Comparative Analysis of Human Growth Hormone in Serum Using SPRi, Nano-SPRi and ELISA Assays
Summary
The proposed work assesses the diagnostic potential of direct and amplified surface plasmon resonance imaging (SPRi) assays, particularly for the detection of recombinant human growth hormone in spiked human serum, by comparing SPRi results directly with commercially available enzyme-linked immunosorbent assay (ELISA) kit.
Abstract
Sensitive and selective methods for the detection of human growth hormone (hGH) over a wide range of concentrations (high levels of 50-100 ng ml−1 and minimum levels of 0.03 ng ml−1) in circulating blood are essential as variable levels may indicate altered physiology. For example, growth disorders occurring in childhood can be diagnosed by measuring levels of hGH in blood. Also, the misuse of recombinant hGH in sports not only poses an ethical issue it also presents serious health threats to the abuser. One popular strategy for measuring hGH misuse, relies on the detection of the ratio of 22 kDa hGH to total hGH, as non-22 kDa endogenous levels drop after exogenous recombinant hGH (rhGH) administration. Surface plasmon resonance imaging (SPRi) is an analytical tool that allows direct (label-free) monitoring and visualization of biomolecular interactions by recording changes of the refractive index adjacent to the sensor surface in real time. In contrast, the most frequently used colorimetric method, enzyme-linked immunosorbent assay (ELISA) uses enzyme labeled detection antibodies to indirectly measure analyte concentration after the addition of a substrate that induces a color change. To increase detection sensitivity, amplified SPRi uses a sandwich assay format and near infrared quantum dots (QDs) to increase signal strength. After direct SPRi detection of recombinant rhGH in spiked human serum, the SPRi signal is amplified by the sequential injection of detection antibody coated with near-infrared QDs (Nano-SPRi). In this study, the diagnostic potential of direct and amplified SPRi was assessed for measuring rhGH spiked in human serum and compared directly with the capabilities of a commercially available ELISA kit.
Introduction
Human growth hormone (hGH) is a 191 amino acid peptide (22 kDa) produced by the pituitary gland and directly released into the bloodstream. Interactions between the hypothalamic peptide growth hormone-releasing hormone (GHRH) and somatotropin induce pulsatile secretions of hGH. As a result, levels of hGH vary from highs in the 50-100 ng/ml to lows in the 0.03 ng/ml range1. Deficiency or excess of hGH in the body can provoke a wide range of abnormal physiological symptoms. For example, excess levels of hGH can lead to gigantism2 and diabetes3. Depleted levels of hGH cause low blood sugar in newborns, and weak bone density and depression in adults4.
The administration of the recombinant form of hGH (rhGH) improves lean muscle mass while reducing body fat. As such, this substance became the drug of choice for professional and amateur athletes as it improves physical strength that confers an advantage in competitive sports. rhGH is banned by the World Anti-Doping Agency (WADA)5,6 and much effort by international researchers has been focused on developing tests that can detect its presence or anabolic effect.
Enzyme-linked immunosorbent assay (ELISA) has been the preferred method for the determination of hGH in whole blood7. Although, ELISA is a reliable technique offering good sensitivity and selectivity, it is relatively time- and labor-intensive. In addition, ELISA relies on the indirect detection of hGH by employing enzymatic tags. In contrast, surface plasmon resonance (SPR) permits detection of hGH directly without the use of labels in real time. The detection principle behind SPR involves a sensing surface consisting of a prism that is coated with a thin metal layer (gold or silver); when a monochromatic polarized light interacts with the metal surface, "surface plasmons" are generated. The binding of an analyte to a surface receptor immobilized on the metal surface perturbs the resonance conditions resulting in a shifted resonance dip, which can then be correlated to the analyte concentration. SPR-based biosensors are now commercially available that offer a real-time, label free technique to monitor biomolecular binding events and biochemical reactions8-10. More recently, SPRi was developed in response to the need for multiplexing (i.e., monitoring multiple binding events simultaneously), which was not possible in classical SPR biosensors. Thus, SPRi has emerged as a tool to monitor several binding events simultaneously. Current SPRi systems are based on microscopic imaging of a surface which is excited with light at a specific angle and wavelength10. The image is then captured onto a charge-coupled device (CCD) array.
To date, there have been a few SPR-based assays developed to detect hGH11-14. One particular strategy, known as the isoform method15, relies on the detection of the ratio of 22 kDa hGH to total hGH, as non-22-kDa endogenous levels drop after exogenous rhGH administration. Recently, de Juan-Franco et al.11 reported on the development of a SPR-based immunosensor for the selective detection of the 22 kDa and 20 kDa hGH isoforms in human serum samples. Monoclonal antibodies specific to each isoform were immobilized directly on the gold sensor permitting the measurement of both isoforms simultaneously in a single injection with a limit of detection at 0.9 ng ml-1. Alternatively, SPR has been used to screen antibodies with high specificity to hGH13. If the concentration of target analyte falls below the SPRi system's limit of detection (<nM), one has to resort to amplifying the SPRi signal via the utilization of nanoparticles (Nano-SPRi). Such SPR-based amplification has been well documented in the literature16-19 for various types of analyte and surfaces.
