Solvent Bonding for Fabrication of PMMA and COP Microfluidic Devices
Summary
Solvent bonding is a simple and versatile method for fabricating thermoplastic microfluidic devices with high quality bonds. We describe a protocol to achieve strong, optically clear bonds in PMMA and COP microfluidic devices that preserve microfeature details, by a judicious combination of pressure, temperature, an appropriate solvent, and device geometry.
Abstract
Thermoplastic microfluidic devices offer many advantages over those made from silicone elastomers, but bonding procedures must be developed for each thermoplastic of interest. Solvent bonding is a simple and versatile method that can be used to fabricate devices from a variety of plastics. An appropriate solvent is added between two device layers to be bonded, and heat and pressure are applied to the device to facilitate the bonding. By using an appropriate combination of solvent, plastic, heat, and pressure, the device can be sealed with a high quality bond, characterized as having high bond coverage, bond strength, optical clarity, durability over time, and low deformation or damage to microfeature geometry. We describe the procedure for bonding devices made from two popular thermoplastics, poly(methyl-methacrylate) (PMMA), and cyclo-olefin polymer (COP), as well as a variety of methods to characterize the quality of the resulting bonds, and strategies to troubleshoot low quality bonds. These methods can be used to develop new solvent bonding protocols for other plastic-solvent systems.
Introduction
Microfluidics has emerged over the past twenty years as a technology well suited for studying chemistry and physics at the microscale1, and with growing promise to significantly contribute to biology research2–4. The majority of microfluidic devices have historically been made from poly(dimethylsiloxane) (PDMS), a silicone elastomer that is easy to use, inexpensive, and offers high quality feature replication5. However, PDMS has well-documented shortcomings and is incompatible with high-volume fabrication processes6,7, and as such, there has been a growing trend toward fabricating microfluidic devices from thermoplastic materials, because of their potential for mass manufacturing and thus commercialization.
One of the major barriers to wider adoption of plastic microfabrication has been achieving easy, high quality bonding of plastic devices. Current strategies employ thermal, adhesive, and solvent bonding techniques, but many suffer from significant challenges. Thermal bonding increases autofluorescence8 and often deforms microchannel geometries9–11, while adhesive techniques require stencils, careful alignment, and ultimately leave the thickness of the adhesive exposed to the microchannel10. Solvent bonding is attractive due to its simplicity, tunability, and low cost10,12–14. In particular, its tunability enables optimization for a variety of plastics, which can yield consistent, high quality bonding that minimizes deformation of microfeatures14.
During solvent bonding, solvent exposure increases the mobility of polymer chains near the surface of the plastic, which enables inter-diffusion of chains across the bonding interface. This causes entanglement via mechanical interlocking of the diffusing chains, and results in a physical bond10. Thermal bonding works in a similar manner, but relies on elevated temperature alone to increase chain mobility. Thus, thermal methods require temperatures near or above the glass transition of the polymer, whereas the use of solvents can significantly reduce the temperature needed for bonding, and thus reduce unwanted deformation.
We provide a specific protocol for bonding both PMMA and COP devices. However, this protocol and method describes a simple, generic approach for solvent bonding of thermoplastic microfluidic devices that can be tailored for other plastic materials, solvents, and available equipment. We describe numerous methods for assessing the quality of bonds (e.g., bond coverage, bond strength, bond durability, and deformation of microfeature geometries), and provide troubleshooting approaches to address these common challenges.
Protocol
Note that all of the steps described below have been developed and performed in a non-cleanroom environment. The solvent bonding steps can certainly be performed in a cleanroom, if available, but this is not required.
1. Preparation of Thermoplastic Microfluidic Device Layers
- Design and fabricate microfluidic device layers from the thermoplastic of choice, using an appropriate fabrication method (e.g., micromilling15, embossing16–18, injection molding).
- Visually inspect device layers to ensure that edges are "clean" (i.e., no burrs or ridges of leftover material from the fabrication process). For best results, check all machined micro-feature edges in addition to the outside edges of the device under an optical microscope.
- If leftover material is found during visual inspection, use a razor blade, or scalpel to carefully remove any material that prevents the device layers from lying flat against one another so that the interfaces of the layers come into conformal contact.
- Clean device surfaces with laboratory soap and water and dry with compressed air. Submerge device layers in 2-propanol for 2 min and dry with compressed air.
2. Solvent Bonding
- Prepare heated press (for PMMA) or hotplate (for COP).
