用的表面图案化微流体系统用于在单细胞水平调查空泡(S) - 细胞的相互作用及产生的生物效应
Summary
A microfluidic chip was fabricated to produce pairs of gold dots for tandem bubble generation and fibronectin-coated islands for single-cell patterning nearby. The resultant flow field was characterized by particle image velocimetry and was employed to study various bioeffects, including cell membrane poration, membrane deformation, and intracellular calcium response.
Abstract
在这个手稿,我们首先描述的微流体芯片的制造协议与金点和纤连蛋白涂布的区域中的相同的玻璃基板上,能精确控制串列式泡沫和具有良好定义的位置和形状的附近形成图案的单个细胞的产生上。然后,我们通过使用两个脉冲激光照射一对金点的有几微秒的时间延迟表明汇接气泡的产生。我们通过可视化的高速成像泡沫泡沫交互和射流形成,并使用粒子图像测速(PIV)描述所生成的流场。最后,我们提出这种技术单细胞分析,包括细胞膜上穿孔用高分子吸收,通过附加整联蛋白结合珠的位移确定的局部膜变形,并从比例成像细胞内钙离子反应的一些应用。我们的结果表明,一个快速和定向喷射流是亲由串联气泡相互作用,其可在接近生长的细胞的表面上施加一个高的局部剪切应力duced。此外,不同的生物效应可通过调节到汇接气泡从细胞相隔距离改变喷射流的强度来诱导。
Introduction
人们越来越认识到细胞的异质性,从基因,蛋白质和代谢物的随机产生的表达,存在较大的细胞群中,并在生物学作为一个基本原则,使细胞适应和进化1。因此,它常常是不精确和不可靠使用基于人口的散装测量理解各个细胞和它们之间的相互作用的功能。因此在生物和药理研究高息的单细胞分析开发新技术是,并且可以使用,例如,以更好地理解在干细胞生物学和癌症治疗2-4键信号通路和过程。在近年来,微流体平台的出现,极大地促进单细胞分析,其中将已与新颖的分析策略5进行定位,处理,并从单个细胞的反应的观察。
气穴起着各种范围的生物医学应用,包括高强度的癌症的治疗中具有重要作用聚焦超声(HIFU)6,由冲击波碎石肾结石的非侵入性碎片(SWL)7,药物或基因递送由sonoporation 8,并且通过水动力空化气泡9,10细胞或组织的最近报告破坏。尽管这样,空化气泡(多个)与生物组织和细胞相互作用的动态过程还没有被很好地理解。这是由于在通过超声,冲击波,和局部液压产生空化起始和气泡动力学的随机性;此外,还有一个缺有利的技术来解决的生物细胞本身固有的复杂和快的反应,特别是在单细胞水平的。
由于这些挑战,这并不奇怪,很少研究有蜂N报调查良好控制的实验条件下,泡沫细胞的相互作用。例如,单个细胞的膜穿孔被困在悬浮液11和人红细胞12的脉冲大变形已在微流体通道使用激光产生的单一气泡证明。后者的技术,但是,可以只在因核13的存在的真核细胞中产生非常小的变形。此外,很难在悬浮处理细胞时监视下游生物效应。在其它研究中,细胞结合的微泡(或超声造影剂)的超声激发用于生产单贴壁细胞的膜穿孔和/或细胞内钙离子的反应已经报道8。单贴壁细胞的膜穿孔,也可以通过使用在薄液层含有光吸收锥虫蓝溶液14激光产生的串联的气泡产生,或通过微秒激光脉冲通过在微容器15的光学吸收基板照射所产生的振荡气体气泡。时相比,光学吸收基底具有的优点在激光吸收台盼蓝溶液,因为后者是细胞毒性。更重要的是,激光产生的气泡是在比声学激发的气泡的气泡尺寸和位置方面更加可控。然而,在所有这些先前的研究中,细胞形状,取向,和粘附条件不加以控制,其可以基本上影响细胞响应和机械应力16产生生物效应。