In this work, the analytical potential of SPRi and Nano-SPRi based biosensors was examined, particularly for the detection of rhGH in spiked human serum, and comparison of its detection capability directly to ELISA. The following parameters will be reviewed and considered: detection time, sensitivity, kinetic profile, reproducibility and specificity.
Protocol
1. Preparation of Solutions and Protein Samples for SPRi
- Prepare 100 ml of low salt phosphate buffered saline (PBS) solution containing 10 mM phosphate, 150 mM sodium chloride, and pH 7.4.
- Prepare 50 ml of high salt PBS solution containing 10 mM phosphate, 750 mM sodium chloride, and pH 7.4.
- Prepare 5 ml of 10 mM sodium acetate.
- Prepare a stock of 5 mg/ml bovine serum albumin (BSA) diluted in low salt PBS solution.
- Prepare a stock solution of recombinant human growth hormone (rhGH) at a concentration of 1 μg/ml in low salt PBS solution.
- Prepare stock solutions of anti-rhGH and negative control antibody at a concentration of 100 μg/ml.
2. Prepare SPRi Chip for Antibody Array
- Clean the chip by sonication in 120 ml of stabilized piranha solution (3:1 concentrated sulfuric acid to 30% hydrogen peroxide) for 90 min at 50 °C and follow by rinsing and sonication with water for 5 min. Then, rinse chip with ethanol and dry with nitrogen stream.
- Place the gold chip in an UV/Ozone chamber for 30 min to remove any contaminants.
- Add 150 mg of 11-mercaptoundecanoic acid to 20 ml ethanol in a test tube that contains a stir bar and a tube cap.
- Place chip in the test tube and microwave tube/chip at 50 W and 50 °C for 5 min.
- Rinse chip with ethanol and soak in ethanol for 5 min.
- Follow by a rinse with water and soak in water for 5 min.
- Add 150 mg of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide to 20 ml water in a test tube that contains a stir bar and a tube cap. Microwave chip as in step 2.4.
- Rinse chip with water and soak in water for 5 min.
- Add 150 mg of N-hydroxysuccinimide in 20 ml water in a test tube that contains a stir bar and a tube cap. Microwave chip as in step 2.4.
- Rinse chip with water and soak in water for 5 min.
- Prepare 250 µM polyethylene glycol (PEG, 800 Da) in 20 ml water in a test tube that contains a stir bar and a tube cap. Microwave chip as in step 2.4.
Note: Microwaving PEG800 on the surface of the sensor is performed as a blocking step to prevent non-specific binding of serum proteins and quantum dot complexes later used in the experiment. This is achieved by PEG800 reacting with non-occupied bare gold sites on the chip. - Rinse chip with water and soak in water for 5 min.
- Prepare 15 μg/ml of anti-rhGH antibody solution and negative control solution of anti-Immunoglobulin G (anti-IgG) and add 10 µl of each antibody solution to a well in the 384 well plate.
- Design the spotting pattern in the microarrayer (4 x 7 quadrants for each sample) and spot chip with antibodies using a 500 μm Teflon tipped pin.
- Incubate spotted chip for 2 hr at RT and under a humid atmosphere of 75% or greater.
- Rinse and soak chip in water for 5 min. Then, dry chip with nitrogen stream.
3. SPRi Experiment Setup and Blocking
- Initialize the instrument and select directory. Select real time camera and start the syringe pump at 1 ml/min for 10 ml of degassed water. Insert chip into the instrument and keep injecting water until all bubbles are removed.
- After rinsing chip with 10 ml of water, start image acquisition, and follow by switching the buffer to low salt PBS and allow it to run at 1 ml/min for 5 ml and then slow flow rate down to 20 µl/min for 20 min.
- Select the highly contrasted image, which shows the immobilized antibodies spotted onto the chip.
- Select and define the 400 µm individual circular spots corresponding to the immobilized antibodies.
- After the instrument traces the plasmon curves, choose a working angle that has the highest slope in the reflectivity curves.
- Flow a running buffer at 20 µl/min of low salt PBS through the system until the signal from all of the selected spots stabilizes.
- Load the BSA (100 µg/ml) in running buffer into the sample loop (150 µl) with the injection valve in the load position. Then switch the valve to the injection position to inject the solution to the flow cell.
Note: BSA is injected to covalently bind to the amine reactive surface, the remaining unbound BSA will wash off the surface through buffer rinse. - Inject 150 µl of a solution of 10 mM sodium acetate over the surface and follow by a 150 µl injection of high salt PBS.
- Inject 150 µl of ethanolamine (1 mM) to deactivate the amine reactive surface.
- Inject 150 µl of 10 mM sodium acetate to wash the surface.
- Then, switch the running buffer to PBS with 50 µg/ml BSA at a flow rate of 250 µl/min for a total of 5 ml.
Note: 50 µg/ml BSA is added to the running buffer for additional blocking of the surface by non-covalently occupying the non-specific sites and this is done to further reduce non-specific binding of serum proteins to the surface. - Change flow rate to 5 µl/min and allow it to stabilize for 20 min.
