Inoculating Anopheles gambiae Mosquitoes with Beads to Induce and Measure the Melanization Immune Response
Summary
Through inoculation with beads, the described technique enables the stimulation of the mosquito melanization response in the hemolymph circulating system. The amount of melanin covering the beads can be measured after dissection as a measure of the immune response.
Abstract
The stimulation of immune responses is a common tool in invertebrate studies to examine the efficacy and the mechanisms of immunity. This stimulation is based on the injection of non-pathogenic particles into insects, as the particles will be detected by the immune system and will induce the production of immune effectors. We focus here on the stimulation of the melanization response in the mosquito Anopheles gambiae. The melanization response results in the encapsulation of foreign particles and parasites with a dark layer of melanin. To stimulate this response, mosquitoes are inoculated with beads in the thoracic cavity using microcapillary glass tubes. Then, after 24 hr, the mosquitoes are dissected to retrieve the beads. The degree of melanization of the bead is measured using image analysis software. Beads do not have the pathogenic effects of parasites, or their capacity to evade or suppress the immune response. These injections are a way to measure immune efficacy and the impact of immune stimulations on other life history traits, such as fecundity or longevity. It is not exactly the same as directly studying host-parasite interactions, but it is an interesting tool to study immunity and its evolutionary ecology.
Introduction
Insects rely on immune responses to protect themselves against parasites and pathogens1–3 that breach through their cuticle or their midgut epithelium4. In mosquitoes, these responses are efficient against bacteria5, viruses6, filarial nematodes7, and malaria parasites1,8,9. In mosquitoes, a key immune response is the encapsulation of foreign particles with melanin10–12. This encapsulation may happen in the midgut or in the hemolymph circulating system10–12. This melanization response is the result of the pro-phenoloxidase cascade10–12, and it can lead to the death of the parasites or to their phagocytosis. In adult mosquitoes, where the number of hemocytes cells is limited, melanization is a humoral response, like against plasmodium parasites or filarial nematodes 7. In some other insects, it is directly the hemocytes cells that gather around the parasite to melanize them7. Besides, melanin is also essential for several other physiological process like egg production and cuticle wounds healing7.
The stimulation of immune responses is used as a tool to study insect immunity in several agricultural and public health model systems13–18. It is used in Anopheles gambiae mosquitoes, the major vector of malaria in Africa, to study host-parasite interactions14–16,19. These techniques are based on the capacity of insects to detect parasites with their pattern recognition receptors (PRR)2. Mosquitoes may also detect other molecules interfering with their biology such as pathogen-associated molecular patterns (PAMPs), or detect their own damaged cells due to the release of collagen and nucleic acids. The mosquito immune cells such as the hemocytes are used for detection20–23. The main immune signaling pathways are Imd, Toll, JAK/STAT24, and ribonucleic acid interference (RNAi)25,26. Both Toll and Imd pathways influence the melanization response and interact with the pro-phenoloxidase cascade10–12.
The standard tool used to stimulate the melanization response is the inoculation of a mosquito with a small bead into the hemolymph of the thoracic cavity. The degree of melanin encapsulation can then be measured19 after retrieving the bead through the dissection of the mosquito. In most studies, only one bead was injected per mosquito15,16,27, but injecting more beads is possible in order to study the limits of the melanization response19. These beads are injected using an injection solution (physiological serum) to limit disturbance of the mosquito physiology and the desiccation of the mosquito15,16,27. A dye is added to this solution to ease bead selection. It is the same for the dissection solution used to retrieve the bead15,16,27.
The advantage of inoculating insects with non-pathogenic stimuli is the ability to focus on the direct effect on the immune response. There are no complicating effects due to parasite pathogenicity28, immunosuppression29–31, or immune evasion31–34. Besides, the consequences of the stimulations on other life history traits, such as longevity or fecundity, can also be studied. Thus, researchers studying evolutionary ecology may require such tools2,35,36. For example, immuno-challenged bumblebees have a shortened life span under starvation. Similar negative effects of immune stimulations and deployments have been observed in different invertebrate models, often resulting in a shorter lifespan or less reproductive success13,27,37. Such studies can be conducted in varying environments2,4,38. Stimulating immunity is also of interest to those focusing directly on immunopathology39,40.
This protocol is based on the inoculation of beads with mosquitoes to stimulate the melanization response and directly measure the amount of melanin. This enables quantitative and qualitative study of the melanization response in different experimental settings. Such a tool can be extended to the stimulation of other immune responses, such as the antibacterial response to heat-killed bacteria41. It can also be conducted in many ecological settings.
