Rapid Quantification of Mitogen-induced Blastogenesis in T Lymphocytes for Identifying Immunomodulatory Drugs
Summary
T-lymphocyte mitogenesis is accompanied by blastogenic transformation, whereupon the cell volume enlarges before cell division. Here, we describe a method to quantify blastogenesis in T lymphocytes using an automated cell counter with the capability of measuring cell diameters.
Abstract
Lymphocyte proliferation in response to antigenic or mitogenic stimulation is a readily quantifiable phenomenon useful for testing immunomodulatory (i.e., immunosuppressive or immunostimulatory) chemical compounds and biologics. One of the earliest steps during mitogenesis is cell enlargement or blastogenic transformation, whereupon the cell volume increases before division. It is usually detectable in the first several hours of T-lymphocyte stimulation. Here, we describe a rapid method to quantify blastogenesis in T lymphocytes isolated from mouse spleens and human peripheral blood mononuclear cells (PBMCs) using an automated cell counter. Various commonly used proliferation assays for the most part are laborious and only reflect the overall population effect rather than individual cellular effects within a population. In contrast, the presented automated cell counter assay provides rapid, direct, and precise measurements of cell diameters that can be used for assessing the effectiveness of various mitogens and immunomodulatory drugs in vitro.
Introduction
T lymphocytes are the primary cells responsible for adaptive immunity in mammals. It is known that they respond to specific antigenic peptides presented by MHC molecules on the surface of antigen-presenting cells. Upon activation of a cognate T-cell receptor (TCR), the cell enlarges in a process termed blastogenic transformation, or blastogenesis. This process is detectable in the first ~6 hr after the stimulus is applied1. During blastogenesis, the volumes of individual T cells increase 2- to 4-fold2-6. Lymphocytes begin to proliferate in a process called clonal expansion, the purpose of which is to generate as many clones of the antigen-specific TCR-bearing cells as possible. The progeny cells then exert their immunologic function by differentiating into cytotoxic (CD8+) or helper (CD4+) effector T lymphocytes. Thus, naïve T lymphocytes in human or mouse blood are in the G0 (resting) phase of the cell cycle and support minimal metabolic activity. Upon exposure to antigens or mitogens, T cells reenter the cell cycle, with a concomitant stimulation of transcription and protein synthesis7-10. Mitogens, such as phorbol 12-myristate 13-acetate (PMA) and ionomycin stimulate the lymphocytes through the activation of protein kinase C (PKC) and Ca2+-dependent signaling pathways1. The activation of T cells with PMA/ionomycin bypasses the TCR signaling steps.
In vitro proliferation assays are widely used for the purpose of evaluating lymphocyte function and response to stimuli. Proliferation readings are typically taken one to three days after the start of T-cell stimulation and reflect the collective state of hundreds or thousands of cells. The potency of various mitogens and immunomodulatory drugs in vitro can be evaluated by simply measuring proliferation rates in the presence of these compounds. Some of these assays and their limitations are discussed below.
For direct cell number counting, the procedure is time consuming, with a high probability of operator errors.
For DNA synthesis, the 3H-thymidine incorporation assay measures DNA synthesis, but its major limitation is its radiotoxicity. A non-radioactive alternative is BrdU, but the range of linear response for the cell growth is limited, and antibody treatment is required, which increases the number of steps in the procedure11,12.
For metabolic activity, tetrazolium salts (MTT, MTS, XTT, and WST-1) and resazurin dye-based colorimetric assays report the general metabolic state of dividing cell populations. However, MTT is not soluble in the culture medium, requiring additional wash steps, thus incorporating errors in the measurement; XTT needs additional components to reduce efficiently; MTS-, WST-1-, and resazurin-based measurements are affected by the culture medium pH and its components serum, albumin or phenol red13-16. These assays do not measure the actual number of viable cells but rather estimate the combined enzyme activities. Therefore, the proliferation rate may not be accurately determined by metabolic assays because of the non-linear correlation between cell number and dye reduction12,17.
For measuring ATP concentration, T-cell activation-induced increases in ATP correlate with proliferation. However, elevation of intracellular ATP is one of the initial steps of T cell activation; many steps behind is the actual proliferation17,18.
