制备及分析<em>体外</em>三维乳腺癌代理项
Summary
We demonstrate a method to generate 3D breast cancer surrogates, which can be cultured using a perfusion bioreactor system to deliver oxygen and nutrients. Following growth, surrogates are fixed and processed to paraffin for evaluation of parameters of interest. The evaluation of one such parameter, cell density, is explained.
Abstract
三维(3D)培养是一个更生理学相关方法细胞行为体外比二维培养建模。癌,包括乳房癌,是癌上皮细胞和间质组分,包括成纤维细胞和胞外基质(ECM)组成的复杂的三维的组织。然而,大多数体外乳腺癌模型由唯一的癌症上皮细胞,省略了基质和,因此, 在活体内肿瘤的三维结构。癌适当的3D建模是肿瘤生物学,行为和对治疗的反应准确的了解非常重要。然而,培养和3D模型的体积的持续时间是由培养中的氧和营养物质的可用性限制。在此,我们展示了一个在其中乳腺癌上皮细胞和基质成纤维细胞掺入的ECM来产生三维乳腺癌的替代,其包括基质和可作为中培养方法固体三维结构或通过使用灌注生物反应器系统以提供氧和营养物质。以下设置和初始生长期,代理人可用于临床前药物测试。可替代地,替代的细胞和基质成分可以被修饰以解决各种生物的问题。培养后,代理人被固定并加工以石蜡,以类似于临床乳腺癌标本的处理的方式,为的感兴趣参数的评价。哪里的ImageJ和CellProfiler图像分析软件系统被施加到代理人的组织切片的显微照片,进行了说明,这样的一个参数,本细胞的密度的评价来量化每区有核细胞的数量。这可以作为在一段时间或在细胞数的变化,从不同的生长条件和治疗导致细胞数的变化的指标。
Introduction
三维能够更准确地模仿肿瘤体系结构和微环境在体内 (3D)培养模型对于旨在解剖细胞及其微环境之间复杂的相互作用,并以测试候选疗法的功效研究很重要。肿瘤维影响氧和养分梯度,药物暴露,间质性压力/血流量,以及3D结构1-4的均匀性。适当的基质微环境的存在有助于肿瘤维和影响细胞-ECM信令和基质细胞和恶性上皮细胞之间的旁分泌信号传导。肿瘤维和细胞功能的微环境的影响被很好地建立,以这两个因素改变药物反应1,3,5-8。此外,细胞的生长动力学,代谢率,以及细胞信号传导在三维二维(2D)培养和培养之间不同,这些因素AFFE电视机细胞反应1,3,8-10。
在体外 ,肿瘤替代微环境可以通过包括代表性的ECM成分和基质细胞群进行调制。恶性上皮细胞被ECM和癌症相关的基质细胞或以协同/保护方式,以促进肿瘤进展或以抑制的方式影响以抑制进一步肿瘤传播5,6,10。在任一情况下,基质可以影响通过旁分泌信号和/或通过在导致肿瘤增加间质的压力降低药物递送1,6-治疗反应和药物递送。因此,在加入ECM和基质细胞的成临床前模型有助于概括不能在二维培养以及建模的肿瘤的各个方面。
在此一方法建立乳腺癌代理人,纳入一个概括性的微环境,包括细胞外基质成分和stromal细胞,在3D体积中描述。在乳腺癌中,基质细胞群主要是由癌相关成纤维细胞(CAF)和基质细胞外基质的主要是由I型胶原的与基质组分的比例较小了在基底膜找到,包括层粘连蛋白和IV型胶原1,4,11-13。因此,乳腺癌微环境的这些组件( 例如,CAF,I型胶原和基底膜)已经被纳入代理人。该方法可用于产生固体,未灌注3D代用品( 图1A)或可适于通过经由生物反应器系统( 图1B)的替代,以包括介质的灌注。这两种方法如下所述。这种方法也可修改为包括其它基质元素,如肿瘤相关巨噬细胞,或通过调节细胞和细胞外基质成分,根据与其他实体瘤模型。