Protocol
1. Saline Solution for Injection and Dissection
- Prepare the saline solution by adding NaCl, KCl, and CaCl2 to distilled water to obtain 1.3 mM NaCl, 0.5 mM KCl, and 0.2 mM CaCl2 at pH = 6.8.
- Add 1 ml of 0.1% methyl green solution to 99 milliliters of the saline solution to color the transparent beads. This is the 0.001% methyl green “injection solution".
- Then, add 5 ml of 0.1% methyl green solution to 45 ml of the saline solution. This 0.01% methyl green “dissection solution” is 10 times more concentrated in methyl green to facilitate the coloration of the beads and their observation during the dissection.
NOTE: Filter sterilize the buffer if necessary.
2. Capillary Preparation
- Heat-pull microcapillary glass tubes to obtain a very fine tip slightly bigger than the smallest beads (φ = 40 µm)42.
- Open and adjust each capillary by breaking the tip with tweezers or by sawing the tip off.
3. Mosquito Rearing
- Rear A. gambiae at 26 ± 1 °C, 70 ± 5% relative humidity, and a 12:12 hr light:dark cycle. Keep adult females and give them access to a 10% sugar solution.
4. Bead Selections with the Capillary
- Pour 0.009 g of negatively charged beads (40-120 µm in diameter) in a 5 cm Petri dish and add 5 ml of injection solution 30 min in advance.
NOTE: The goal is to let the dye color the beads to make bead selection for injection easier. Autoclave the beads if they don't come in a sterile jar. - Using a capillary mounted in a capillary pipette bulb, visually select the smallest bead in 0.1 µl of the saline solution under a binocular microscope.
NOTE: When inoculating several beads simultaneously, select three beads from a total volume of 0.1 µl.
5. Mosquito Handling and Inoculation
- Using an insect aspirator, place each mosquito in a 50 ml tube.
- Chill each mosquito briefly by placing the tube in crushed ice (2-5 min).
- Place a mosquito on its right side under the binocular microscope.
- Under a stereomicroscope, inoculate the mosquito with the capillary by firmly piercing through the left side of the thoracic cavity cuticle and then injecting the liquid and the bead.
NOTE: Take care not to damage the flight muscles by holding the capillary as perpendicularly to the mosquito as possible. - Remove the capillary.
- Using featherweight entomology forceps, place each female individually in a 180 ml plastic cup covered with mosquito netting; give them access to a 10% sugar solution.
NOTE: Caution is required with the sugar solution, as the mosquito may get stuck on sugar solution drops.
6. Mosquito Dissection and Bead Photography
- Check if the mosquitoes are alive 24 hr after the inoculation. If so, check their condition and keep only the mosquitoes that are able to fly.
- Freeze these mosquitoes at -20 °C.
NOTE: They can be kept several days to months before dissection, if needed. - Remove the mosquitoes' wings using tweezers under a binocular microscope at 32X magnification. Fix the wings onto glass slides with transparent tape. Write down the code used for each individual mosquito.
- Put the glass slides in a scanner and scan them at 1,200 dpi.
NOTE: Check that the code is readable for each individual mosquito. - On a glass microscope slide and in dissection solution, use forceps to separate the thorax from the head and abdomen.
- Open the thorax with forceps to retrieve the beads.
NOTE: Beads that do not move to the abdomen, are not in contact with each other and most of them float freely in the hemolymph. Be careful not to break the bead with the forceps, as a non-melanized bead may be tough to find. Wait for 1 to 5 min for beads to obtain color. - Transfer the beads in a droplet of dissection solution on a glass microscope slide, before taking the picture.
NOTE: No need to wash the beads. - Using a microscope equipped with a camera, take a digital image of each bead at a standard lighting setting and 400X magnification.
NOTE: Do not change the illumination between the different pictures to enable their comparison.
7. Picture Analysis: Bead Melanization
- Open the image analysis software. Click on "file" and then on "open" to find a bead image.
- Click on "analyze." Click on "set measurements." Tick the "mean grey value" box in the "set measurements" window.
- Click on the circle selection tool icon. Select the bead perimeter; remain inside the bead and avoid the luminous halo around it.
- Click on the "analyze" menu. Click on "measure." Obtain the mean grey value and size of the cross-sectional area of the bead.
- To obtain the melanization value of each bead, perform the following operation using a calculator or calculator software: "256 – mean grey value." The melanization measure is a value between 0 and 256, with 0 representing a totally white image.