为了克服在以前的研究这些缺点,我们最近开发了气泡的产生,细胞图案化,气泡的气泡 – 细胞相互作用,和实时通过使用微加工TECHN的一个独特的组合构成的微流控芯片细胞应答的生物测定法的实验系统iques。从其他外地区分我们的实验系统的三个主要功能有:1)在玻璃基板上的微米级金的点的图案形成,以使气泡生成17的局部激光吸收; 2)在同一基板上的细胞粘附的细胞外基质(ECM)的微米大小的岛的图案化,以控制这两个位置和各个单元的几何形状;和3)从3D气泡泡沫 – 细胞相互作用域的尺寸与准二维空间压缩,以促进在面内的可视化气泡气泡的相互作用,喷射流场,细胞变形,和生物效应,在所有捕获一个改进的成像序列( 图1d)。
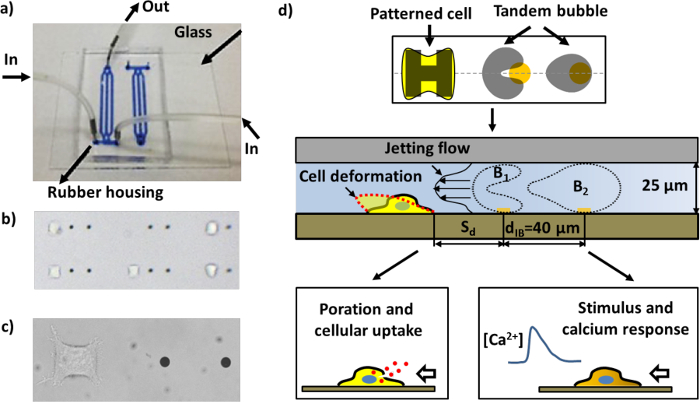
图1:微流体芯片和不同测定的示意图。 a)在频道的组装微流控芯片充满了蓝墨水可视化。 b)在图案化的细胞和金的点在微流体芯片内的区域(在邻近的两金点之间的距离为40微米)。工作单位的许多对可以被布置在一个通道。 三)特写由一对金点的并粘附于细胞图案化区域中的HeLa细胞的单一工作单元的图像。 d)该设备的操作示意图。单个细胞附着和扩散上涂覆有纤连蛋白的“H”形岛。一对空化气泡(串联气泡)与抗相振荡的被照亮的金点的脉冲激光束(参见图4a),导致了快速和局部的喷射朝向目标小区移动附近的发生而产生。细胞可能会变形,中蕴含大分子摄入,和/或与钙反应的刺激,这取决于细胞的串联气泡的偏离距离(S D)。F =“http://ecsource.jove.com/files/ftp_upload/55106/55106fig1large.jpg”目标=“_空白”>点击此处查看该图的放大版本。
这个平台可以与荧光测定法和附着到细胞表面的气蚀引起的生物效应官能珠被进一步组合。特别是,该平台将打开在单细胞水平可靠和可定量测定的方式。到现在为止,我们已经使用了装置串联气泡诱导细胞膜变形,细胞穿孔和细胞内摄取,生存能力,细胞凋亡和细胞内钙响应的分析。在下面的协议中,我们描述了芯片制造的过程以及用于分析上述的各种生物效应的过程。此外,还描述了芯片的操作。
Protocol
Representative Results
Discussion
单细胞分析,在用活细胞成像的组合,大大提高了在单个细胞,如表型的发展和免疫反应23动态和常变量过程的理解。在对比中菜或瓶的现有的细胞培养,微流体系统使微环境的精确控制,下至单细胞水平,在现实的时间。因此,先进的微流体技术和工艺在很大程度上改善了单细胞分析的吞吐量和重现性。通过整合软光刻和表面图案化,微流体系统可进一步设计成便于单个小区对spatiotempor…
Divulgations
The authors have nothing to disclose.
Acknowledgements
We would like to acknowledge the use of the clean room facility SMIF at Duke University. We also want to thank Hao Qiang for his assistance in measuring the jet velocity. The authors thank Todd Rumbaugh of Hadland Imaging for providing the Shimadzu HPV-X camera used in this study.The work was funded in part by NIH through grants 5R03EB017886-02 and 4R37DK052985-20.