<p这里描述类=“jove_content”>对于乳腺癌替代,我们已利用了MDA-MB-231(231),乳腺癌细胞系,CAF先前从人乳腺癌14分离,和一个ECM组成90%的胶原蛋白I的( 6毫克/毫升)和10%的生长因子减少基底膜材料(BM)。代孕是在8孔玻片(固体代理)或者种植或生物反应器系统是利用提供持续的营养灌注(灌注替代)。任何灌注生物反应器系统,该系统可容纳含有ECM的细胞的体积可以使用15。作为一个例子,我们描述了我们的生物反应器系统的组织替代物的准备。这个系统是在内部开发并且不是可商购的。因为我们的重点是在这里对3D组织替代物的制备和分析,我们还没有进入对我们的生物反应器系统的制造和装配的具体细节丰富。然而,详细描述此系统及其发展已经出版16。在该生物反应器系统中,聚二甲基硅氧烷(PDMS)流动通道用于容纳所述替代,这是由一个PDMS泡沫支持的(使用类似于由Calcagnile 等 17中所述的方法形成的)。此体积是由4微通道(每个400微米的直径),其被连续地由介质经由microphysiologic泵灌注供应氧气和营养物的替代穿透。的替代物的合适的分析是至关重要获得关于对治疗的反应或其他操作的细胞功能相关的信息。代理人可通过各种方法,包括完整的替代物的直接成像使用共聚焦显微镜或非侵入性成像的其他方法,通过测定条件培养基,或灌洗液间接细胞分析进行分析,对于分泌的产品,或固定和加工后的组织切片的分析至石蜡。可以在组织学切片进行评估一个这样的参数是细胞密度。我们提出了以测量细胞密度的一种方法( 即,每截面积的有核细胞的数目),使用施加到用苏木精和曙红(H&E)染色的替代组织切片的显微照片半自动化图像处理技术。细胞密度可以用作细胞数随时间的相对变化的一个指标,或从不同的生长条件和处理结果。
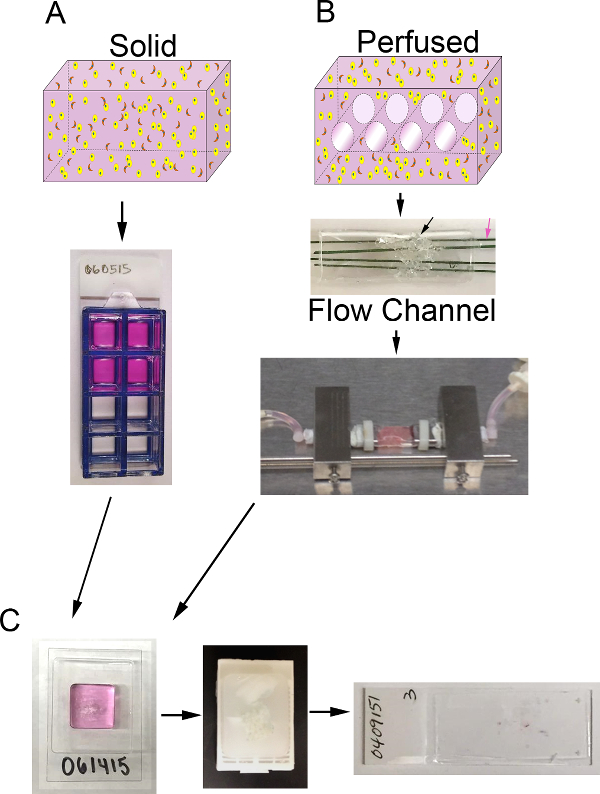
图1. 3D体积和生物反应器系统。 A)的过程示意图产生固体3D代理人。上图:含ECM(粉红色),上皮细胞(黄色)和CAF(橙色)固体体积的3D卡通;下图:含8孔腔滑代理人的俯视图B)原理的过程来产生3D灌注代理人。上图:CA具有通道3D体积的rtoon以允许介质灌注和含有ECM(粉),上皮癌细胞(黄色),和CAF(橙色);中间:含PDMS的泡沫(黑色箭头)的PDMS流动通道的图像与细胞中+ ECM被注入并通过聚合物涂布的不锈钢丝(粉红色箭头)直径为400微米的侵入;下:含有替代,并连接到生物反应器系统,以允许连续介质灌注的PDMS流动通道的图像(蠕动泵和介质贮存器未示出)℃)培养后固体和灌注代理人的处理步骤的图像。左图:包含样品处理凝胶和替代的cryomold的形象;中东:含固定和加工替代石蜡块的图像;右:用替代的H&E染色组织切片载玻片上的图像,请点击这里查看更大的版本这个数字。
Protocol
Representative Results
Discussion
这里,已经描述了三维培养物的方法,该方法结合了组织微环境的组件,包括细胞外基质(ECM)和人类间质成纤维细胞,在一个体积更接近的模型人乳腺癌,以允许一个概括性三维形态的发展。所描述的3D培养方法是更具有代表性的人类疾病比在多种细胞类型的传统的二维细胞培养物的掺入ECM的3D体积。人们已经注意到,这些参数( 即,多种类型的细胞,细胞外基质,和3D体积)提供更拨上…
Divulgations
The authors have nothing to disclose.