Representative Results
Mosquitoes did not all melanize the beads in the same way, as some beads were less covered with melanin than others (Figure 1). Indeed, some beads remained blue because of a lack of melanization, whereas others were completely dark (Figure 1). The melanization value was standardized by linear interpolation to a value between 0 (which corresponded to a blue and unmelanized bead) and 100 (corresponding to a dark and heavily melanized bead) (Figure 2a and 2b). These values indicate the degree of melanization of the beads and thus, the strength of the immune response.
We can compare beads to each other by comparing their melanization values. The melanization response generally follows a Gaussian distribution, which enables classical statistical analyses like ANOVA or Gaussian glm.
We studied the variation of the melanization response with respect to the number of beads simultaneously inoculated in a mosquito. The goal was to gain a better understanding of the variability and limits of the melanization response. The size of the bead did not have any effect.
The average bead melanization value decreased from 71 to 50 when the number of beads inoculated increased (Figure 2). There are some limits of the melanization response in a mosquito when the immune challenge increases. Furthermore, the melanization response decreased for the least-melanized bead in each mosquito, from 71 to 35 for one to three beads, respectively. However, the melanization value of the most strongly melanized bead stayed about constant. The variability of melanization increased with the number of beads. The melanization effort was not uniform amongst all beads in a given mosquito, as one bead was prioritized over others. This injection technique is useful to study the variability of immune responses in mosquitoes.
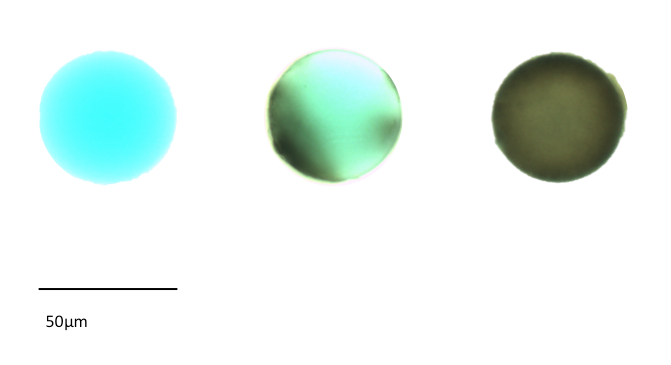
Figure 1: Picture examples of a non-melanized, a partially melanized, and a highly melanized bead after dissection. On the left, middle, and right are a non-melanized bead, a partially melanized bead, and a highly melanized bead, respectively.
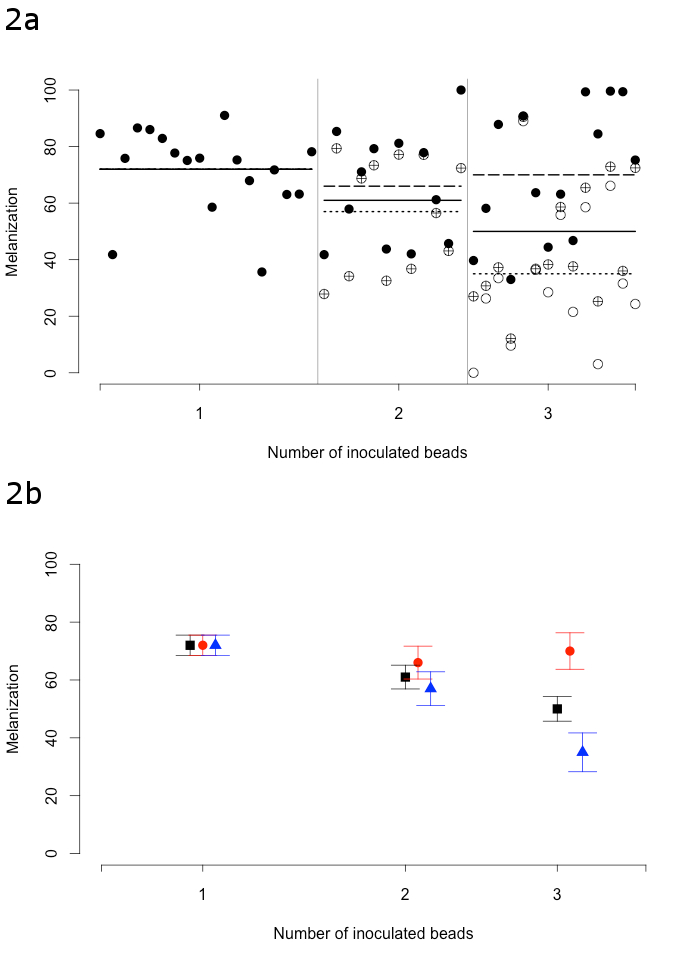
Figure 2: Bead melanization as a function of the number of inoculated beads. (a) Each point shows the melanization of a single bead (with melanization values ranging from 0 for non-melanized beads to 100 for heavily melanized ones) and each column of points represent an individual mosquito. Each mosquito was inoculated with one, two, or three beads. The solid points represent the highest melanization value per mosquito, the crossed points represent the intermediate melanization value, and the empty points represent the lowest melanization value. The solid lines represent the mean melanization per bead treatment, the dashed lines show the mean of the highest melanization, and the dotted lines indicate the mean of the lowest melanization. (b) Mean and standard error of the melanization, the highest melanization and the lowest melanization per bead inoculation treatment. The black represent the mean bead melanization as a function of the number of beads (with melanization values ranging from 0 for non-melanized beads to 100 for heavily melanized ones). The red point represents the mean of the highest melanization. The blue triangle represents the mean of the lowest melanization. The bars are the standard errors. Modified from reference19.
