热可逆交联橡胶通过的Diels-Alder反应的化学制备与性能
Summary
A simple two-step approach involving rubber modification and cross-linking yields fully reworkable, elastic rubber products.
Abstract
论证一种利用狄尔斯阿尔德热可逆化学为橡胶制品交联方法工具。在这项工作中,一个商用乙烯 – 丙烯橡胶,马来酸酐接枝,是热可逆分两步交联的。挂起的酸酐部分首先与糠胺接枝呋喃基的橡胶主链改性。这些挂件呋喃基是然后经由狄尔斯 – 阿尔德偶联反应的双 – 马来酰亚胺交联的。两个反应可以在广泛的实验条件下进行,并且可以很容易地在大范围的应用。所得狄尔斯 – 阿尔德交联橡胶的材料性质类似于过氧化物固化的乙烯/丙烯/二烯橡胶(EPDM)参考。该交联通过复古Diels-Alder反应在升高的温度下破裂(> 150℃),并且可以通过在较低温度下热退火(50-70℃)进行改革。该系统的可逆性被证明机智ħ红外光谱,溶解度试验和机械性能。该材料的可回收也显示在一个实际的方式, 也就是说,通过切割的交联样品分成小份并压缩它们成型为新的样本显示可比的机械性能,这是不可能的常规交联的橡胶。
Introduction
硫磺硫化和过氧化物固化是目前在橡胶工业中的主要工业交联技术,产生不可逆的化学交联,以防止熔融后处理:1,2 A'摇篮到摇篮“的方式回收交联的橡胶,需要一种材料表现在提供服务的条件类似于永久交联的橡胶,而具有热塑性的高温下的加工性和完整的可回收性。要达到这样的可回收的方法使用与该对外部刺激作出反应的可逆交联,如温度(从未来的工业应用的观点出发最可行的)的橡胶状网络。3-5这些交联的以相对较低的服务的形成温度是必需的橡胶的良好的机械特性,而在高温下(类似于原始的非交联的化合物的处理温度)的裂解允许当r该材料的ecycling。
一些具体的材料可以是可逆地通过经由缩聚反应6或通过所谓的可逆网络拓扑利用所谓的动态共价网络经由酯交换反应冷冻交联的。7-9这些方法的缺点是设计的必要性和合成新的聚合物,而不是修改已经具有所需性质的现有商业橡胶。技术来热-可逆交联橡胶涉及氢键,离子相互作用和共价键,如通过热活化的二硫化物重排。10-13最近,热可逆交联通过狄尔斯-阿尔德(DA)的化学开发的。14 -21 DA化学可以应用于范围广泛的聚合物,并代表一个流行的选择,特别是因为对DA反应允许相对快的动力学和反应条件温和。17,22-24钍EIR低耦合和高的去耦的温度使呋喃和马来酰亚胺优良候选可逆聚合物交联。18-20,25-28
本工作的目的是提供一种用于使用DA化学作为用于工业橡胶产品( 图1)的热可逆交联的工具的方法。5首先,饱和烃类弹性体,如乙烯的反应性/丙烯橡胶(EPM),必须增加。促进这方面的一个商业相关的例子是马来酸酐(MA)的过氧化物引发自由基接枝29-34其次,呋喃组可以通过插入糠(FFA)插入挂件酸酐接枝到这样一种马来EPM橡胶以形成酰亚胺。35,36最后,从而被附接到橡胶骨架上的呋喃基部分可以再参加热可逆DA化学作为富电子二烯。25,37的电子婆或双-马来酰亚胺(BM)是该交联反应的合适的亲二烯体。19,26,38
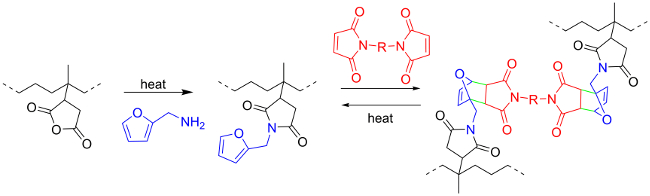
图1. 反应方案。呋喃嫁接和EPM-G-MA橡胶(从5许可转载)双马来酰亚胺的交联。 请点击此处查看该图的放大版本。
Protocol
Representative Results
Discussion
商业EPM-G-MA橡胶是热可逆的交联在一个简单的两步骤方法。该马来化的橡胶首先用FFA改性接枝呋喃基到橡胶骨架。所得未决呋喃显示反应性狄尔斯 – 阿尔德二烯。脂族的BM被用作交联剂,得到两个呋喃部分之间的热可逆桥。两个反应均成功使用根据红外光谱,元素分析好的转换(> 80%)。交联是通过溶胀和溶解性试验,得到100%的凝胶含量示出。
为描述的协议的成功执行,…
Divulgations
The authors have nothing to disclose.