Acknowledgements
The University of Alabama at Birmingham Center for Metabolic Bone Disease performed the histologic processing and sectioning of surrogates. Southern Research (Birmingham, AL) provided support for the manufacture of the bioreactor system. Funding was provided by the United States Department of Defense Breast Cancer Research Program (BC121367).
Materials
| Dulbecco's Modified Eagel Medium 1x (DMEM) | Corning CellGro | 10-014-CV | |
| Fetal Bovine Serum (FBS) | Atlanta Biologicals | S11150 | |
| 0.25% Trypsin + 2.21 mM EDTA 1x | Corning | 25-053-CI | |
| Tissue Culture plates, 100mm | CellTreat Scientific Products | 229620 | Sterile |
| Tissue Culture plates, 35mm | CellTreat Scientific Products | 229638 | For PDMS foam formation |
| 9" Glass pipette | Fisher | 13-678-20D | Sterile |
| 10 ml pipette | CellTreat Scientific Products | 229210B | Sterile |
| 1000 µl piptette tips | FisherBrand | 02-717-166 | Sterile Filtered |
| 200 µl pipette tips | FisherBrand | 02-717-141 | Sterile Filtered |
| 10 µl pipette tips | FisherBrand | 02-717-158 | Sterile Filtered |
| 15 ml conical tubes | CellTreat Scientific Products | 229410 | Sterile |
| 50 ml conical tubes | CellTreat Scientific Products | 229422 | Sterile |
| 1.5 ml microcentrifuge tubes | FisherBrand | 05-408-129 | Sterile |
| Trypan blue | Corning Cellgro | 25-900-CI | Sterile |
| Sylgard 184 | Electron Microscopy Sciences | 24236-10 | PDMS elastomer and curing agent. Used for our in-house bioreactor. |
| PDMS Foam | Made in-house for use in our in-house bioreactor. | ||
| High Concentration Bovine Collagen Type I | Advanced Biomatrix | 5133-A | FibriCol ~10 mg/mL |
| Growth Factor Reduced Matrigel (Basement Membrane) | Corning | 354230 | Basement membrane material |
| Sodium Bicarbonate | Sigma | S8761 | |
| Molecular Biology Grade Water | Fisher | BP2819-1 | |
| DMEM 10x | Sigma-Aldrich | D2429 | |
| Nunc Lab-Tek Chamber Slide System | Thermo Scientific | 177402 | 8-well |
| Bioreactor | Made in-house. | ||
| Spring-Back 304 Stainless Steel—Coated with PTFE polymer | McMaster-Carr | 1749T19 | Stainless steel wires to generate microchannels in our in-house bioreactor system. 0.016" Diameter |
| BioPharm Plus platinum-cured silicone pump tubing, L/S 14 | Masterflex | EW-96440-14 | For use in our in-house bioreactor system. Tubing ID: 1.6 mm, Hose barb size: 1/16 in. |
| 2-Stop Tubing Sets, non-flared PVC, 1.52 mm ID | Cole-Parmer | EW-74906-36 | For use in our in-house bioreactor system (with microperistalitic pump). |
| Six Channel precision micro peristaltic pump | Cole-Parmer | EW-74906-04 | For use with our in-house bioreactor system |
| Labtainer BPC Bag – 2 Ports, Luer Lock 50mL |
Thermo Scientific | SH3065711 | Example Media Reservoir |
| Tuberculin Syringes | BD Medical | 309625 | 26 gauge 3/8 in. needle; Sterile |
| Dissecting Tissue Forceps | FisherBrand | 13-812-36 | 5.5 inch |
| Mini Tube Rotator | Boekel Scientific | 260750 | Equipment option for surrogate rotation. Used with carousel for 50 ml tubes (model number 260753) |
| 50 ml tube carousel | Boekel Scientific | 260753 | Used with mini tube rotator |
| Bambino Hybridization Oven | Boekel Scientific | 230301 | Equipment option for surrogate rotation |
| HistoGel Specimen Processing Gel | Thermo Scientific | HG-4000-012 | Specimen Processing Gel described in Step 5.2 |
| Cryomold | Andwin Scientific | 4566 | 15 mm x 15 mm x 5 mm |
| Tissue Marking Dye | Cancer Diagnostics, inc. | 03000P | Can be used to mark surrogates, allowing multiple samples to be included in one tissue cassette |
| Hinged tissue cassettes | FisherBrand | 22-272-416 | |
| Formalin | Fisher | 23-245-685 | |
| GoldSeal Plain Glass Slides | Thermo Scientific | 3048-002 | |
| Xylene | Fisher | X3P-1GAL | |
| Ethanol, 200 proof (100%), USP | Decon Laboratories, Inc. | 2805M | |
| Hematoxylin | Thermo Scientific Richard-Allan Scientific | 7211 | |
| Clarifier | Thermo Scientific Richard-Allan Scientific | 7401 | |
| Bluing Solution | Thermo Scientific Richard-Allan Scientific | 7301 | |
| Eosin Y | Thermo Scientific Richard-Allan Scientific | 7111 | |
| Cytoseal XYL mounting media | Thermo Scientific Richard-Allan Scientific | 83124 | |
| Coverslips | Fisher Scientific | 12-548-5G |
References
- Hakanson, M., Textor, M., Charnley, M. Engineered 3D environments to elucidate the effect of environmental parameters on drug response in cancer. Integr Biol (Camb). 3 (1), 31-38 (2011).