For dye dilution assay, CFSE fluorescent dye stains cells by covalently binding to intracellular proteins. The dye shows a proliferation-dependent decrease in fluorescent intensity, which can track the number of cell divisions. However, because of covalent protein labeling, the functions of these proteins can be compromised. The dye is toxic to the cells at higher concentrations. At lower dye concentrations, however, the initial fluorescence intensity is reduced, decreasing the number of cell divisions that can be tracked. Additionally, after labeling with CFSE, there is a proliferation-independent ~50% loss of initial fluorescence during the first 24 to 48 hr period, which limits the dynamic range of this assay19,20.
Most of these assays reflect the collective state of large numbers of cells and require the treatment of the cells with fluorescent dyes. Necrotic and apoptotic cells might also contribute to these measurements, unless they are removed from the analysis by staining with chemicals or antibodies.
Lymphocyte blastogenesis can be evaluated by a variety of methods, such as optical microscopy or flow cytometry4,21,22. Here, we describe a rapid method for the measurement of T-cell sizes using an automated cell counter, which collects real-time cell images that are stored and can be re-analyzed at a later time. In addition to size measurements, this device provides precise cell numbers and the percentage of viable cells, as determined by trypan blue stain exclusion. The device used in this protocol is commercially available, and the manufacturer tested the precision of the instrument using three different instruments and several concentration and viability controls. Results of these studies demonstrated a coefficient of variance that was generally below 6%. As noted in the protocol, the device is calibrated on a regular basis with 6 µm and 8 µm diameter polystyrene beads. The advantages of using a cell counter to differentiate between resting T cells and T lymphoblasts based on cell diameter is the ease of use and the automated nature of the analysis. The software is capable of drawing a circle around each cell and calculating the cell diameter. Additionally, the images are visible to the operator, who can verify the accuracy of the instrument in identifying the cells and correctly drawing a circle around them. In terms of limitations, the instrument cannot per se differentiate between debris and cells; therefore, it is important that the operator views every image as it is being processed. There is a potential for incorporating air bubbles, which will decrease the number of usable fields for analysis; however, this is rare if the regular flushing maintenance is performed.
In this study, groups of splenic T lymphocytes were stimulated with ionomycin and increasing concentrations of PMA for 12-48 hr. PMA concentrations as low as 2 ng/ml induced both a robust blastogenic response and significant proliferation. Measurements of the effects of several drugs, such as the immunosuppressants cyclosporine A (CsA), FK506 (tacrolimus), and rapamycin (sirolimus), as well as ion channel blockers TRAM-34 and FTY720 (fingolimod), on blastogenesis demonstrated good agreement with reported effects on proliferation. The blastogenic response of human PBMCs to PMA/ionomycin and murine T-cell stimulation with anti-CD3 and anti-CD28 antibody-coated magnetic beads were also measured.
The cell counter assay quantifies both blastogenesis and the proliferation rate (cell density) simultaneously but separately, unlike the abovementioned methods, which look at a combination of these effects. The presented protocol provides a rapid and robust technique for evaluating the potency of mitogenic and immunomodulatory agents.
Protocol
All experiments are carried out in accordance with protocols approved by the Wright State University Lab Animal Care and Use Committee and Institutional Review Board.
NOTE: Human PBMCs are isolated by the Ficoll density gradient centrifugation method5.
1. Spleen Harvest
- House adult female ICR mice in standard lab conditions with the food, water, and bedding requirements specific to the species.
NOTE: Animals had a mean age of 2.9 months and a mean weight of 30.4 g at the time of euthanasia. - On the date of isolation (DOI), euthanize the animals individually without other conspecific animals present. Make all efforts to ensure minimal stress to the mouse. Euthanize using CO2 with secondary cervical dislocation to ensure death.
- Place the animal, still inside the transfer cage, into the CO2 chamber and start the gas flow at 5 L/min. This flow rate displaces oxygen at the recommended 10-30% per min.
- After the animal is unconscious, increase the CO2 flow rate to 15 L/min. Check the animal for cessation of breathing and leave in the chamber for an additional 2 min.
- Apply cervical dislocation with distraction force to ensure death.
- Harvest the spleen from the animal using aseptic technique within 10 min of death.
- Sterilize two sets of scissors and forceps for the removal of the spleen in an autoclave oven prior to use. Sterilize the dissection surface by applying 70% ethanol.