4. SPRi Detection of Human Growth Hormone
- Prepare a solution of a set concentration of rhGH (30,000 pg/ml; 250 pg/ml; 25 pg/mL; 2.5 pg/ml and 0.25 pg/ml) in 10% serum and diluted in PBS containing 1 mg/ml BSA.
- Prepare a 2:1 solution by adding 1 µl of biotin labeled anti-rhGH detection antibodies (6 µM) to 3 µl of streptavidin coated near infrared quantum dots (1 µM) in a 0.5 ml microcentrifuge tube and incubate for 30 min for effective coupling.
- Inject 150 µl of the hGH spiked human serum solution in the injection loop. This results in a strong increase in signal followed by a slow drop from the non-specifically bound serum rinsing off the surface.
- Once the signal stabilizes, wash the surface with a solution of 450 mM sodium chloride added to the running buffer.
- Dilute the solution of anti-rhGH detection antibodies and quantum dots to a concentration of 10 nM (2:1) with running buffer (PBS with 50 µg/ml BSA) and inject into the flow cell.
5. SPRi Data Analysis
Note: Following the injection of the rhGH spiked human serum and quantum dot enhancer, an increase in reflectance change occurs due to the amplified shift of the plasmon curves. This results in a difference image showing a gray scale image correlating to the signal of on the chip.
- Import the data into a data analysis program. Plot the SPRi signal (% Reflectivity) versus time (s) and determine the difference between the anti-rhGH and negative control antibody spots after the high salt wash.
6. ELISA Protocol (Day 1)
- Store all assay components included in the rhGH ELISA kit at 2-8 °C. This includes the anti-rhGH antibody coated well plates, standards (0-5) in sheep serum, controls (1 &2) in human serum, conjugate buffer, (200x) wash buffer, chromogen TMB (Tetramethylbenzidine) and stop reagent.
- Setup the reagents and follow the methods as described in the commercial ELISA kit protocol.
- First, allow the anti-rhGH antibody coated 96-wells to warm to RT prior to opening the foil package.
- Then remove the desired number of 8-well strips for assay and reseal the foil package containing the remaining wells and store at 2-8 °C.
- Prepare standards by reconstitution in 2 ml of distilled water for standard #0, in 1 ml of distilled water for standards (1-5), and in 1 ml of distilled water for the controls (1&2). Below is a table of the corresponding concentrations of the standards and controls (Table 1):
| Standards | Concentration (ng/ml) |
| 0 | 0 |
| 1 | 0.17 |
| 2 | 0.94 |
| 3 | 2.63 |
| 4 | 10.4 |
| 5 | 26.9 |
| Control #1 | 1.22 ± 0.33 ng/ml |
| Control #2 | 4.97 ± 1.25 ng/ml |
Table 1. Concentrations of the standards and controls included in the commercial ELISA kit.
- Prepare the samples which consist of the rhGH hormone in 10% human serum at the following concentrations (Table 2):
| Sample | Concentration (ng/ml) |
| 1 | 0.025 |
| 2 | 0.2 |
| 3 | 0.5 |
| 4 | 2.5 |
| 5 | 10 |
| 6 | 30 |
Table 2. Concentrations of the rhGH samples prepared in 10% Serum.
- Add 50 μl of each standard, control and sample to each well (in triplicates). Seal the wells and incubate with the standards, controls, and rhGH samples O/N at 4 °C under gentle shaking.
7. ELISA Protocol (Day 2)
- Remove the plate from the shaker and allow it to warm to RT (15-20 min) prior to proceeding with the assay.
- Remove the Anti-rhGH-HRP, conjugate buffer, wash buffer (200x), chromogen TMB (tetramethylbenzidine), and stop reagent from the refrigerator and allow warming to RT.
- Reagent preparation: (according to manufacturer's specifications)
- Dilute the Anti-rhGH-HRP 40x with the conjugate buffer. Prepare the wash buffer by diluting 200x with distilled water.
- Add 50 μl of Anti-rhGH-HRP conjugate into each well, seal the wells and incubate at RT for 30 min while gently shaking.
- Decant the solution from the wells and invert the plate and tap dry onto an absorbent tissue. Then add 200 μl of wash buffer into each well.
- Decant the wash solution and tap dry onto an absorbent tissue. Repeat this step 3 times.
- Add 100 μl of Chromogen into each well within 15 min following the wash step. Incubate for 30 min at RT in the dark while gently shaking. The solutions turn from colorless to blue.
- Add 100 μl of the stop reagent into each well. The solutions turn from blue to yellow. Immediately, read the absorbance of each well at 450 nm using a microplate reader.
- Plot the standard curve for the provided standards (0-5). Then plot the optical density of the rhGH in 10% serum samples versus the concentration of each sample.
Representative Results
The performance of SPRi and Nano-SPRi (SPRi employing the NanoEnhancers) was compared with ELISA for the detection of rhGH in a complex environment. The differences in the setup of these methods are described here briefly. For SPRi (direct detection, Figure 1), the capture antibody is immobilized on the surface and then the sample is injected and binding of analyte to the sensor surface is measured directly in real time and label-free manner. However, with Nano-SPRi (Figure 1), after the analyte binds to the sensor surface, a consecutive injection is followed with quantum dots coated with detection antibodies to amplify the SPRi signal.
