 365-367 (1987).
- Lewis, S. J., et al. Excitotoxin-induced degeneration of rat vagal afferent neurons. Neurosciences. 34 (2), 331-339 (1990).
- Wallick, D. W., Dunlap, M. E., Stuesse, S. S., Thames, M. D. Denervation of vagal cardiopulmonary receptors by injection of kainic acid into the nodose ganglia in dogs. Auton. Neurosci. 102 (1-2), 85-89 (2002).
- Muroi, Y., et al. Selective inhibition of vagal afferent nerve pathways regulating cough using Nav 1.7 shRNA silencing in guinea pig nodose ganglia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304 (11), 1017-1023 (2011).
- Kollarik, M., et al. Transgene expression and effective gene silencing in vagal afferent neurons in vivo using recombinant adeno-associated virus vectors. J. Physiol. 588 (21), 4303-4315 (2010).
- Zhang, Z., Zhang, C., Zhou, M., Xu, F. Activation of opioid mu-receptors, but not delta- or kappa-receptors, switches pulmonary C-fiber-mediated rapid shallow breathing into an apnea in anesthetized rats). Respir. Physiol. Neurobiol. 183 (3), 211-217 (2012).
- Calik, M. W., Radulovacki, M., Carley, D. W. Intranodose ganglion injections of dronabinol attenuate serotonin-induced apnea in Sprague-Dawley rat. Respir. Physiol. Neurobiol. 190, 20-24 (2014).
- Yoshioka, M., Goda, Y., Togashi, H., Matsumoto, M., Saito, H. Pharmacological characterization of 5-hydroxytryptamine-induced apnea in the rat. J. Pharmacol. Exp. Ther. 260 (2), 917-924 (1992).
- Kopczynska, B., Szereda-Przestaszewska, M. 5HT2 and 5HT3 receptors’ contribution to modeling of post-serotonin respiratory pattern in cats. Life Sci. 75 (19), 2281-2290 (2004).
- Fan, P. Cannabinoid agonists inhibit the activation of 5-HT3 receptors in rat nodose ganglion neurons. J. Neurophysiol. 73 (2), 907-910 (1995).
- Jespersen, B., Knupp, L., Northcott, C. A. Femoral arterial and venous catheterization for blood sampling, drug administration and conscious blood pressure and heart rate measurements. J. Vis. Exp. (59), (2012).
- Barann, M., et al. Direct inhibition by cannabinoids of human 5-HT3A receptors: probable involvement of an allosteric modulatory site. Br. J. Pharmacol. 137 (5), 589-596 (2002).
- Bonaz, B., Picq, C., Sinniger, V., Mayol, J. F., Clarencon, D. Vagus nerve stimulation: from epilepsy to the cholinergic anti-inflammatory pathway. Neurogastroenterol. Motil. 25 (3), 208-221 (2013).