- Apply 70% ethanol to the mouse abdomen. Using one set of scissors and forceps, make a cut in the fur and skin from the left side of the abdomen, then pull apart and peel back the skin to reveal the peritoneum. Once exposed, apply 4% chlorhexidine solution before opening the peritoneum.
- Using the second set of scissors and forceps, make a 2- to 3-cm cut along the central abdomen. Open the peritoneum and remove the spleen by cutting away visceral attachments and excess fat. Place the spleen in Dulbecco's phosphate-buffered saline (DPBS).
2. Preparation of Raw Splenocytes
NOTE: Perform all steps under a laminar flow biosafety cabinet using aseptic technique.
- Soak the harvested spleens in 10 ml of deionized sterile H2O for 5 min in a 10 cm culture dish to lyse the surface erythrocytes.
NOTE: This step can be omitted if the spleen was damaged during isolation. - To release the splenocytes, crush the spleens between two sterilized glass slides (frosted side facing the spleen) over a fresh 10 cm culture dish. Mix with 10 ml of RPMI-1640 supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 50 IU/ml penicillin, and 50 µg/ml streptomycin (below referred to as complete RPMI).
- Filter the cells through a sterile 40 µm nylon cell strainer into a new 10 cm culture dish to remove the connective tissue and debris.
- Lyse the remaining erythrocytes by adding 20 ml of RBC lysis buffer composed of 155 mM NH4Cl, 10 mM NaHCO3, and 0.1 mM EDTA at pH 7.4 and ~300 mOsm/L.
NOTE: Osmolality is measured by a freezing point or a vapor pressure osmometer.- Transfer the cells from the 10-cm plate to a 50 ml conical tube and centrifuge at 250 x g for 10 min (4 °C).
- Remove the supernatant, add 20 ml of RBC lysis buffer, and re-suspend the pelleted cells by pipetting.
- Incubate at room temperature in the lysis buffer for 10 min with gentle rocking.
- Centrifuge for 10 min at 250 x g (4 °C) to pellet the cells. Pour off the lysis buffer.
- Re-suspend cells in 10 ml of complete RPMI and centrifuge again (250 x g, 10 min, 4 °C). Discard the supernatant.
- Re-suspend the splenocytes in 2 ml of complete RPMI warmed to 37 °C for transfer to the nylon wool fiber column.
3. Purification of T Lymphocytes
- Wash the nylon wool columns twice with 5 ml of complete RPMI. Incubate at 37 °C in a 5% CO2 cell culture incubator for 1 hr.
NOTE: It is imperative to keep the nylon wool wet for the duration of the experiment and to use complete RPMI warmed to 37 °C for all nylon wool isolation steps. - Add isolated splenocytes to the column and incubate for 1 hr to allow the B cells, fibroblasts, and accessory cells to adhere to the nylon wool.
- Add 2 ml of RPMI containing the splenocytes into the column and pass through until the top of the nylon wool is reached.
- Add 2 ml of complete RPMI warmed to 37 °C on top of the nylon wool and pass the medium through the wool until the liquid level reaches the top surface.
- Add 3 ml of warm complete RPMI to the column to completely cover the nylon wool.
- Incubate the loaded column undisturbed in a 37 °C, 5% CO2 cell culture incubator for 1 hr.
- Elute the cells by washing the column twice with 5 ml of complete RPMI. Perform an additional wash of the eluted cells by centrifugation.
- Fill the column to the top 2 times with complete RPMI and allow the solution in the column to flow through into a 50 ml sterile conical tube.
NOTE: Avoid tapping or bumping the column, which can dislodge the B cells, accessory cells, and fibroblasts adhering to the nylon wool. - Collect the flow-through fraction and spin at 250 x g for 10 min (4 °C) to pellet the cells. Wash once with 10 ml of complete RPMI. Pellet the cells again (250 x g, 10 min, 4 °C) and re-suspend in 2 ml of complete RPMI.
- Measure the cell density using a hemocytometer and dilute in complete RPMI to 0.5 x 106 cells/ml.
- Seed 1 or 2 ml aliquots of cells into each well of a 24- or 6-well cell culture plate, respectively, and culture the cells at 37 °C with 5% CO2.
NOTE: 1 mM 1,4-dithiothreitol (DTT) can be added to each well at this stage to improve T-cell survival. - Optional: Confirm the efficiency of the isolation protocol by flow cytometry. This protocol normally yields ~80% T lymphocytes23.