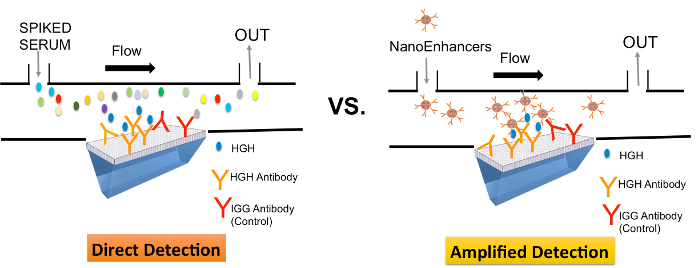
Figure 1. A schematic representation comparing direct-mode (SPRi) and amplified-mode (Nano-SPRi) of detection of rhGH in crude samples. Ligands (rhGH or IgG specific antibodies) were immobilized in an array format on the SPRi biochip. Target protein (rhGh) spiked in human serum introduced to the sensor surface are directly detected (SPRi) and sequentially highlighted with NanoEnhancers (Nano-SPRi). Please click here to view a larger version of this figure.
As for ELISA, the multiwell plates arrive already pre-functionalized with capture antibody and then sample is introduced, the analyte of interest will bind. A detection antibody is introduced followed by substrate addition. The optical density is then measured at 450 nm. In this study, a commercial ELISA kit (Figure 2) was used to measure rhGH spiked in 10% human serum.
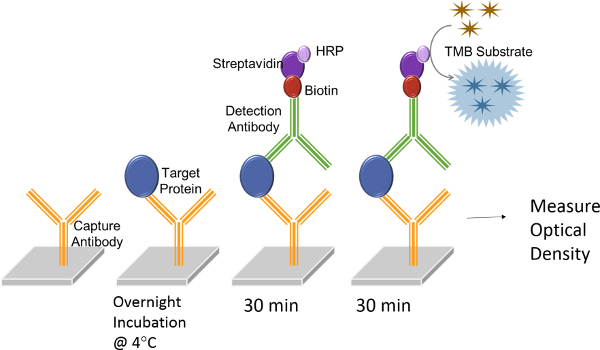
Figure 2. A schematic representation of ELISA assay procedure. rhGH protein (blue ovals) is introduced to wells that have been pre-functionalized with monoclonal antibodies (yellow) specific to rhGH. Non-specific interactions are eliminated by rinsing the wells with wash buffer followed by the introduction of a detection antibody prefunctionalized with horseradish peroxidase (HRP, purple). The solution will change in color after adding the substrate tetramethylbenzidine (TMB, gold). Please click here to view a larger version of this figure.
Figure 3 represents the titration curve of rhGH spiked in 10% human serum and is plotted against the obtained OD at 450 nm. A good linear response was observed and the limit of detection was determined to be 1 ng/ml. The coefficient of variation (CV) was 6.5% suggesting good reproducibility.
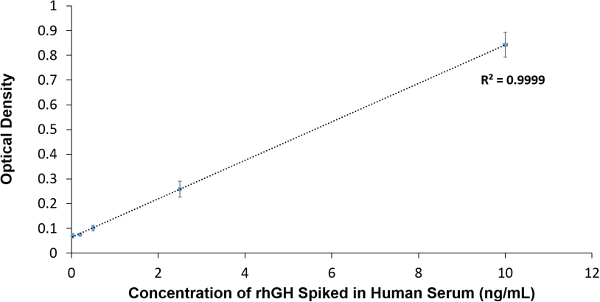
Figure 3. ELISA data Analysis. Concentration of rhGH spiked in serum is plotted against the obtained OD at 450 nm. Please click here to view a larger version of this figure.
Next, the detection of rhGH spiked in human serum was assessed with SPRi. Direct detection of rhGH resulted with the corresponding concentration gradient curve (Figure 4), each point represents the average value of the reflectivity difference calculated from three SPRi kinetic curves for each concentration. The limit of detection (LOD) was determined to be 3.61 ng/ml. The SPRi direct detection assay was highly reproducible as the CV of the assay was only 4.1%.
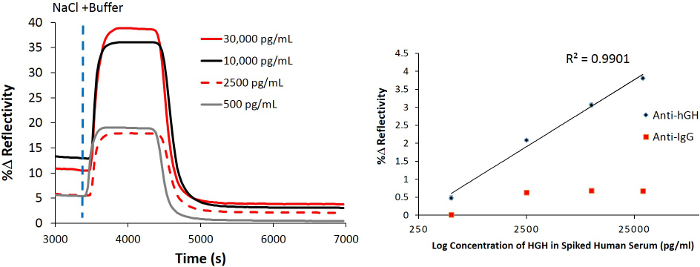
Figure 4. Direct SPRi detection of hGH spiked in 10% human serum. The resultant normalized SPRi kinetic plot after the injection of various amounts of hGH spiked in human serum followed by the injection of a high salt buffer (dashed vertical line) to remove non-specific interactions . A concentration gradient curve representing the binding of various amounts of hGH spiked in human serum to the sensor surface that has been prefunctionalized with biotinylated hGH-specific-Antibody . Please click here to view a larger version of this figure.