Discussion
This injection technique is useful to stimulate and study the melanization response in mosquitoes. For example, here we studied the effect of the immune stimuli load.
The critical step in this procedure is to properly inoculate the mosquito. Any excessive damage to the flight muscles or to the mosquito itself may prevent the mosquito from feeding or may kill it before the dissection. A second key step is to keep the mosquitoes on ice long enough to knock them out without killing them. A small container will ease and speed up the process, as it enables closer contact with the ice. Finally, special care is needed during dissection, as tweezers may otherwise break the beads. Beads embedded in mosquito tissue may take more time to be colored by the dissection solution.
Using multiple kinds of beads (like neutrally, positively, or negatively charged beads or glass beads) will lead to different results, as they may not all be melanized to the same degree27,43. Indeed, compared to neutrally charged beads, negatively charged beads lead to more variability in the melanization response between mosquitoes27. Glass beads, on the other hand, are inert in mosquitoes and do not trigger the melanization response at all27. Negatively charged beads may thus be preferred, but comparisons between different beads can also be pertinent. However, depending on the kind of bead, a different dye may be required for the injection and dissection solutions; for example, cresyl violet dye should be used instead of methyl green when using glass beads27.
This injection technique is invasive for the mosquito. Even if damage and injury can be minimized and controlled, alternate tools may be a better option, depending on the experimental design. It is difficult to stimulate and simultaneously measure several immune responses in the same mosquito. Working on families or groups of mosquitoes is required when looking at the correlations between immune responses28. Besides, beads are only partially similar to the actual presence of parasites in an insect.
First, the immune response is stimulated, and then the resulting phenotypic response, the encapsulation, is measured, but what happens in between is unknown. Coupling injections with physiological or molecular tools10–12 can improve our understanding of this melanization response. To study the underlying mechanisms of the melanization response, the expression of key enzymes or molecules produced during the pro-phenoloxidase cascade can be measured10–12. However, focusing solely on the up- or down-regulation of such molecules, without knowing the actual consequences of the final encapsulation, may lead to an inaccurate evaluation of the melanization process. Therefore, studying encapsulation and the molecular mechanisms together would be highly informative, despite the fact that they are each a valid scientific technique when used separately.
To conclude, this is a technique to study immunity and the evolution of immunity. There is, for example, increasing evidence for a link between insecticide resistance and mosquito susceptibility to malaria parasites. This injection technique could be used in such a context, as well as in field trials. A second work direction would be to combine immune stimulation with gene knockdowns to study immune pathways in detail.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This research was possible through funding from the University of Neuchâtel. We would like to thank all the students that helped in improving this technique, namely our colleague Kevin Thievent. We would also like to thank the members of the Thomas Lab for making their laboratory available. We would like to thank Janet Teeple for her help with mosquito rearing. We would also like to thanks Loyal Hall in the laboratory of Pr. Tom Baker for his help in the preparation of the micro capillary glass tubes.