- For PMMA (cast acrylic, glass transition temperature of ~100-110 °C)18 preheat press to 70 °C, and allow temperature to stabilize.
- For COP (glass transition temperature of 102 °C, from manufacturer), preheat hotplate to 25 °C, and allow temperature to stabilize.
- Prepare solvent for bonding process.
- For PMMA, measure 0.5 ml of ethanol per square inch of bonding area.
- For COP, prepare a 65:35 mixture of 2-propanol and cyclohexane, with a total volume of 0.5 ml of the mixture per square inch of bonding area.
NOTE: For COP, use glass pipettes and containers, as cyclohexane will dissolve common polypropylene labware. Perform all mixing and bonding in a fume hood, as cyclohexane is toxic.
- Dispense 0.1 ml of solvent per square inch of bonding area between cleaned plastic layers and bring the layers together. Visually inspect for air bubbles at the bonding interface, which are common, and should be removed as much as possible.
NOTE: It is beneficial to work quickly once the solvent has been dispensed, as volatile solvents will begin to evaporate (and hence, solvent mixtures will change in composition).- If bubbles are present, slide the two plastic layers along the bonding interface so that they nearly come apart (but remain in contact), and then slide them back together.
- Align the layers of the device with alignment pins, a custom jig, or simply by hand (see Discussion section for further details).
- If using alignment pins, align the holes for the pins, and insert the pins into the device stack.
- If using a custom jig, insert the device stack into the jig and tighten around the device.
- If aligning by hand, use fingers to align the outer edges of the device.
- Place the device with solvent into the pre-heated press (for PMMA) or onto the pre-heated hotplate (for COP).
- For PMMA, apply 2,300 kPa of pressure for 2 min.
- For COP, apply 350 kPa of pressure. Increase the temperature from 25 °C to 70 °C at a rate of 5 °C/min. After reaching 70 °C (after 9 min), bond for an additional 15 min.
- Use tweezers to safely remove the hot device for inspection. Bonding is now complete.
- Remove any remaining liquid in the device (in microchannels or other features).
- For PMMA, remove any remaining liquid with compressed air. For COP, place bonded device on hotplate and bake at 45 °C for 24 hr to remove any remaining cyclohexane.
Representative Results
A schematic of the general solvent bonding procedure is shown in Figure 1. The easiest way to assess bond quality is to visually inspect bond coverage, since poor bond coverage is easily visible as regions of unbonded plastic, and is indicative of weak bonding. Such regions are typically near free edges (e.g., periphery of device, or near open ports or microchannels), and can also often appear around any particles of dirt or dust at the bonding interface. Poor bond coverage due to weak bonding is typically seen during the protocol development stage, before the optimal solvent composition and bonding conditions have been found, and examples are shown in Figure 2A. Typically, weak bonding suggests a need for one or more of: (i) a more aggressive solvent (i.e., a higher concentration solution, or a different solvent), (ii) a higher bonding temperature, and (iii) a higher bonding pressure.
Conversely, overly aggressive bonding conditions can lead to excellent bond coverage with high bond strength, but also damaged or sealed microfeatures, as shown in Figure 2B. This is most often due to the solvent being too aggressive, although high temperature (approaching the glass transition temperature of the plastic) can also cause significant deformation.
A high quality, well-bonded device has both good bond coverage and minimal deformation of microfeatures, as shown in Figure 2C. Depending on the solvent and temperature of choice, it may be difficult to achieve good bond coverage near free edges of the device if the solvent is very volatile and thus evaporates quickly. In these situations, adding small microgrooves to the design of the device, along the edges of interest, can help mitigate solvent evaporation and thus improve bond coverage, as shown in Figure 2D. We typically use grooves with cross-sectional dimensions of 500 x 500 µm, placed 300 µm from the edge.14
In addition to general visual inspection of bond coverage, both destructive and non-destructive tests should be used to further investigate bond quality and microfeature damage. Two destructive tests that are useful during the protocol development stage are (i) cross-sectioning and (ii) wedging apart bonded devices, to assess microfeature geometry and bond strength, respectively. We prefer using micromilling to section our devices, as we find this offers a good combination of convenience, accuracy, and relatively clean surfaces. In the absence of a milling machine other approaches can be used, although they will present different challenges. Examples include cutting with a diamond saw or band saw (leaves rough surfaces), carefully snapping a device along scored lines (can be difficult due to thickness, and shear stress may cause device to delaminate), or simply sanding away part of the device (time-consuming). Rough surfaces from cutting can be smoothed with sanding.
