开发和维护的临床前患者来源的肿瘤异种移植模型为新的抗癌疗法的调查
Summary
在皮下临床前模型利用患者来源的肿瘤是研究新的治疗方法,预测生物标志物的发现,以及耐药性途径的有效性的好方法。这种模式,在药物开发过程中,在事先确定了许多新的抗癌疗法的命运临床研究至关重要。
Abstract
Patient derived tumor xenograft (PDTX) models provide a necessary platform in facilitating anti-cancer drug development prior to human trials. Human tumor pieces are injected subcutaneously into athymic nude mice (immunocompromised, T cell deficient) to create a bank of tumors and subsequently are passaged into different generations of mice in order to maintain these tumors from patients. Importantly, cellular heterogeneity of the original tumor is closely emulated in this model, which provides a more clinically relevant model for evaluation of drug efficacy studies (single agent and combination), biomarker analysis, resistant pathways and cancer stem cell biology. Some limitations of the PDTX model include the replacement of the human stroma with mouse stroma after the first generation in mice, inability to investigate treatment effects on metastasis due to the subcutaneous injections of the tumors, and the lack of evaluation of immunotherapies due to the use of immunocompromised mice. However, even with these limitations, the PDTX model provides a powerful preclinical platform in the drug discovery process.
Introduction
结直肠癌(CRC)是一个显著贡献在美国癌症死亡。 2015年,有CRC估计有132 700新病例有49700人死亡1。虽然患者的预后与局部的疾病是优秀的,晚期患者有不良后果,以新颖的疗法的发展使这是一个主要的优先事项。尽管护理化疗方案和部署防治这一疾病新型生物制剂的标准,出现了只在总生存期的增量增加。因此,存在对于理解参与促进在这种疾病的肿瘤生长的驱动通路一个显著努力。癌症基因组图谱网络最近发现了有牵连的CRC失调,包括众多的主要途径:WNT,磷酸肌醇3-激酶(PI3K),RAS,转化生长因子β(TGF-β)和TP53 2。总之,与调查描述OT她的途径,在CRC使可能的增长已经点燃了旨在改善显著的生存在这个病人的人口3-5新疗法的发展。在肿瘤学药物开发利用临床前模型已在此过程中预测这些新化合物的临床活性是必不可少的。
各种临床前模型已被用于在药物开发过程。考虑到临床前的转基因动物模型和永生细胞系已经确定的新型抗肿瘤药治疗的临床活动不成功,主要是由于其无法反映人类肿瘤的复杂性,患者来源的肿瘤异种移植(PDTX)模型已经建立。这种模式的最大好处是,肿瘤异质性保持不变,并密切反映了原始病人肿瘤6-9的分子特征和克隆。 PDTX模型提供的体内极好临床前研究平台,研究新型药物,耐药性的途径,组合策略,和癌症干细胞生物学10。
该PDTX过程的一般概述在图1中示出,它开始在临床上,同意患者以允许他们的一些过量的肿瘤组织用于本研究。接着,在外科手术,一块肿瘤是由一个病理学家票房并放入介质被运送到研究人员。紧接在此之后,将肿瘤的部分被切成小块并移植到免疫缺陷小鼠皮下。一旦肿瘤生长,这是传代到小鼠的不同世代以维持肿瘤10。典型地,F3代后的肿瘤可以扩展为一个治疗研究,其中被评估的新化合物和/或组合疗法。利用下一代序列(外显子组序列,RNA测序和SNP阵列)潜在的预测生物标志物发现编了协助的可从特定治疗中获益的患者的选择。
使用PDTX模型的总体目标是:1)评估新疗法作为单一药剂或组合的有效性和2)确定前临床研究敏感性或抗性的预测生物标记。在这个手稿,我们提供一个CRC PDTX银行的启动和维护的方法和提供该模型在药物开发发现的优点和局限性。
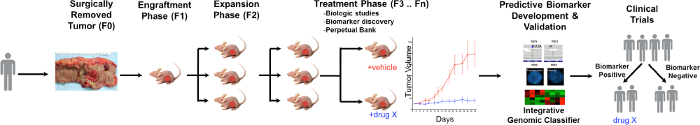
图 1. CRC PDTX示范协议概述。患者来源的肿瘤手术收到并立即注入无胸腺裸鼠皮下。一旦肿瘤生长被扩大到后代,并最终扩大了治疗研究。治疗respo国家科学教育标准进行评估和预测生物标志物被确定可在病人的选择提供帮助。 请点击此处查看该图的放大版本。
Protocol
Representative Results
Discussion
该PDTX药物发现平台提供了一个改进的模型,以用于预测新化合物的临床活动不可靠等临床前模型的缺点。重要的是,在此模型中的肿瘤在生物学上稳定的,保留转移潜能,并从代显示出到代类似药物的响应。在这种模式下,患者来源的肿瘤被注入无胸腺裸鼠,传代,随后在治疗评价中使用。有一个成功的PDTX银行,其中包括几个关键步骤:1)一个有凝聚力的临床小组,以查明/同意病人和切除和肿…
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by grant 1R01CA152303-01.