Acknowledgements
This research forms part of the research program of the Dutch Polymer Institute, project #749.
Materials
| ENB-EPDM | LANXESS Elastomers B.V. | Keltan 8550C | |
| EPM-g-MA | LANXESS Elastomers B.V. | Keltan DE5005 | Vacuum oven for one hour at 175 °C |
| furfurylamine | Sigma-Aldrich | F20009 | Freshly distillated before use |
| di-dodecylamine | Sigma-Aldrich | 36784 | |
| maleic anhydride | Sigma-Aldrich | M0357 | |
| octadecyl-1-(3,5-di-tert.-butyl-4-hydroxyphenyl)propionate | Sigma-Aldrich | 367079 | |
| bis(tert.-butylperoxy-iso-propyl) benzene | Sigma-Aldrich | 531685 | |
| tetrahydrofuran | Sigma-Aldrich | 401757 | |
| decalin | Sigma-Aldrich | 294772 | |
| acetone | Sigma-Aldrich | 320110 |
References
- Myhre, M., MacKillop, D. A. Rubber recycling. Rubber Chem Technol. 75 (3), 429-474 (2002).
- Baranwal, K. C., Stephens, H. L. . Basic Elastomer Technology. , (2001).
- Such, G. K., Johnston, A. P. R., Liang, K., Caruso, F. Synthesis and functionalization of nanoengineered materials using click chemistry. Prog Polym Sci. 37 (7), 985-1003 (2012).
- Kloxin, C. J., Scott, T. F., Adzima, B. J., Bowman, C. N. Covalent Adaptable Networks (CANS): A Unique Paradigm in Cross-Linked Polymers. Macromol. 43 (6), 2643-2653 (2010).
- Polgar, L. M., van Duin, M., Broekhuis, A. A., Picchioni, F. The use of Diels-Alder chemistry for thermo-reversible cross-linking of rubbers: the next step towards recycling of rubber products. Macromol. 48 (19), 7096-7105 (2015).
- Garcia, J. M., et al. Recyclable, strong thermosets and organogels via paraformaldehyde condensation with diamines. Sci. 344 (6185), 732-735 (2014).
- Montarnal, D., Capelot, M., Tournilhac, F., Leibler, L. Silica-like malleable materials from permanent organic networks. Sci. 334 (6058), 965-968 (2011).
- Capelot, M., Montarnal, D., Tournilhac, F., Leibler, L. Metal-catalyzed transesterification for healing and assembling of thermosets. J Am Chem Soc. 134 (18), 7664-7667 (2012).
- Cordier, P., Tournilhac, F., Soulie-Ziakovic, C., Leibler, L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature. 451 (7181), 977-980 (2008).
- Imbernon, L., Oikonomou, E. K., Norvez, S., Leibler, L. Chemically crosslinked yet reprocessable epoxidized natural rubber via thermo-activated disulfide rearrangements. Polym Chem. 6 (23), 4271-4278 (2015).
- Van der Mee, M. A. J., Goossens, J. G. P., van Duin, M. Thermoreversible cross-linking of maleated ethylene/propylene copolymers with diamines and amino-alcohols. Polym. 49 (5), 1239-1248 (2008).
- Van der Mee, M. A. J., Goossens, J. G. P., Van Duin, M. Thermoreversible covalent crosslinking of maleated ethylene/propylene copolymers with diols. J Polym Sci A-Polym Chem. 46 (5), 1810-1825 (2008).
- Das, A., et al. Ionic modification turns commercial rubber into a self-healing material. Acs Appl Mater Interf. 7 (37), 20623-20630 (2015).
- Gandini, A. The furan/maleimide Diels-Alder reaction: A versatile click-unclick tool in macromolecular synthesis. Prog Polym Sci. 38 (1), 1-29 (2013).
- Toncelli, C., De Reus, D. C., Picchioni, F., Broekhuis, A. A. Properties of reversible Diels-Alder furan/maleimide polymer networks as function of crosslink density. Macromol Chem Phys. 213 (2), 157-165 (2012).