Whatever the method used, devices should be sectioned perpendicular to microfeatures of interest, and can be visually inspected using an optical microscope. The size and shape of the microfeature cross-sections will indicate how much deformation has occurred due to bonding. Less aggressive solvents, and lower temperatures and pressures, will better preserve sharp corners and straight walls, while more aggressive conditions will cause rounding of corners and walls, as well as a decrease in cross-sectional area due to polymer swelling. Images of typical results are shown in Figure 3.
Bond strength can be measured by partially wedging apart the bonded layers with a shim and measuring the distance from the edge of the shim to the edge of the delamination region. The bond strength can be calculated from this distance, the thickness of the layers and the shim, and the elastic modulus of the plastic.14,19 If the specific bond strength value is not important, wedging the layers apart provides a qualitative method to assess whether the bond is strong enough for the typical forces and handling the device will encounter during experiments.
Nondestructive tests are useful for verifying the quality of a device while leaving it usable for its intended purpose. A simple and useful method is to inspect via microscopy, focusing primarily on bond coverage near microfeature edges, or on small bonding areas. Unbonded regions appear slightly darker than bonded regions due to the thin air gap between the plastic layers, and as shown in Figure 4, should be noticeable by careful inspection with a microscope. Finding regions of unbonded plastic can help direct a second iteration of localized bonding to seal critical parts of the device before use.
A useful technique for achieving strong bonds with an aggressive solvent, but while minimizing damage to microchannels, is to add grooves with access ports to the device design, and only add solvent to the grooves (instead of flooding the bonding surface with solvent). This greatly reduces contact between the microchannel and the liquid solvent (vapor still enters the microchannel), and consequently reduces deformation as shown in Figure 5.
Depending on the intended device application, longer-term durability testing may be required. For example, many of our devices are used for biological experiments, and can reside in a cell incubator environment (37 °C, 100% humidity) for up to several weeks. Durability can be assessed by placing test devices in the required conditions for the required duration, and inspected afterward for a visual decrease in bond coverage (delamination of the device), or weakened bond strength that allows the device to be easily wedged apart. Examples are shown in Figure 6, and indicate the need to modify the bonding procedure to achieve higher bond strength.
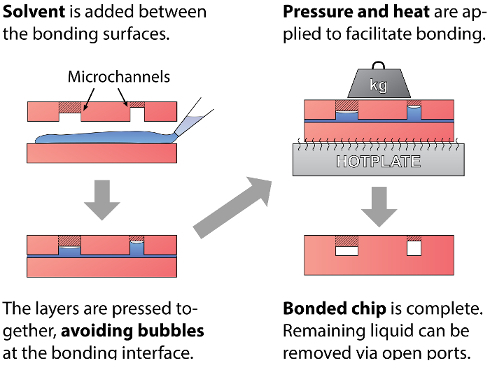
Figure 1: Schematic of Bonding Process. The general solvent bonding process is shown. Liquid solvent is added between the two thermoplastic device layers to be bonded. The layers are brought together and bubbles are removed from the liquid at the bonding interface. Pressure and heat are applied to the device for the required duration, and the bonded device is complete. Any remaining liquid can be removed from open ports. Please click here to view a larger version of this figure.
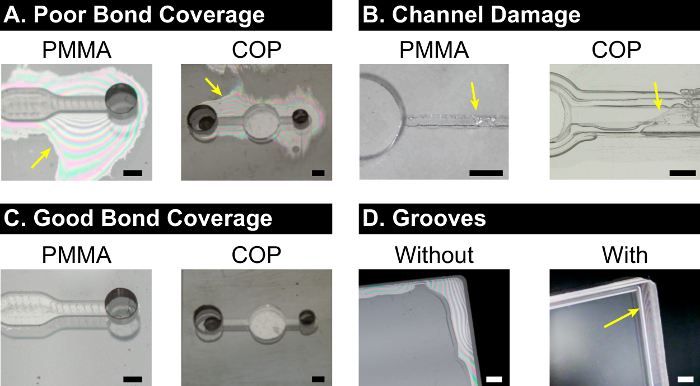
Figure 2: Visual Examples of Varying Bond Quality. (A) A common challenge with many liquid solvent bonding approaches is that rapid evaporation occurs near free edges of the device during the heated bonding step due to solvent volatility. This often leads to regions of unbonded material near these edges, resulting in poor bond coverage, leaky microfluidic features, and low overall bond strength. These regions are visible as lighter patches with colored interference fringes (yellow arrows). (B) Conversely, overly aggressive solvent bonding yields excellent bond coverage, but can also cause significant damage to microfeatures, thereby deforming or closing off device channels (yellow arrows). (C) An optimized solvent-plastic system achieves good bond coverage and strength, and minimizes damage to microfeatures while still properly sealing them. (D) In some cases, adding solvent retention grooves parallel to device edges (yellow arrow) can help improve bond coverage and properly seal microfeatures. Grooves may be needed if better bond coverage is required, but it is undesirable to increase solvent strength (due to the resulting increased damage to microfeatures). Scale bars = 1 mm. Please click here to view a larger version of this figure.