Materials
| RPMI or DMEM | Corning | 10-040-CV | |
| Penicillin-Streptomycin | Corning | 30-002-CI | |
| Non-essential Amino Acids | Corning | 25-025-CI | |
| Fetal Bovine Serum | Corning | 35-010-CV | Thaw in -4 °C, then activate for 30 minutes at 60 °C water bath |
| CPT blood tube | BD vacutainer | 362761 | |
| Microcentrifuge tube | Surelock | A-7002 | |
| Phospate-Buffered Saline | Corning | 21-040-CV | |
| Cyrogenic vials | Cyroking | C0732901 | |
| Plastic tumor cutting dish | Trueline | TR4001 | |
| Scissors | Roboz | RS-5881 | |
| Forceps | Roboz | RS-5135 | |
| Matrigel (gelatinous protein mixture) | Corning | 354234 | Store at -20 or -80 °C, then thaw on ice, do not leave at room temperature |
| 10% Formalin cups | Protocol | 032-059 | |
| Liquid Nitrogen Dewar Storage | Thermolyne | CY50900 | |
| Portable liquid nitrogen dewar | Nalgene | 4150-2000 | |
| Dimethyl Sulfoxide | Fischer | 67-68-5 | |
| Freezing container: Mr Frosty | Nalgene | 5100-0001 | |
| Isopropyl Alcohol | Decon | 64-17-5 | |
| Trocars | Innovative Research of America | MP-182 | |
| Anesthesia machine | Patterson Veterinary | none | |
| Anesthesia box | Patterson Veterinary | none | |
| Isoflurane | Vet one | 1038005 | |
| F-Air Canister | Bickford Omnicon | 80120 | |
| Meloxicam | Vet one | 5182-90C | |
| Calipers | Fowler | 54-100-167 | |
| Weight scale | Ohaus | Scout Pro SP601 |
References
- Siegel, R. L., Miller, K. D., Jemal, A. Cancer statistics. 2015. CA Cancer J Clin. 65 (1), 5-29 (2015).
- . Comprehensive molecular characterization of human colon and rectal cancer. Nature. 487 (7407), 330-337 (2012).
- Arcaroli, J. J., et al. Tumours with elevated levels of the Notch and Wnt pathways exhibit efficacy to PF-03084014, a gamma-secretase inhibitor, in a preclinical colorectal explant model. Br J Cancer. 109 (3), 667-675 (2013).
- Hubbard, J., Grothey, A. Antiangiogenesis agents in colorectal cancer. Curr Opin Oncol. 22 (4), 374-380 (2010).
- van Es, J. H., et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 435 (7044), 959-963 (2005).
- Cassidy, J. W., Caldas, C., Bruna, A. Maintaining Tumor Heterogeneity in Patient-Derived Tumor Xenografts. Cancer Res. 75 (15), 2963-2968 (2015).
- Jin, K., et al. Patient-derived human tumour tissue xenografts in immunodeficient mice: a systematic review. Clin Transl Oncol. 12 (7), 473-480 (2010).
- Julien, S., et al. Characterization of a large panel of patient-derived tumor xenografts representing the clinical heterogeneity of human colorectal cancer. Clin Cancer Res. 18 (19), 5314-5328 (2012).