To increase the sensitivity of the SPRi biosensor, NanoEnhancers (QDs pre-functionalized with detection antibodies) are sequentially introduced to the sensor surface in order to highlight the presence of rhGH spiked in human serum. After background subtraction, the NanoEnhancers were able to amplify the biosensor response up to 7.9%, however, with minimal signal change (Immunoglobulin G (IgG) -specific antibody, 0.38% change in reflectivity; Figure 5 on controlled regions of interest. Imaging of the sensor surface revealed that only regions of interest that have rhGH-specific antibodies immobilized experience the largest contrast change corroborating directly with the kinetic sensorgram response.
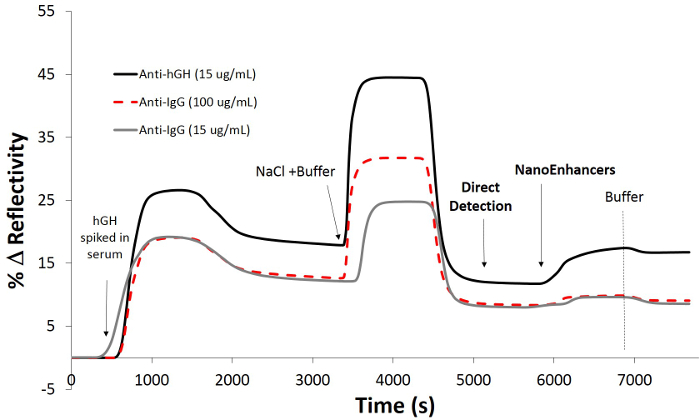
Figure 5. Detection of rhGH using a sandwich assay spiked in 10% human serum. SPRi kinetic plot after the injection of rhGH (30 ng/ml) in buffer (10 mM PBS, 150 mM sodium chloride, pH = 7.4) onto a pre-functionalized chip with 11-mercaptoundecanoic acid / rhGH-specific antibody and control IgG-antibody then blocked with BSA followed by the addition of detection antibody-coated quantum dots (NanoEnhancers). Please click here to view a larger version of this figure.
To demonstrate the practicability of the Nano-SPRi biosensor, the measuring range of rhGH in crude samples was assessed. An extended working range from 30,000 pg/ml to 0.25 pg/ml resulted as a response to the addition of NanoEnhancers (Figure 6). It is worth noting that each point on the titration curve is averaged from three independent experiments. Consequently, the lower limit of detection was calculated to be 9.20 pg/ml and the coefficient of variation was 20%.
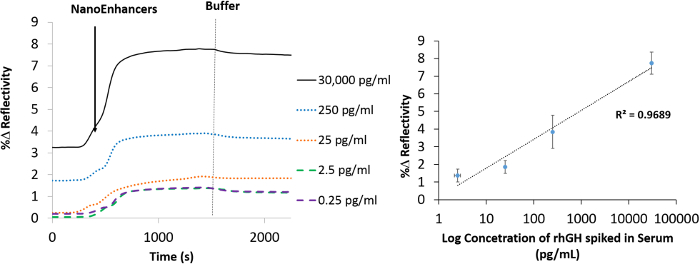
Figure 6. Nano-SPRi detection of hGH spiked in human serum. Normalized SPRi kinetic plot representation of hGH_specific_Anti-QDs-amplified signal for human serum samples spiked with different concentrations of hGH. A vertical dashed line (grey) represents the injection point of the running buffer. (b) A concentration gradient curve representing the binding of NanoEnhancers (hGH_specific_Anti-QDs) after the injection of various amounts of hGH spiked in human serum to the sensor surface that has been prefunctionalized with 11-mercaptoundecanoic acid /hGH-specific antibody. Please click here to view a larger version of this figure.
Next, the change that occurs in the SPR reflectivity curve as a function of the concentration was compared between the direct and the NanoEnhancer bioassay method (Figure 7). For the direct detection technique between 25 and 0.25 pg/ml, the signal started to plateau and this is not surprising as these concentrations fall below the LOD of 3 ng/ml (Figure 7). Similarly, for the amplified technique, concentrations below the LOD of 9.2 pg/ml signal started to plateau and showed virtually no variation.
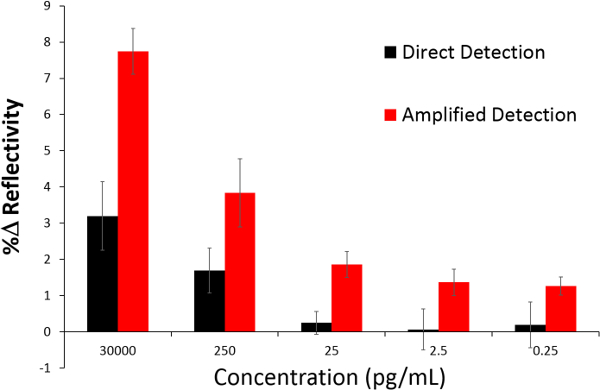
Figure 7. A comparative analysis of SPRi with Nano-SPRi. This bar graph depicts the percent change in reflectivity (%R) after introduction of rhGH spiked in human serum (direct detection) followed by the injection of NanoEnhancers (amplified detection) for 30,000 pg/ml, 250 pg/ml, 25 pg/ml, 2.5 pg/ml and 0.25 pg/ml. Please click here to view a larger version of this figure.