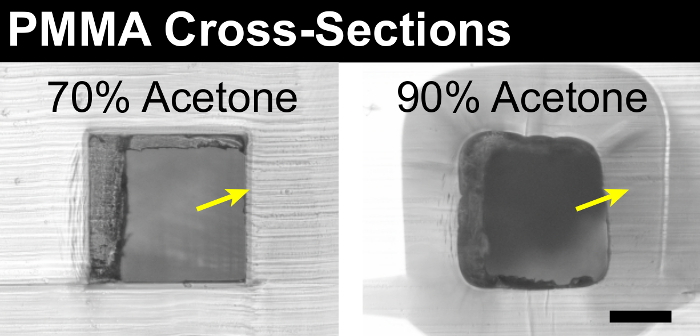
Figure 3: Cross-Sections of Bonded Devices. The solvent bonding process can deform microfeatures if the solvent is too aggressive, or if the bonding temperature or pressure are too high. Inspecting cross-sections of bonded devices will reveal incidents of microfeature deformation. Performing such inspections for each specific plastic-solvent system can help establish the optimal parameters for achieving the desired microchannel quality. Representative images are shown for cross-sections of a 500-µm square PMMA channel bonded with appropriate (left) and overly aggressive (right) solvent mixtures. Two indicators of the extent of solvent-induced deformation are the rounding of corners and straight walls, and the thickness of a solvent-rich layer of plastic at surfaces that were in contact with the solvent (yellow arrows). Scale bar = 200 µm. Please click here to view a larger version of this figure.
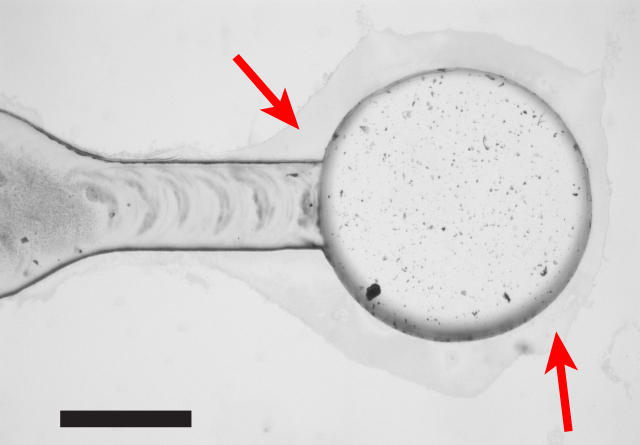
Figure 4: Non-destructive Inspection via Microscopy. Careful inspection with an optical microscope can identify regions of unbonded plastic, particularly near microfeature edges, or on small bonding areas. Unbonded regions (red arrows) appear slightly darker than bonded regions due to the thin air gap between the plastic layers, and colored interference fringes are sometimes also visible in these areas. Identifying regions of poor bonding can indicate the need for a second iteration of bonding, with solvent locally added to trouble spots. Scale bar = 1 mm.