- Siolas, D., Hannon, G. J. Patient-derived tumor xenografts: transforming clinical samples into mouse models. Cancer Res. 73 (17), 5315-5319 (2013).
- Tentler, J. J., et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 9 (6), 338-350 (2012).
- Carson, F. L. . Histotechnology: A Self-Assessment Workbook. , (1996).
- Arcaroli, J. J., et al. Common PIK3CA mutants and a novel 3′ UTR mutation are associated with increased sensitivity to saracatinib. Clin Cancer Res. 18 (9), 2704-2714 (2012).
- Arcaroli, J. J., et al. A NOTCH1 gene copy number gain is a prognostic indicator of worse survival and a predictive biomarker to a Notch1 targeting antibody in colorectal cancer. Int J Cancer. 138 (1), 195-205 (2016).
- Arcaroli, J. J., et al. Gene array and fluorescence in situ hybridization biomarkers of activity of saracatinib (AZD0530), a Src inhibitor, in a preclinical model of colorectal cancer. Clin Cancer Res. 16 (16), 4165-4177 (2010).
- Lieu, C. H., et al. Antitumor activity of a potent MEK inhibitor, TAK-733, against colorectal cancer cell lines and patient derived xenografts. Oncotarget. 6 (33), 34561-34572 (2015).
- Pitts, T. M., et al. Association of the epithelial-to-mesenchymal transition phenotype with responsiveness to the p21-activated kinase inhibitor, PF-3758309, in colon cancer models. Front Pharmacol. 4, 35 (2013).
- Song, E. K., et al. Potent antitumor activity of cabozantinib, a c-MET and VEGFR2 inhibitor, in a colorectal cancer patient-derived tumor explant model. Int J Cancer. 136 (8), 1967-1975 (2015).
- Tentler, J. J., et al. Identification of predictive markers of response to the MEK1/2 inhibitor selumetinib (AZD6244) in K-ras-mutated colorectal cancer. Mol Cancer Ther. 9 (12), 3351-3362 (2010).
- Bardelli, A., et al. Amplification of the MET receptor drives resistance to anti-EGFR therapies in colorectal cancer. Cancer Discov. 3 (6), 658-673 (2013).
- Bertotti, A., et al. A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 1 (6), 508-523 (2011).
- Davis, S. L., et al. Combined inhibition of MEK and Aurora A kinase in KRAS/PIK3CA double-mutant colorectal cancer models. Front Pharmacol. 6, 120 (2015).
- Morelli, M. P., et al. Preclinical activity of the rational combination of selumetinib (AZD6244) in combination with vorinostat in KRAS-mutant colorectal cancer models. Clin Cancer Res. 18 (4), 1051-1062 (2012).
- Pitts, T. M., et al. Dual pharmacological targeting of the MAP kinase and PI3K/mTOR pathway in preclinical models of colorectal cancer. PLoS One. 9 (11), e113037 (2014).
- Spreafico, A., et al. Rational combination of a MEK inhibitor, selumetinib, and the Wnt/calcium pathway modulator, cyclosporin A, in preclinical models of colorectal cancer. Clin Cancer Res. 19 (15), 4149-4162 (2013).
- Arcaroli, J. J., et al. ALDH+ tumor-initiating cells exhibiting gain in NOTCH1 gene copy number have enhanced regrowth sensitivity to a gamma-secretase inhibitor and irinotecan in colorectal cancer. Mol Oncol. 6 (3), 370-381 (2012).
- Hoey, T., et al. DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell Stem Cell. 5 (2), 168-177 (2009).
- Ikebuchi, F., et al. Dissociation of c-Met phosphotyrosine sites in human cells in response to mouse hepatocyte growth factor but not human hepatocyte growth factor: the possible roles of different amino acids in different species. Cell Biochem Funct. 31 (4), 298-304 (2013).
- Zhang, Y. W., et al. Enhanced growth of human met-expressing xenografts in a new strain of immunocompromised mice transgenic for human hepatocyte growth factor/scatter factor. Oncogene. 24 (1), 101-106 (2005).