Furthermore, the specificity of the Nano-SPRi biosensor was assessed. Insulin-like growth factor-1 (IGF-1) spiked in human serum was injected as a control since hGH stimulates the secretion of IGF-1 through the growth hormone receptor on the hepatocyte membrane. After the injection of the IGF-1 in serum, the NanoEnhancers were sequentially injected and the SPR signal response did not show any specific binding (Figure 8). However, when rhGH spiked in serum sample was injected to the very same spot functionalized with rhGH antibody, signal enhancement was observed. In conclusion, the Nano-SPRi platform has demonstrated excellent specificity and selectivity for rhGH.
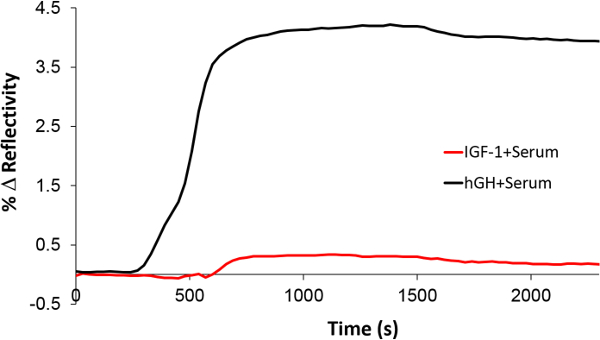
Figure 8. Assessment of Nano-SPRi selectivity towards rhGH. The Nano-SPRi biosensor response after the injection of rhGH (black) and IGF-1 (red) in spiked serum. Please click here to view a larger version of this figure.
A correlation analysis was performed using the Pearson correlation coefficient to determine the correlation between the SPRi signal intensity and ELISA optical density values (Figure 9). A p-value <0.01 was considered significant. As illustrated in this graph, there is a good correlation between the rhGH levels in spiked human serum measured by SPRi and ELISA. The r-value was 0.9263 for 9 different samples.
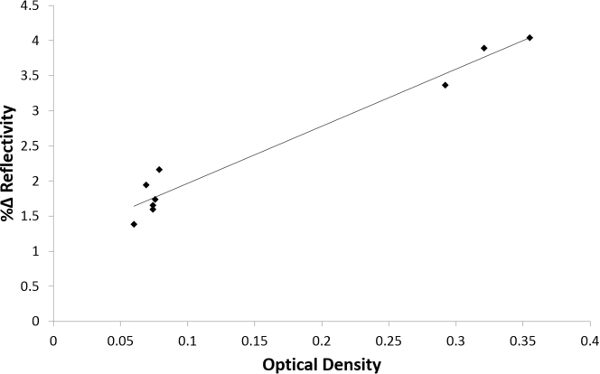
Figure 9. Pearson correlation analysis between ELISA and Nano-SPRi. The plot correlates Nano-SPRi signal intensity (y-axis) with ELISA optical density (x-axis) values. (Pearson correlation coefficient n = 9, r = 0.9263, p = 0.00000183). Please click here to view a larger version of this figure.
The equilibrium dissociation constant (KD) was determined using the graphpad software for ELISA. The calculated KD value was approximately 79 nM (Table 3). The on (Ka) and off (Kd) rates could not be determined by ELISA. However, using the data analysis software, the direct detection method resulted with KD value of 23 pM using the molecular mass of 22 kDa that corresponds to one rhGH molecule. The on and off rates were calculated to be 6.1 x107 M-1s-1 and 1.33 x 10-3 sec-1, respectively. This inherently translates that 0.13% of the rhGH and antibody complexes decay per second. As for the amplified SPRi experiment, a stronger overall interaction was observed between NanoEnhancers and rhGH as the calculated binding affinity was determined to be 4.3 pM. In addition, a stronger association rate was observed for NanoEnhancers and rhGH than capture antibody/rhGH, however the dissociation rate suggests that 0.26% of NanoEnhancer/rhGH/Capture antibody decay per second.
| Method | KD (Affinity) | Ka (on-rate) | Kd (off-rate) | LOD |
| ELISA | 79.45 x 10-9 M | NA | NA | 1 ng/ml |
| SPRi | 23.2 x 10-12 M | 6.1 x 107 M-1sec-1 | 1.33 x10-3 sec-1 | 3.61 ng/ml |
| Nano-SPRi | 4.33 x 10-12 M | 7.54 x108 M-1sec-1 | 2.62 x10-3 sec-1 | 0.0092 ng/ml |
Table 3. Full kinetic data analysis. Evaluation of the affinity, on-rate and off-rate of the antibody responses using graphpad (ELISA) and data analysis (SPRi and Nano-SPRi) software. The determination of the avidity using the molecular mass of 22 kDa that corresponds to one rhGH molecule was chosen. The limits of detection were determined for all three studies using excel.