Materials
| Reagent/Materials | |||
| 75x38mm Plain Microscope Slides | Corning | 2947-75X38 | |
| Acetone | Sigma Aldrich, Co. | 320110 | ACS reagent, ≥99.5% |
| Isopropyl alcohol | Sigma Aldrich, Co. | W292907 | ≥99.7%, FCC, FG |
| Sulfuric acid | Sigma Aldrich, Co. | 320501 | ACS reagent, 95.0-98.0% |
| Hydrogen peroxide | Sigma Aldrich, Co. | 216763 | 30 wt.% in H2O |
| Primer P-20 | Microchem | MCC Primer 80/20 | |
| NFR photoresist | JSR | NFR016D2 | |
| Photomask | Photoplotstore | N/A | 4×4 Direct write mask |
| MF-319 Developer | Shipley (Rohm and Haas) | Microposit MF-319 | |
| 1165 Photoresist Remover | Dow Chemical, Co. | DEM-10018073 | 1-methyl-2-pyrrolidinone based |
| S1813 photoresist | Shipley (Rohm and Haas) | S1813 | |
| PLL-g-PEG | SuSoS | PLL(20)-g[3.5]- PEG(2) | |
| HEPES | ThermoFisher Scientific | 15630080 | |
| Paraffin film | HACH | 251764 | |
| SU-2025 photoresist | Microchem | SU-2025 | |
| PDMS | Dow Corning | 184 SIL ELAST KIT 0.5KG | |
| Microbore Tubing | Saint-Gobain PPL Corp. | S-54-HL | |
| Metal pins | New England Small Tube | NE-1300-01 | Cut Tube (straight), 0.025” OD x 0.017” ID x 0.50” Long |
| HeLa cells | Duke Cell Culture Facility | (307-CCL-2) HeLa, p.148 | |
| DPBS(1X) buffer | ThermoFisher Scientific | 14190144 | |
| DMEM culture medium | ThermoFisher Scientific | 11995065 | |
| Fibronectin Bovine Protein, Plasma | ThermoFisher Scientific | 33010018 | |
| 0.25% Trypsin-EDTA (1X) | ThermoFisher Scientific | 25200056 | |
| Propidium Iodide | ThermoFisher Scientific | P21493 | |
| Carboxylate Microspheres 1.00μm | Polysciences, Inc | 08226-15 | |
| Carboxylate Microspheres 2.00μm | Polysciences, Inc | 18327-10 | |
| EDC (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride) | ThermoFisher Scientific | 22980 | |
| Sulfo-NHS | Sulfo-NHS (N-hydroxysulfosuccinimide) | 24510 | |
| Peptite-2000 | Advanced BioMatrix | 5020-5MG | |
| FITC Annexin V | ThermoFisher Scientific | A13199 | |
| Fura-2, AM | ThermoFisher Scientific | F1221 | |
| DMSO | Sigma Aldrich, Co. | D2650 | |
| F-127 | invitrogen | P6866 | 0.2 µm filtered (10% Solution in Water) |
| Reduced serum media | ThermoFisher Scientific | 11058021 | |
| Name | Company | Catalog Number | Comments |
| Equipment | |||
| Plasma asher | Emitech | K-1050X | O2 / Ar plasma ashing of photoresist and other organic materials |
| Mask aligner | SUSS MicroTec | Karl Suss MA6/BA6 | |
| E-beam evaporator | CHA Industries | CHA Industries Solution E-Beam | |
| RIE | Trion Technology | Trion Technology Phantom II | (oxide/ nitride/ polymer) etching |
| Stereoscope | AmScope | American Scope SM-4TZ-FRL | Stereo Microscope |
| Syringe pump | Chemyx Inc | NanoJet | |
| Cell culture incubator | NuAire | AutoFlow NU-8500 Water Jacket CO2 Incubator | |
| Biological Safety Cabinets | NuAire | NU-425-400 | |
| Water bath | VWR | 1122s | |
| Centrifuge | IEC | Centra CL2 | |
| Microscope | Zeiss | Axio Observer Z1 | |
| Nd:YAG laser (laser 1) | New Wave Research | Tempest | |
| Nd:YAG laser (laser 2) | New Wave Research | Orion | |
| Delay generator | Berkeley Nucleonics | BNC 565-8c | |
| Flash lamp | Dyna-Lite | ML1000 fiber-coupled flashtube | |
| high speed camera | DRS Hadland | Imacon 200 | |
| high speed camera | Shimadzu | HPV-X | |
| high speed camera | Vision Research | Phantom V7.3 | |
| PIV software | LaVision | DaVis 7.2 | |
| camera | Zeiss | AxioCam MRc 5 | |
| software | Zeiss | AxioVision | |
| PTI system | Horiba | S/N: 1705 RAM-X | |
| EasyRatio software | Horiba | Easy Ratio Pro 2 | version 2.3.125.86 |
| 63× objective | Zeiss | LD Plan Neofluar |
References
- Wang, D., Bodovitz, S. Single cell analysis: the new frontier in ‘omics. Trends Biotechnol. 28 (6), 281-290 (2010).
- Weaver, W. M., et al. Advances in high-throughput single-cell microtechnologies. Curr Opin Biotechnol. 25, 114-123 (2014).
- Gossett, D. R., et al. Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proc Natl Acad Sci USA. 109 (20), 7630-7635 (2012).
- Spiller, D. G., Wood, C. D., Rand, D. A., White, M. R. H. Measurement of single-cell dynamics. Nature. 465 (7299), 736-745 (2010).
- Lecault, V., White, A. K., Singhal, A., Hansen, C. L. Microfluidic single cell analysis: from promise to practice. Curr Opin Chem Biol. 16 (3-4), 381-390 (2012).
- Kennedy, J. E. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 5 (4), 321-327 (2005).
- Zhu, S., Cocks, F. H., Preminger, G. M., Zhong, P. The role of stress waves and cavitation in stone comminution in shock wave lithotripsy. Ultrasound Med Biol. 28 (5), 661-671 (2002).