- Tian, Q., Rong, M. Z., Zhang, M. Q., Yuan, Y. C. Synthesis and characterization of epoxy with improved thermal remendability based on Diels-Alder reaction. Polym Int. 59 (10), 1339-1345 (2010).
- Franc, G., Kakkar, A. K. Diels-Alder “click” chemistry in designing dendritic macromolecules. Chem-a Eur J. 15 (23), 5630-5639 (2009).
- Goiti, E., Huglin, M. B., Rego, J. M. Thermal breakdown by the retro Diels-Alder reaction of crosslinking in poly[styrene-co-(furfuryl methacrylate). Macromol Rapid Comm. 24 (11), 692-696 (2003).
- Gheneim, R., Perez-Berumen, C., Gandini, A. Diels-Alder reactions with novel polymeric dienes and dienophiles: Synthesis of reversibly cross-linked elastomers. Macromol. 35 (19), 7246-7253 (2002).
- Moustafa, M. M. A. R., Gillies, E. R. Rubber functionalization by Diels-Alder chemistry: from cross-linking to multifunctional graft copolymer synthesis. Macromol. 46 (15), 6024-6030 (2013).
- Scheltjens, G., Diaz, M. M., Brancart, J., Van Assche, G., Van Mele, B. A self-healing polymer network based on reversible covalent bonding. React Funct Polym. 73 (2), 413-420 (2013).
- Gandini, A., Silvestre, A. J. D., Coelho, D. Reversible click chemistry at the service of macromolecular materials. Polym Chem. 2 (8), 1713-1719 (2011).
- Nandivada, H., Jiang, X., Lahann, J. Click chemistry: Versatility and control in the hands of materials scientists. Adv Mater. 19 (17), 2197-2208 (2007).
- Chen, X. X., et al. A thermally re-mendable cross-linked polymeric material. Sci. 295 (5560), 1698-1702 (2002).
- Laita, H., Boufi, S., Gandini, A. The application of the Diels-Alder reaction to polymers bearing furan moieties .1. Reactions with maleimides. Eur Polym J. 33 (8), 1203-1211 (1997).
- Gandini, A., Coelho, D., Silvestre, A. J. D. Reversible click chemistry at the service of macromolecular materials. Part 1: Kinetics of the Diels-Alder reaction applied to furan-maleimide model compounds and linear polymerizations. Eur Polym J. 44 (12), 4029-4036 (2008).
- Ax, J., Wenz, G. Thermoreversible networks by Diels-Alder Reaction of cellulose furoates with bismaleimides. Macromol Chem Phys. 213 (2), 182-186 (2012).
- Canary, S. A., Stevens, M. P. Thermally reversible cross-linking of polystyrene via the furan-maleimide Diels-Alder reaction. J Polym Sci A-Polym Chem. 30 (8), 1755-1760 (1992).
- Burlett, D. J., Lindt, J. T. Reactive processing of rubbers. Rubber Chem Technol. 66 (3), 411-434 (1993).
- Saelao, J., Phinyocheep, P. Influence of styrene on grafting efficiency of maleic anhydride onto natural rubber. J Appl Polym Sci. 95 (1), 28-38 (2005).
- Guldogan, Y., Egri, S., Rzaev, Z. M. O., Piskin, E. Comparison of maleic anhydride grafting onto powder and granular polypropylene in the melt by reactive extrusion. J Appl Polym Sci. 92 (6), 3675-3684 (2004).
- Van Duin, M. Grafting of polyolefins with maleic anhydride: Alchemy or technology. Macromol Symp. 202, 1-10 (2003).
- Barra, G. M. O., Crespo, J. S., Bertolino, J. R., Soldi, V., Pires, A. T. N. Maleic anhydride grafting on EPDM: Qualitative and quantitative determination. J Braz Chem Soc. 10 (1), 31-34 (1999).
- Oostenbrink, A. J., Gaymans, R. J. Maleic-anhydride grafting on epdm rubber in the melt. Polym. 33 (14), 3086-3088 (1992).
- Schmidt, U., Zschoche, S., Werner, C. Modification of poly(octadecene-alt-maleic anhydride) films by reaction with functional amines. J Appl Polym Sci. 87 (8), 1255-1266 (2003).