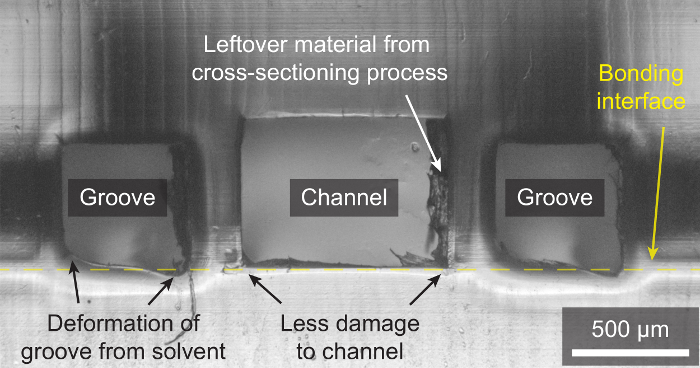
Figure 5: Minimizing Damage to Channels by Adding Solvent Only to Grooves. The microscope image shows a cross-section of a PMMA channel surrounded by two grooves. Solvent can be added to grooves while the two device layers are pressed together, rather than flooding the bonding interface with solvent. This greatly minimizes solvent contact with the inside of the microchannel, and thus minimizes solvent-induced deformation. This approach is useful when an aggressive solvent is needed to ensure high bond strength and coverage, but contact between the solvent and microchannels needs to be minimized. Please click here to view a larger version of this figure.
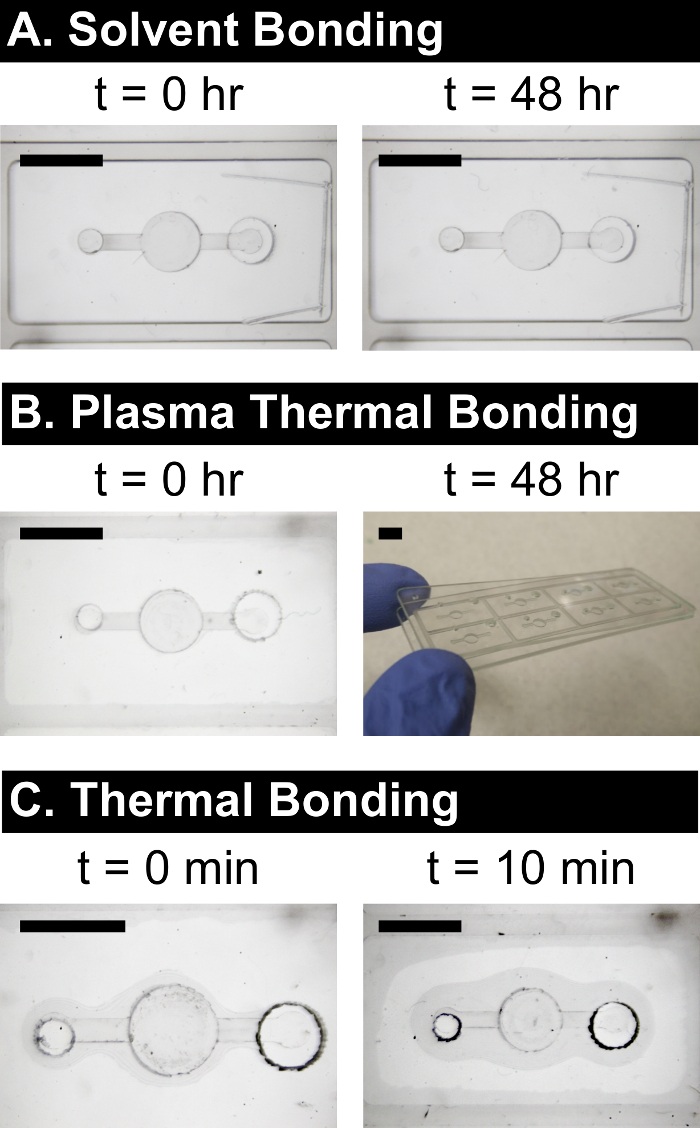
Figure 6: Durability and Comparison to (Plasma-Assisted) Thermal Bonding. Solvent bonding (A) yields better bond quality than plasma-assisted thermal bonding (B) or thermal bonding (C) techniques, and also better durability in cell incubator conditions (37 °C, 100% humidity). Both thermal techniques routinely leave significant "halos" of unbonded plastic surrounding microfeatures, with plasma-assisted thermal bonding being better than regular thermal bonding. These bonds also degrade over time, with the unbonded regions growing in size. While the solvent bonded COP device showed no change in bonding over 48 hours in an incubator (A), the plasma thermal bonded device delaminated completely (B). The regular thermal bonded device began to delaminate within 10 min in ambient conditions (C), and also delaminated completely in the incubator. Scale bars = 5 mm. Please click here to view a larger version of this figure.