Materials
| Microcapillary glass tubes GB120TF-10 | science-products.com | GB120TF-10 | http://www.science-products.com/Products/CatalogG/Glass/Glass.html |
| Microcaps Capillary pipette bulb | Drumond | 1-000-9000 | |
| negatively charged Sephadex CM C-25 beads | Sigma-Aldrich, Steinheim, Germany | C25120 SIGMA | need few to start |
| Methyl green | Sigma-Aldrich | 323829 ALDRICH | need few to start |
| Software ImageJ | opensource | Version 1.47f7 or later |
References
- Dong, Y., Aguilar, R., Xi, Z., Warr, E., Mongin, E., Dimopoulos, G. Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS pathog. 2 (6), e52 (2006).
- Sadd, B. M., Schmid-Hempel, P. PERSPECTIVE: Principles of ecological immunology. Evolutionary Appl. 2 (1), 113-121 (2008).
- Crompton, P. D., Moebius, J., et al. Malaria Immunity in Man and Mosquito: Insights into Unsolved Mysteries of a Deadly Infectious Disease*. Annu Rev Immuno. 32 (1), 157-187 (2014).
- Schmid-Hempel, P. EVOLUTIONARY ECOLOGY OF INSECT IMMUNE DEFENSES. Annu Rev Entomol. 50 (1), 529-551 (2005).
- Hillyer, J. F., Schmidt, S. L., Christensen, B. M. Rapid phagocytosis and melanization of bacteria and Plasmodium sporozoites by hemocytes of the mosquito Aedes aegypti. J parasito. 89 (1), 62-69 (2003).
- Carissimo, G., Pondeville, E., et al. Antiviral immunity of Anopheles gambiae is highly compartmentalized, with distinct roles for RNA interference and gut microbiota. PNAS. 112 (2), E176-E185 (2015).
- Christensen, B. M., Li, J., Chen, C. -. C., Nappi, A. J. Melanization immune responses in mosquito vectors. Trends parasito. 21 (4), 192-199 (2005).
- Collins, F., Sakai, R., et al. Genetic selection of a Plasmodium-refractory strain of the malaria vector Anopheles gambiae. Science. 234 (4776), 607-610 (1986).
- Warr, E., Lambrechts, L., Koella, J. C., Bourgouin, C., Dimopoulos, G. Anopheles gambiae immune responses to Sephadex beads: Involvement of anti-Plasmodium factors in regulating melanization. Insect Biochem Molec. 36 (10), 769-778 (2006).
- Fuchs, S., Behrends, V., Bundy, J. G., Crisanti, A., Nolan, T. Phenylalanine metabolism regulates reproduction and parasite melanization in the malaria mosquito. PloS one. 9 (1), e84865 (2014).
- Cerenius, L., Söderhäll, K. The prophenoloxidase-activating system in invertebrates. Immuno Rev. 198, 116-126 (2004).
- Cerenius, L., Lee, B. L., Söderhäll, K. The proPO-system: pros and cons for its role in invertebrate immunity. Trend Immuno. 29 (6), 263-271 (2008).
- Moret, Y., Schmid-Hempel, P. . Survival for immunity: the price of immune system activation for bumblebee workers. , 1166-1168 (2000).
- Suwanchaichinda, C., Paskewitz, S. M. Effects of Larval Nutrition, Adult Body Size, and Adult Temperature on the Ability of Anopheles gambiae(Diptera: Culicidae) to Melanize Sephadex Beads. J Med Entomol. 35 (2), 157-161 (1998).
- Chun, J., Riehle, M., Paskewitz, S. M. Effect of Mosquito Age and Reproductive Status on Melanization of Sephadex Beads in Plasmodium-Refractory and -Susceptible Strains of Anopheles gambiae. J Invertebr Pathol. 66 (1), 11-17 (1995).
- Schwartz, A., Koella, J. C. Melanization of Plasmodium falciparum and C-25 Sephadex Beads by Field-Caught Anopheles gambiae (Diptera: Culicidae) from Southern Tanzania. J Med Entomol. 39 (1), 84-88 (2002).
- Zahedi, M., Denham, D. A., Ham, P. J. Encapsulation and melanization responses of Armigeres subalbatus against inoculated Sephadex beads. J Invertebr Pathol. 59 (3), 258-263 (1992).
- Laughton, A. M., Garcia, J. R., Altincicek, B., Strand, M. R., Gerardo, N. M. Characterisation of immune responses in the pea aphid, Acyrthosiphon pisum. J insect physiol. 57 (6), 830-839 (2011).
- Barreaux, A. M. G., Barreaux, P., Koella, J. C. Overloading the immunity of the mosquito Anopheles gambiae with multiple immune challenges. Parasite Vector. 9 (1), 210 (2016).