- Horning, J. L., et al. 3-D tumor model for in vitro evaluation of anticancer drugs. Mol Pharm. 5 (5), 849-862 (2008).
- Dhiman, H. K., Ray, A. R., Panda, A. K. Three-dimensional chitosan scaffold-based MCF-7 cell culture for the determination of the cytotoxicity of tamoxifen. Biomaterials. 26 (9), 979-986 (2005).
- Place, A. E., Jin Huh, S., Polyak, K. The microenvironment in breast cancer progression: biology and implications for treatment. Breast Cancer Res. 13 (6), 227 (2011).
- Mao, Y., Keller, E. T., Garfield, D. H., Shen, K., Wang, J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 32 (1-2), 303-315 (2013).
- Paulsson, J., Micke, P. Prognostic relevance of cancer-associated fibroblasts in human cancer. Semin Cancer Biol. 25, 61-68 (2014).
- Roskelley, C. D., Desprez, P. Y., Bissell, M. J. Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc. Natl. Acad. Sci. 91, 12378-12382 (1994).
- Pickl, M., Ries, C. H. Comparison of 3D and 2D tumor models reveals enhanced HER2 activation in 3D associated with an increased response to trastuzumab. Oncogene. 28 (3), 461-468 (2008).
- Ivascu, A., Kubbies, M. Rapid generation of single-tumor spheroids for high-throughput cell function and toxicity analysis. J Biomol Screen. 11 (8), 922-932 (2006).
- Lovitt, C. J., Shelper, T. B., Avery, V. M. Advanced cell culture techniques for cancer drug discovery. Biology (Basel). 3 (2), 345-367 (2014).
- Bergamaschi, A., et al. Extracellular matrix signature identifies breast cancer subgroups with different clinical outcome. J Pathol. 214 (3), 357-367 (2008).
- Oskarsson, T. Extracellular matrix components in breast cancer progression and metastasis. The Breast. 22, S66-S72 (2013).
- Kelley, L. C., Lohmer, L. L., Hagedorn, E. J., Sherwood, D. R. Traversing the basement membrane in vivo: A diversity of strategies. JBC. 204 (3), 291-301 (2014).
- Sadlonova, A., et al. Breast fibroblasts modulate epithelial cell proliferation in three-dimensional in vitro co-culture. Breast Cancer Res. 4, (2004).
- Wendt, D., Marsano, A., Jakob, M., Heberer, M., Martin, I. Oscillating perfusion of cell suspensions through three-dimensional scaffolds enhances cell seeding efficiency and uniformity. Biotechnol Bioeng. 84 (2), 205-214 (2003).
- Marshall, L. E., et al. Flow-perfusion bioreactor system for engineered breast cancer surrogates to be used in preclinical testing. J Tissue Eng Regen Med. , (2015).
- Calcagnile, P., Fragouli, D., Mele, E., Ruffilli, R., Athanassiou, A. Polymeric foams with functional nanocomposite cells. RSC Adv. 4, 19177-19182 (2014).
- Naba, A., Clauser, K. R., Lamar, J. M., Carr, S. A., Hynes, R. O. Extracellular matrix signatures of human mammary carcinoma identify novel metastasis promoters. eLife. 3, (2014).