Discussion
Irregular levels of hGH, a naturally occurring hormone, have been linked to numerous medical disorders that affect human growth and development. Moreover, exogenous administration of rhGH is commonly used by athletes, even though it is forbidden, as a doping agent to enhance their performance. Challenges in detecting rhGH misuse result from the difficulty in distinguishing exogenous hGH from endogenous form. As such, the current approved technique for detecting exogenous hGH relies on measuring the ratio of the 22 kDa hGH isoform in relation to the 20 kDa isoform. Since the isoform test demands for the measurement of multiple hGH isoforms simultaneously within a short period of time in a wide range of concentrations, therefore, we considered the SPRi platform as a perfect match. In addition, endogenous hGH level fluctuate to a very low level (0.03 ng/ml) in the bloodstream therefore the detection system must be able to measure this range comfortably with high specificity. As a result, we also investigated in this study the potential of Nano-SPRi as a diagnostic tool for hGH and compared it directly with SPRi and the classical immunoassay ELISA.
Based on results obtained from this study, the main advantage of the SPRi and Nano-SPRi method is that rhGH concentrations can be measured in a quicker manner in comparison to the more conventional method ELISA. A standard duration for measuring rhGH levels in one sample with the direct detection method was 1 hr whereas the Nano-SPRi required 2 hr due to the additional steps in the process. Overall, with SPRi and Nano-SPRi experiments, before the injection of a sample, a calibration step is highly recommended. In addition, the injection of a crude sample like human serum results in some non-specific interactions as a result it is imperative to inject a high salt wash buffer to only reveal specific interactions. It is also worth noting that a wash step is absolutely necessary after the introduction of blocking molecules to the sensor surface, to remove unbound molecules. As for ELISA time requirements are far greater (~16-18 hr) for the analysis of one sample. A longer incubation time is needed as the sensitivity of the assay is enhanced especially for this study, as the focus was to compare the lower limit of detection.
The choice of surface chemistry will vary from one application to another and this could be realized as one of the limitation of the SPRi technique. In this study, a wide range and combination of chemical linkers and blocking molecules were assessed to achieve the right combination in order to observe optimal binding efficiency of rhGH to the sensor surface. For example, in this study, the combination of BSA and PEG served well in minimizing non-specific interactions. However, in a previous study17, where the capture ligand was an aptamers, PEG alone served as the best blocking molecule. The variables that affect binding efficiency of analyte to ligand are also dependent on pH, buffer and temperature. Therefore, with any application, these variables need to be optimized. In addition, it is critical to determine the optimal spotting concentration of the ligand to the chip surface. A titration experiment with a range of concentrations of immobilized ligands is performed before initiating the study. As for ELISA, a crucial step in the procedure was to decant the wash buffer from wells by tapping the microplate as this ensured no residual liquid is leftover. Removing the buffer wash with pipette was not sufficient as any residual liquid interfered with the signal reading of the target sample.
In reference to sensitivity, ELISA (1 ng/ml) is comparable to SPRi (3.61 ng/ml) but nano-SPRi (9.20 pg/ml) improves sensitivity by three orders of magnitude, thereby enabling measurements at the lower biological levels of rhGH 0.03 ng/ml. As we previously reported16,17, the signal enhancement imparted by the NanoEnhancers is attributed to a mass loading effect and the strong coupling that exists between NIR fluorophores and propagating surface plasmons for gold film nanostructures. Even though, Nano-SPRi adds an extra step to the procedure, this level of sensitivity can widen the applications of SPRi technology in various outlets.
SPRi provides scientists a full kinetic profile (KD, Ka and Kd) of antibody/rhGH interaction whereas ELISA can report only affinity values. The coefficient of variation (CV) was below 10% for SPRi (4.1%) and ELISA (6.5%), suggesting good reproducibility. The ELISA and SPRi affinity values are different because the capture antibodies immobilized on the sensor chip are different from antibodies immobilized in the ELISA 96-well plate. As for Nano-SPRi a higher CV value (20%) was observed. There are several parameters that can contribute as a source of errors for CV determination. For example, with the Nano-SPRi experiment a much lower concentration of analyte is being measured, the addition of NanoEnhancers adds another step in the procedure and the experiment was performed manually. A very good correlation between Nano-SPRi and ELISA was achieved for the detection of rhGh in spiked human serum. Finally, ELISA can be a reliable technique however the method itself is time consuming which makes it difficult to use in situations requiring real time monitoring and multiplexing, as it is the case with hGH. In addition, a more attractive feature that was not investigated directly in this study that SPRi offers over ELISA, is the ability to measure hundreds of interactions simultaneously in real time. Therefore in the future, Nano-SPRi method will be assessed to detect multiple biomarkers simultaneously in real time (multiplexing) present in serum at various concentrations in order to examine its potential as a viable clinical diagnostic tool.
Divulgations
The authors have nothing to disclose.
Acknowledgements
NanoManufacturing Innovation Consortium for funding support.