- Lazzaro, B. P., Rolff, J. Danger, Microbes, and Homeostasis. Science. 332 (6025), 43-44 (2011).
- Arrighi, R. B. G., Faye, I. Plasmodium falciparum GPI toxin: a common foe for man and mosquito. Acta trop. 114 (3), 162-165 (2010).
- Michel, K., Kafatos, F. C. Mosquito immunity against Plasmodium. Insect Biochem Molec. 35 (7), 677-689 (2005).
- Osta, M. A., Christophides, G. K., Vlachou, D., Kafatos, F. C. Innate immunity in the malaria vector Anopheles gambiae: comparative and functional genomics. J Exp Biol. 207 (15), 2551-2563 (2004).
- Christophides, G. K., Vlachou, D., Kafatos, F. C. Comparative and functional genomics of the innate immune system in the malaria vector Anopheles gambiae. Immunol Rev. 198 (1), 127-148 (2004).
- Blair, C. D. Mosquito RNAi is the major innate immune pathway controlling arbovirus infection and transmission. Future microbiol. 6 (3), 265-277 (2011).
- Fragkoudis, R., Attarzadeh-Yazdi, G., Nash, A. A., Fazakerley, J. K., Kohl, A. Advances in dissecting mosquito innate immune responses to arbovirus infection. J Gen Virol. , (2009).
- Schwartz, A., Koella, J. C. The cost of immunity in the yellow fever mosquito, Aedes aegypti depends on immune activation. J evol biol. 17 (4), 834-840 (2004).
- Lambrechts, L., Vulule, J. M., Koella, J. C. Genetic correlation between melanization and antibaterial immune responses in a natural population of the malaria vector Anopheles gambiae. Evolution. 58 (10), 2377 (2004).
- Boete, C., Paul, R. E. L., Koella, J. C. Direct and indirect immunosuppression by a malaria parasite in its mosquito vector. P Roy Soc B-Biol Sci. 271 (1548), 1611-1615 (2004).
- Sacks, D., Sher, A. Evasion of innate immunity by parasitic protozoa. Nat immunol. 3 (11), 1041-1047 (2002).
- Zambrano-Villa, S., Rosales-Borjas, D., Carrero, J. C., Ortiz-Ortiz, L. How protozoan parasites evade the immune response. Trend Parasito. 18 (6), 272-278 (2002).
- Damian, R. T. Parasite immune evasion and exploitation: reflections and projections. Parasitology. 115, S169-S175 (1997).
- Schmid-Hempel, P. Parasite immune evasion: a momentous molecular war. Trend ecol evol. 23 (6), 318-326 (2008).
- Schmid-Hempel, P. Immune defence, parasite evasion strategies and their relevance for "macroscopic phenomena" such as virulence. P Roy Soc B-Biol Sci. 364 (1513), 85-98 (2009).
- Stearns, S. C., Koella, J. C. The evolution of phenotypic plasticity in life history traits- predictions of reaction norms for age and size at maturity. Evolution. 40 (5), 893-913 (1986).
- Stearns, S. C. Life-history tactics: a review of the ideas. Q rev biol. 51 (1), 3-47 (1976).
- Valtonen, T. M., Kleino, A., Ramet, M., Rantala, M. J. Starvation Reveals Maintenance Cost of Humoral Immunity. Evol Biol. 37 (1), 49-57 (2010).
- Sheldon, B. C., Verhulst, S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trend Ecol Evo. 11 (8), 317-321 (1996).
- Graham, A. L., Allen, J. E., Read, A. F. Evolutionary causes and consequences of immunopathology. Annu Rev Ecol Evol S. 36, 373-397 (2005).
- Best, A., Long, G., White, A., Boots, M. The implications of immunopathology for parasite evolution. P Roy Soc B-Biol Sci. 279 (1741), 3234-3240 (2012).
- Cator, L. J., George, J., et al. 34;Manipulation" without the parasite: altered feeding behaviour of mosquitoes is not dependent on infection with malaria parasites. P Roy Soc B-Biol Sci. 280 (1763), 20130711 (2013).
- Voordouw, M. J., Lambrechts, L., Koella, J. No maternal effects after stimulation of the melanization response in the yellow fever mosquito Aedes aegypti. Oikos. 117 (8), 1269-1279 (2008).
- Paskewitz, S., Riehle, M. A. Response of Plasmodium refractory and susceptible strains of Anopheles gambiae to inoculated Sephadex beads. Dev comp immunol. 18 (5), 369-375 (1994).