- Lochter, A., Bissell, M. J. Involvement of extracellular matrix constituents in breast cancer. Semin Cancer Biol. 6 (3), 165-173 (1995).
- Joiner, K. S., Spangler, E. A. Evaluation of HistoGel-embedded specimens for use in veterinary diagnostic pathology. J Vet Diagn Invest. 24 (4), 710-715 (2012).
- Varsegi, G. M., Shidham, V. Cell Block Preparation from Cytology Specimen with Predominance of Individually Scattered Cells. J Vis Exp. (29), e1316 (2009).
- Sadlonova, A., et al. Human Breast Fibroblasts Inhibit Growth of the MCF10AT Xenograft Model of Proliferative Breast Disease. Am J Pathol. 170 (3), (2007).
- Otali, D., He, Q., Stockard, C. R., Grizzle, W. E. Preservation of immunorecognition by transferring cells from 10% neutral buffered formalin to 70% ethanol. Biotech Histochem. 88, 170-180 (2013).
- Webster, S. S., Jenkins, L., Burg, K. J. L. Histological Techniques for Porous, Absorbable, Polymeric Scaffolds, Used in Tissue Engineering. J Histotechnol. 26 (1), 57-65 (2003).
- Troy, T. -. C., Arabzadeh, A., Enikanolaiye, A., Lariviere, N., Turksen, K. Immunohistochemistry on Paraffin Sections of Mouse Epidermis Using Fluorescent Antibodies. J Vis Exp. (11), (2008).
- Carpenter, A. E., et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 7 (10), R100 (2006).
- Kwon, Y. -. J., et al. Gli1 enhances migration and invasion via up-regulation of MMP-11 and promotes metastasis in ERa negative breast cancer cell lines. Clin Exp Metastasis. (28), (2011).
- Evilsizor, M. N., Ray-Jones, H. F., Lifshitz, J., Ziebell, J. Primer for Immunohistochemistry on Cryosectioned Rat Brain Tissue: Example Staining for Microglia and Neurons. J Vis Exp. (99), e52293 (2015).
- Pal, A., Kleer, C. G. Three dimensional cultures: a tool to study normal acinar architecture vs. malignant transformation of breast cells. J Vis Exp. (86), e51311 (2014).
- Hasselbach, L. A., et al. Optimization of High Grade Glioma Cell Culture from Surgical Specimens for Use in Clinically Relevant Animal Models and 3D Immunochemistry. J Vis Exp. (83), e51088 (2014).
- Foty, R. A Simple Hanging Drop Cell Culture Protocol for Generation of 3D Spheroids. J Vis Exp. (51), e2720 (2011).
- Materne, E. -. M., et al. The Multi-organ Chip – A Microfluidic Platform for Long-term Multi-tissue Coculture. J Vis Exp. (98), e52526 (2015).
- Sadlonova, A., et al. Identification of Molecular Distinctions Between Normal Breast-Associated Fibroblasts and Breast Cancer-Associated Fibroblasts. Cancer Microenviron. 2, 9-21 (2009).
- Wang, J. D., Douville, N. J., Takayama, S., ElSayed, M. Quantitative analysis of molecular absorption into PDMS microfluidic channels. Ann Biomed Eng. 40 (9), 1862-1873 (2012).
- Halldorsson, S., Lucumi, E., Gomez-Sjoberg, R., Fleming, R. M. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens Bioelectron. 63, 218-231 (2015).
- Regehr, K. J., et al. Biological implications of polydimethylsiloxane-based microfluidic cell culture. Lab Chip. 9 (15), 2132-2139 (2009).
- Burdett, E., Kasper, F. K., Mikos, A. G., Ludwig, J. A. Engineering tumors: a tissue engineering perspective in cancer biology. Tissue Eng Part B Rev. 16 (3), 351-359 (2010).
- Caruso, R. A., et al. Mechanisms of coagulative necrosis in malignant epithelial tumors (Review). Oncol Lett. 8 (4), 1397-1402 (2014).
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol Pathol. 35 (4), 495-516 (2007).
- Majno, G., Joris, I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 146 (1), 3-15 (1995).
- Ogino, S., et al. Molecular pathological epidemiology of epigenetics: emerging integrative science to analyze environment, host, and disease. Mod Pathol. 26 (4), 465-484 (2013).
- Otali, D., et al. Combined effects of formalin fixation and tissue processing on immunorecognition. Biotech Histochem. 84 (5), 223-247 (2009).