- Vermeesch, I., Groeninckx, G. Chemical modification of poly(styrene-co-maleic anhydride) with primary N-alkylamines by reactive extrusion. J Appl Polym Sci. 53 (10), 1365-1373 (1994).
- Zhang, Y., Broekhuis, A. A., Picchioni, F. Thermally self-healing polymeric materials: the next step to recycling thermoset polymers. Macromol. 42 (6), 1906-1912 (2009).
- Gousse, C., Gandini, A., Hodge, P. Application of the Diels-Alder reaction to polymers bearing furan moieties. 2. Diels-Alder and retro-Diels-Alder reactions involving furan rings in some styrene copolymers. Macromol. 31 (2), (1998).
- Mikroyannidis, J. A. Synthesis and Diels-Alder polymerization of furfurylidene and furfuryl-substituted maleamic acids. J Polym Sci A-Polym Chem. 30 (1), 125-132 (1992).
- Kossmehl, G., Nagel, H., Pahl, A. Cross-linking reactions on polyamides by bis- and tris(maleimide)s. Angew Makromol Chem. 227 (1), 139-157 (1995).
- Liu, X., et al. Kinetic study of Diels-Alder reaction involving in maleimide-furan compounds and linear polyurethane. Polym Bull. 70 (8), 2319-2335 (2013).
- Stamboliyska, B. A., Binev, Y. I., Radomirska, V. B., Tsenov, J. A., Juchnovski, I. N. IR spectra and structure of 2,5-pyrrolidinedione (succinimide) and of its nitranion: experimental and ab initio MO studies. J Molec Struct. 516 (2-3), 237-245 (2000).
- Sombatsompop, N., Kumnuantip, C. Rheology, cure characteristics, physical and mechanical properties of tire tread reclaimed rubber/natural rubber compounds. J Appl Polym Sci. 87 (10), 1723-1731 (2003).
- Kim, J. K., Lee, S. H. New technology of crumb rubber compounding for recycling of waste tires. J Appl Polym Sci. 78 (8), 1573-1577 (2000).
- Dikland, H. G., van Duin, A. Miscibility of EPM-EPDM blends. Rubber Chem Technol. 76 (2), 495-506 (2003).
- Klots, T. D., Chirico, R. D., Steele, W. V. Complete vapor-phase assignment for the fundamental vibrations of furan, pyrrole and thiophene. Spectrochim Acta A-Mol Biomol Spectr. 50 (4), 765-795 (1994).
- Litvinov, V. M., Barendswaard, W., van Duin, M. The density of chemical crosslinks and chain entanglements in unfilled EPDM vulcanizates as studied with low resolution, solid state 1H-NMR. Rubber Chem Technol. 71 (1), 105-118 (1998).
- Orza, R. A., Magusin, P. C. M. M., Litvinov, V. M., van Duin, M., Michels, M. A. J. Solid-state 1H-NMR study on chemical cross-links, chain entanglements, and network heterogeneity in peroxide-cured EPDM rubbers. Macromol. 40 (25), 8999-9008 (2007).
- Henssler, J. T., Matzger, A. J. Regiochemical effects of furan substitution on the electronic properties and solid-state structure of partial fused-ring oligothiophenes. J Org Chem. 77 (20), 9298-9303 (2012).
- Hofmann, W. . Rubber Technology Handbook. , (1989).
- Chen, Y., Xu, C. Stress-strain behaviors and crosslinked networks Studies of natural rubber-zinc dimethacrylate composites. J Macromol Sci B-Phys. 51 (7), 1384-1400 (2012).
- Pritchard, R. H., Terentjev, E. M. Swelling and de-swelling of gels under external elastic deformation. Polym. 54 (26), 6954-6960 (2013).
- Tizard, G. A., Dillard, D. A., Norris, A. W., Shephard, N. Development of a high precision method to characterize Poisson’s ratios of encapsulant gels using a flat disk configuration. Exp Mech. 52 (9), 1397-1405 (2012).
- Dijkhuis, K. A. J., Babu, I., Lopulissa, J. S., Noordermeer, J. W. M., Dierkes, W. K. A mechanistic approach to EPDM devulcanization. Rubber Chem. Technol. 81 (2), 865-880 (2008).
- Sutanto, P., Picchioni, E., Janssen, L. P. B. M., Dijkhuis, K. A. J., Dierkes, W. K., Noordermeer, J. W. M. State of the art: Recycling of EPDM rubber vulcanizates. Int Polym Proc. 21 (2), (2006).