- Fan, Z., Liu, H., Mayer, M., Deng, C. X. Spatiotemporally controlled single cell sonoporation. Proc Natl Acad Sci U S A. 109 (41), 16486-16491 (2012).
- Itah, Z., et al. Hydrodynamic cavitation kills prostate cells and ablates benign prostatic hyperplasia tissue. Exp Biol Med. 238 (11), 1242-1250 (2013).
- Kosar, A., Sesen, M., Oral, O., Itah, Z., Gozuacik, D. Bubbly cavitating flow generation and investigation of its erosional nature for biomedical applications. IEEE Trans Biomed Eng. 58 (5), 1337-1346 (2011).
- Li, Z. G., Liu, A. Q., Klaseboer, E., Zhang, J. B., Ohl, C. D. Single cell membrane poration by bubble-induced microjets in a microfluidic chip. Lab Chip. 13 (6), 1144-1150 (2013).
- Li, F. F., Chan, C. U., Ohl, C. D. Yield Strength of Human Erythrocyte Membranes to Impulsive Stretching. Biophys J. 105 (4), 872-879 (2013).
- Li, F., M, M., Ohl, C. .. D. .. Shear stress induced stretching of red blood cells by oscillating bubbles within a narrow gap. Bull Am Phys Soc. 58, (2013).
- Sankin, G. N., Yuan, F., Zhong, P. Pulsating tandem microbubble for localized and directional single-cell membrane poration. Phys. Rev. Lett. 105 (7), 078101 (2010).
- Fan, Q., Hu, W., Ohta, A. T. Laser-induced microbubble poration of localized single cells. Lab Chip. 14 (9), 1572-1578 (2014).
- Chen, C. S., Mrksich, M., Huang, S., Whitesides, G. M., Ingber, D. E. Geometric control of cell life and death. Science. 276 (5317), 1425-1428 (1997).
- Yuan, F., Sankin, G., Zhong, P. Dynamics of tandem bubble interaction in a microfluidic channel. J Acoust Soc Am. 130 (5), 3339-3346 (2011).
- Yang, C. . Analysis of Tandem Bubble Interaction and Jet Formation in a Microfluidic Channel. , (2013).
- Simon, S. I., Schmid-Schonbein, G. W. Cytoplasmic strains and strain rates in motile polymorphonuclear leukocytes. Biophys J. 58 (2), 319-332 (1990).
- Barbee, K. A., Macarak, E. J., Thibault, L. E. Strain measurements in cultured vascular smooth muscle cells subjected to mechanical deformation. Ann Biomed Eng. 22 (1), 14-22 (1994).
- Yuan, F., Yang, C., Zhong, P. Cell membrane deformation and bioeffects produced by tandem bubble-induced jetting flow. Proc. Natl. Acad. Sci. U.S.A. 112 (51), E7039-E7047 (2015).
- Yin, H., Marshall, D. Microfluidics for single cell analysis. Curr Opin Biotechnol. 23 (1), 110-119 (2012).
- Tay, S., et al. Single-cell NF-kappa B dynamics reveal digital activation and analogue information processing. Nature. 466 (7303), 267-271 (2010).
- Rand, R. P., Burton, A. C. Mechanical Properties of the Red Cell Membrane: I. Membrane Stiffness and Intracellular Pressure. Biophys J. 4 (2), 115-135 (1964).
- Lim, C. T., Dao, M., Suresh, S., Sow, C. H., Chew, K. T. Large deformation of living cells using laser traps. Acta Mater. 52 (7), 1837-1845 (2004).
- Puig-de-Morales-Marinkovic, M., Turner, K. T., Butler, J. P., Fredberg, J. J., Suresh, S. Viscoelasticity of the human red blood cell. Am J Physiol Cell Physiol. 293 (2), C597-C605 (2007).
- Kudo, N., Okada, K., Yamamoto, K. Sonoporation by single-shot pulsed ultrasound with microbubbles adjacent to cells. Biophys J. 96 (12), 4866-4876 (2009).
- van Wamel, A., et al. Vibrating microbubbles poking individual cells: Drug transfer into cells via sonoporation. J Control Release. 112 (2), 149-155 (2006).
- Hu, Y., Wan, J. M., Yu, A. C. Membrane perforation and recovery dynamics in microbubble-mediated sonoporation. Ultrasound Med Biol. 39 (12), 2393-2405 (2013).
- Dijkink, R., et al. Controlled cavitation-cell interaction: trans-membrane transport and viability studies. Phys Med Biol. 53 (2), 375-390 (2008).
- Rau, K. R., Quinto-Su, P. A., Hellman, A. N., Venugopalan, V. Pulsed laser microbeam-induced cell lysis: Time-resolved imaging and analysis of hydrodynamic effects. Biophys J. 91 (1), 317-329 (2006).