| Plastic | Solvent | Temperature (°C) | Pressure (kPa) | Duration (min) | Notes | ||
| PMMA | 100% Ethanol | 70 | 2,300 | 2 | Best option if a heated press is available (required for high pressure). Consistently yields excellent bond coverage (without the use of solvent retention grooves), with low deformation of microfeatures. Requires very little solvent, and is very insensitive to bubbles and dust/dirt at the bonding interface due to high pressure. | ||
| PMMA | 75% Acetone/25%Water | 40 | 30 | 20 | Inferior consistency to above method and takes longer, but does not require heated press (can be done with hotplate and free weights). Bond coverage is greatly aided by use of solvent retention grooves. | ||
| COP | 35% Cyclohexane/65% 2-propanol | 25 -> 70* | 350 | 15* | We have found that pre-heating the hotplate causes too much initial evaporation, and also leads to whitening of the plastic. Instead, we place the device on a room temperature hotplate and then increase the temperature to 70 °C (ramp rate of 5 °C/min). After the temperature stabilizes at 70 °C (in 9 min), we bond at this temperature for an additional 15 min. | ||
Table 1: Solvent Bonding Parameters. Summary of combinations of plastics, solvents, temperatures, and pressures for PMMA and COP solvent bonding protocol.
Discussion
The feasibility of potential bonding strategies depends on available equipment. While hotplates are relatively common and free weights can be purchased inexpensively, high pressure strategies will require the use of a heated press. For example, our optimal PMMA bonding recipe requires high pressure to bond with ethanol (see Table 1), and the required pressure is not attainable for typical device sizes using free weights. Thus, if only a hotplate and weights are available, PMMA can instead be bonded with a different solvent (75% acetone in water). Additionally, the use of solvents that require a fume hood may also limit feasibility, particularly if high pressures are needed, as such strategies would require both the use of a heated press and the need to fit the press inside a fume hood. These considerations can help guide solvent choices when developing new solvent-plastic systems, with preference given to less harmful solvents and lower pressures that still offer high quality bonding.
After a solvent bonding method has been optimized for a specific plastic, there may be remaining challenges with poor bond coverage near device edges, due to evaporation effects during the bonding process. As mentioned in the Representative Results section, one strategy that can mitigate these issues is the addition of a solvent retention groove that runs parallel to the edges of interest (i.e., the outer edge of the device, and alongside any channels or ports that typically exhibit bonding difficulty). Additionally, two or more through-hole ports can be added to each groove (and/or grooves can be connected), such that solvent can be added directly into the grooves with a pipette. This has two potential uses: (i) solvent can be added to the grooves instead of flooding the bonding interface, which greatly minimizes contact between the solvent and the walls of the microchannels (see Figure 5), and (ii) if the initial attempt at bonding leaves some areas with poor bond coverage, more solvent can be added locally for a second round of bonding, which often seals any remaining critical areas around microfeatures. It is worth noting that grooves can add to fabrication time, and since they take up space on the device, may not be amenable to some high-density designs.
One common challenge with bonding strategies is the alignment of the device layers during bonding. Several strategies are possible, depending on accuracy requirements. If alignment is not critical (i.e., if all microfeatures are on one layer, which is bonded to a flat underlying substrate layer), then manual alignment by hand is sufficient. For more demanding alignment requirements, hand alignment may still be sufficient, although this depends somewhat on the skill of the individual researcher. We can routinely achieve alignment accuracy to within 100 µm, and clever decisions in device design can relax the requirements for alignment accuracy (i.e., by designing tolerances into the microfeatures of the device if possible, such that slight misalignments do not affect device performance). A useful technique to help with hand alignment is to use as little solvent as possible to cover the bonding surface. With excessive amounts of solvent, the plastic layers "float" on the thin layer of liquid, and do not maintain their alignment when the device is transferred to the hotplate or heated press. In contrast, a very thin layer of solvent helps to "stick" the plastic layers together and maintain alignment after they are adjusted by hand.
If more accurate alignment is required, possible strategies include L-shaped corner brackets to hold the device layers together, alignment pins that go through the device layers, using tape on the outside of the device to secure layer positions, or custom-built jigs that can hold the device during the bonding step. For all of these strategies, note that the alignment hardware must typically be shorter than the total stack height of the device such that pressure can be applied to the face of the device during bonding.