Materials
| Somatotropin Growth Hormone (GH1) capture antibody | Acris Antibodies | DM1015 | |
| Bovine Serum Albumin | Fisher Bioreagents | BP671-10 | |
| Biotin labeled Somatotropin Growth Hormone (GH1) detection antibody | Acris Antibodies | AM09304BT-N | |
| 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide | TCI America | D1601 | |
| Ethanolamine | Acros Organics | 141-43-5 | |
| Ethanol | Fisher Chemicals | BP2818-4 | |
| Human HGH ELISA Kit | invitrogen | KAQ1081 | |
| Human Serum | Sigma Aldrich | H4522 | |
| Rabbit IgG Antibody | Sigma Aldrich | I5006 | |
| 11-mercaptoundecanoic acid | Sigma Aldrich | 450561 | |
| Nanostrip | Cyantek | ||
| N-hydroxysuccinimide | Sigma Aldrich | 130672 | |
| Phosphate Buffer Saline | Invitrogen | 00-3002 | |
| Polyethylene glycol (800 Da) | Sigma Aldrich | 729108 | |
| Recombinant Human Growth Hormone | Calbiochem | 869008 | |
| Sodium Acetate | Sigma Aldrich | 127-09-3 | |
| Sodium Chloride | Fisher Chemicals | 7647-14-5 | |
| Streptavidin coated near infrared quantum dots | Life Technologies | Q10171MP | |
| UV/Ozone Procleaner | Bioforce Nanosciences | ||
| Microwave reactor | CEM Corporation | Discover system | |
| SPRi-Arrayer | LabNext Xactll Microarray System | ||
| SPR biochip | HORIBA | ||
| SPRi-Lab+ | HORIBA | ||
| Synergy Mx Multimode Microplate reader | BioTek | ||
| ScrubberGen | HORIBA | Data Analysis software |
References
- Popii, V., Baumann, G. Laboratory measurement of growth hormone. Clinica Chimica Acta. 350 (1-2), 1-16 (2004).
- Higham, C. E., Trainer, P. J. Growth hormone excess and the development of growth hormone receptor antagonists. Exp Physiol. 93 (11), 1157-1169 (2008).
- Sönksen, P., Salomon, F., Cuneo, R. Metabolic effects of hypopituitarism and acromegaly. Horm Res. 36, 27-31 (1991).
- Molitch, M., et al. Evaluation and Treatment of Adult Growth Hormone Deficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 91 (5), 1621-1634 (2006).
- . . The World Anti-Doping Code: The 2015 Prohibited List International Standard. , (2015).
- . . The 2014 prohibited list world anti-doping code. , (2014).
- Langkamp, M., Weber, K., Ranke, M. B. Human growth hormone measurement by means of a sensitive ELISA of whole blood spots on filter paper. Growth Horm IGF Res. 18 (6), 526-532 (2008).
- Rich, R. L., Myszka, D. G. Survey of the year 2007 commercial optical biosensor literature. J Mol Recognit. 21 (6), 355-400 (2008).
- Homola, J. Present and future of surface plasmon resonance biosensors. Anal Bioanal Chem. 377 (3), 528-539 (2003).
- Hoa, X. D., Kirk, A. G., Tabrizian, M. Toward Integrated Surface Plasmon Resonance Biosensors: A Review of Recent Progress. Biosens Bioelectron. 23 (2), 151-160 (2007).
- de Juan-Franco, E., Rodrìguez-Frade, J. M., Mellado, M., Lechuga, L. M. Implementation of a SPR immunosensor for the simultaneous detection of the 22K and 20K hGH isoforms in human serum samples. Talanta. 114, 268-275 (2013).
- de Juan-Franco, E., Caruz, A., Pedrajas, J. R., Lechuga, L. M. Site-directed antibody immobilization using a protein A-gold binding domain fusion protein for enhanced SPR immunosensing. Analyst. 138 (7), 2023-2031 (2013).
- Du, H., et al. Immunological screening and characterization of highly specific monoclonal antibodies against 20 kDa hGH. Bioanalysis. 4 (17), 2161-2168 (2012).
- Treviño, J., Calle, A., Rodrìguez-Frade, J. M., Mellado, M., Lechuga, L. M. Surface plasmon resonance immunoassay analysis of pituitary hormones in urine and serum samples. Clin Chim Acta. 403 (1-2), 56-62 (2009).
- Wu, Z., Bidlingmaier, M., Dall, R., Strasburger, C. J. Detection of doping with human growth hormone. Lancet. 353 (9156), 895 (1999).
- Malic, L., Sandros, M. G., Tabrizian, M. Designed biointerface using near-infrared quantum dots for ultrasensitive surface plasmon resonance imaging biosensors. Anal. Chem. 83 (13), 5222-5229 (2011).
- Vance, S. A., Sandros, M. G. Zeptomole Detection of C-Reactive Protein in Serum by a Nanoparticle Amplified Surface Plasmon Resonance Imaging Aptasensor. Sci. Rep. 4 (5129), 1-7 (2014).
- Uludag, Y., Tothill, I. E. Cancer biomarker detection in serum samples using surface plasmon resonance and quartz crystal microbalance sensors with nanoparticle signal amplification. Anal. Chem. 84 (14), 5898-5904 (2012).
- Špringer, T., Homola, J. Biofunctionalized gold nanoparticles for SPR-biosensor-based detection of CEA in blood plasma. Anal Bioanal Chem. 404 (10), 2869-2875 (2012).