The challenge for solvent bonding is usually not the width, but the depth of microchannels because shallow microchannels can be collapsed by an overly aggressive solvent or by excessively high temperatures and pressures. For a given depth, wider channels are more susceptible to collapse than narrow channels. Although we have not fabricated channels below 50 µm in width (due to limitations of our CNC milling machine that restrict the minimum endmill diameter we can use), we have routinely bonded channels that are separated by distances on the order of 25 µm. In terms of depth, we have also successfully bonded very shallow channels (~15 µm) using this method.
Finally, a comparison of liquid phase solvent bonding to other bonding techniques is warranted. Three other common bonding techniques are vapor phase solvent bonding, thermal diffusion bonding, and plasma-assisted thermal diffusion bonding. Vapor phase solvent bonding shares many of the characteristics of liquid phase solvent bonding, but is in our experience more difficult to perform and less tunable, leading to less consistent results. Applying solvent in the vapor phase requires either a vacuum chamber or a vapor chamber with a device holder, and we find the process is less controllable than using liquid. In addition, vapor phase techniques are rarely amenable for use with solvent mixtures, since nearly all mixtures are non-azeotropic, and thus will change their composition from liquid to gas phase, depending on ambient pressure and temperature.
Thermal diffusion bonding involves pressing the device layers together at elevated temperature near the glass transition temperature (Tg) of the plastic. The high temperature increases polymer chain mobility, allowing chains to interdiffuse across the bonding interface and form a bond.10 However, because the entire device is heated to the same temperature, chain mobility is increased everywhere, and microfeatures become distorted and rounded9,10. Thus, increasing bond strength (due to higher bonding temperature) comes at the direct cost of damage to microfeatures. One modification to thermal bonding is the use of a pre-bonding oxygen plasma surface treatment, which lowers the local (surface) Tg of the polymer20. This plasma-assisted thermal bonding thus enables bonding to occur at lower temperatures, further away from the bulk Tg. While this does reduce the amount of deformation to microfeatures when compared to thermal bonding, we have found that initial bond coverage and strength are still much lower when compared to solvent bonding, and importantly, the bonds degrade over subsequent hours. As shown in Figure 6, plasma-assisted thermal bonded devices showed significant loss of bond coverage over 48 hours in a cell incubator, while solvent bonded devices showed no changes.
Here, we described a simple, efficient protocol that relies on liquid-based solvent bonding to achieve quality bonds for PMMA and COP microfluidic devices. Our results demonstrated that specific steps for PMMA and COP bonding will lead to strong and optically clear bonds with negligible deformation of microfeatures, and that use of grooves and alignment hardware can circumvent common practical issues during the solvent bonding procedure. Incorporating this method into the fabrication process will accelerate development of PMMA and COP-based microfluidic devices, and enable researchers to more easily adopt thermoplastics into their microfluidic device designs.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We acknowledge financial support from the Natural Sciences and Engineering Research Council of Canada (NSERC, #436117-2013), the Cancer Research Society (CRS, #20172), Myeloma Canada, and Grand Challenges Canada.
Materials
| COP | Zeonor | 604Z1020R080 | 20 kg COP Pellets – 1020R. Multiple suppliers can be used, but may affect bonding characteristics. |
| PMMA | McMaster Carr | 8560K173 | 1.5 mm sheet thickness for our typical applications. Multiple suppliers can be used, but may affect bonding characteristics. |
| Cyclohexane | Sigma-Aldrich | 227048 | Cyclohexane, anhydrous, 99.5%. Multiple suppliers can be used. Toxic, requires fumehood. |
| Ethanol | Sigma-Aldrich | 24102 | Ethanol, absolute, ≥99.8% (GC). Multiple suppliers can be used. |
| Acetone | Sigma-Aldrich | 179124 | Acetone, ACS reagent, ≥99.5%. Multiple suppliers can be used. |
| 2-Propanol | Sigma-Aldrich | 278475 | 2-Propanol, anhydrous, 99.5%. Multiple suppliers can be used. |
| Hot plate(s) | Torrey Pines Scientific | HP60 | Fully programmable digital hotplate. Multiple suppliers can be used. |
| Free weights | Cap Barbell | RPG#2 | Standard cast iron plate. Multiple suppliers and different weights can be used. |
| Heated press | Carver | Auto CH | Auto series heated hydraulic press. Multiple suppliers can be used. A press that fits in a fumehood would allow the most flexibility (this model does not). |
| CNC Milling Machine | Tormach | PCNC 770 | 3 Axis CNC mill. Multiple suppliers can be used. |
| Endmills | Various | Various | Required sizes depend on designs. Multiple suppliers can be used. |
References
- Beebe, D. J., Mensing, G. A., Walker, G. M. Physics and applications of microfluidics in biology. Annual Review of Biomedical Engineering. 4, 261-286 (2002).
- Situma, C., Hashimoto, M., Soper, S. a. Merging microfluidics with microarray-based bioassays. Biomolecular Engineering. 23 (5), 213-231 (2006).
- Paguirigan, A. L., Beebe, D. J. Microfluidics meet cell biology: Bridging the gap by validation and application of microscale techniques for cell biological assays. BioEssays. 30 (9), 811-821 (2008).
- Young, E. W. K., Beebe, D. J. Fundamentals of microfluidic cell culture in controlled microenvironments. Chemical Society Reviews. 39 (3), 1036-1048 (2010).
- Duffy, D. C., McDonald, J. C., Schueller, O. J. A., Whitesides, G. M. Rapid Prototyping of Microfluidic Systems in Poly(dimethylsiloxane). Analytical Chemistry. 70 (23), 4974-4984 (1998).
- Berthier, E., Young, E. W. K., Beebe, D. Engineers are from PDMS-land, Biologists are from Polystyrenia. Lab on a Chip. 12 (7), 1224-1237 (2012).
- Sackmann, E. K., Fulton, A. L., Beebe, D. J. The present and future role of microfluidics in biomedical research. Nature. 507 (7491), 181-189 (2014).
- Young, E. W. K., Berthier, E., Beebe, D. J. Assessment of enhanced autofluorescence and impact on cell microscopy for microfabricated thermoplastic devices. Analytical Chemistry. 85 (1), 44-49 (2013).
- Wallow, T. I., Morales, A. M., et al. Low-distortion, high-strength bonding of thermoplastic microfluidic devices employing case-II diffusion-mediated permeant activation. Lab on a Chip. 7 (12), 1825-1831 (2007).
- Tsao, C. W., DeVoe, D. L. Bonding of thermoplastic polymer microfluidics. Microfluidics and Nanofluidics. 6 (1), 1-16 (2009).
- Young, E. W. K., Berthier, E., et al. Rapid prototyping of arrayed microfluidic systems in polystyrene for cell-based assays. Analytical Chemistry. 83 (4), 1408-1417 (2011).
- Truckenmüller, R., Henzi, P., Herrmann, D., Saile, V., Schomburg, W. K. Bonding of polymer microstructures by UV irradiation and subsequent welding at low temperatures. Microsystem Technologies. 10 (5), 372-374 (2004).
- Tsao, C. W., Hromada, L., Liu, J., Kumar, P., DeVoe, D. L. Low temperature bonding of PMMA and COC microfluidic substrates using UV/ozone surface treatment. Lab on a Chip. 7 (4), 499-505 (2007).
- Wan, A. M. D., Sadri, A., Young, E. W. K. Liquid phase solvent bonding of plastic microfluidic devices assisted by retention grooves. Lab on a Chip. 15 (18), 3785-3792 (2015).
- Guckenberger, D. J., de Groot, T. E., Wan, A. M. D., Beebe, D. J., Young, E. W. K. Micromilling: a method for ultra-rapid prototyping of plastic microfluidic devices. Lab on a Chip. 15 (11), 2364-2378 (2015).
- Cameron, N. S., Roberge, H., Veres, T., Jakeway, S. C., John Crabtree, H. High fidelity, high yield production of microfluidic devices by hot embossing lithography: rheology and stiction. Lab on a Chip. 6 (7), 936 (2006).
- Yang, S., Devoe, D. L. Microfluidic device fabrication by thermoplastic hot-embossing. Methods in Molecular Biology. 949, 115-123 (2013).
- Konstantinou, D., Shirazi, A., Sadri, A., Young, E. W. K. Combined hot embossing and milling for medium volume production of thermoplastic microfluidic devices. Sensors and Actuators B: Chemical. 234, 209-221 (2016).
- Maszara, W. P., Goetz, G., Caviglia, A., McKitterick, J. B. Bonding of silicon wafers for silicon-on-insulator. Journal of Applied Physics. 64 (10), 4943 (1988).
- Bhattacharyya, A., Klapperich, C. M. Mechanical and chemical analysis of plasma and ultraviolet-ozone surface treatments for thermal bonding of polymeric microfluidic devices. Lab on a Chip. 7 (7), 876-882 (2007).