NOTE: Nylon wool-purified cells contain approximately 50% CD4+ and 23% CD8+ cells. To purify the CD4+ and CD8+ subpopulations further, a single immunomagnetic depletion step can be performed using eight antibodies and magnetic beads24. Alternatively, purer CD4+ and CD8+ T-cell populations can be isolated by positive or negative selection with specific antibodies.
- Fill the column to the top 2 times with complete RPMI and allow the solution in the column to flow through into a 50 ml sterile conical tube.
4. Activation of T Lymphocytes and Experimental Drug Exposure
- Activate T lymphocytes within 24 hr of isolation through the addition of PMA and ionomycin calcium salt or magnetic beads coated with anti-CD3 and anti-CD28 antibodies23,25,26. Add experimental drugs (e.g., CsA, FK506, rapamycin, TRAM-34, and FTY720) at the time of activation.
NOTE: Here, PMA concentrations varied from 2 to 250 ng/ml, and the ionomycin concentration was kept at 250 nM. If possible, dilutions should be prepared from stock aliquots of each drug so that the volume added to each well of cells is ≥1 µl to ensure reproducibility. The anti-CD3/anti-CD28-coated magnetic beads are added at a 1:1 bead-to-cell ratio. Increasing the number of beads per cell (i.e., the bead-to-cell ratio) will increase the intensity of stimulation25,26. The beads are washed and re-suspended in complete RPMI before their addition to the cells. Note that trypan blue stains the beads. - Culture the cells for 12-72 hr at 37 °C with 5% CO2 before analyzing with the automated cell counter.
5. Automated Cell Counter Data Collection
- Before running the sample, make sure that the cells are gently mixed with a serological pipette to avoid clumps. This is particularly important for activated lymphocytes because of their increased adhesiveness.
- For cultures that are activated with anti-CD3/CD28 beads, after pipetting, transfer the sample to a 1.5 ml or 2 ml centrifuge tube. Separate the beads by keeping the tube on a magnet for 1-2 min. Use the supernatant containing the cells for analysis.
NOTE: Analyze both resting and activated cells within 1 hr to account for any pre-activation of the resting cells by serum present in the culture medium. After 12 hr of PMA/ionomycin stimulation, a small increase in cell size was detectable (Figure 2). At 72 hr, no significant cell size increase compared to 48 hr was observed (data not shown).
- For cultures that are activated with anti-CD3/CD28 beads, after pipetting, transfer the sample to a 1.5 ml or 2 ml centrifuge tube. Separate the beads by keeping the tube on a magnet for 1-2 min. Use the supernatant containing the cells for analysis.
- Transfer 1 ml of the cell suspension into a sample cup and run it through the automated cell counter according to the instructions in the manual.
NOTE: For each of the trials, a minimum of 1 ml (maximum of 2 ml) of cell culture should be used. The automated cell counter uses a syringe to mix trypan blue with the cell suspension and passes the cell-trypan blue mixture over a field that is imaged and analyzed by the software. The software detects trypan blue-stained cells, draws a circle around each cell, and determines the diameter. 100 images are collected from each sample, and cell viability and sizes are determined. The detection threshold of the cell counter used here has a lower limit of 5 µm. - Transfer data from each run to a spreadsheet for further analysis.
- Calibrate the device on a regular basis using a 1 ml sample of 6 µm and 8 µm diameter polystyrene beads.
NOTE: The actual bead sizes measured by the manufacturer for each batch should be used, not the nominal size. 6 and 8 µm beads are convenient because these diameters are close to the expected diameters of resting and activated T cells (see Figure 1).
6. Data and Statistical Analysis
- Analyze the data using appropriate statistical and graphing software.
NOTE: The spreadsheet for each run contains the number of cells with each diameter (range: 5 µm to 70 µm). The cells above a 17 µm diameter are removed from the analysis to exclude dust particles and small bubbles, which can be read as viable cells by the instrument. - Perform hypothesis testing using Welch's t-test for data sets with unequal variance; results are considered statistically significant if p < 0.001. The formula used was,
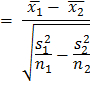
where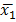 and
and 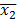 are sample means,
are sample means, 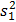 and
and 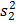 are sample variances, and
are sample variances, and 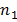 and
and 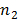 are sample sizes for each data set. The number of degrees of freedom is conservatively estimated using the smaller of the two sample sizes for each comparison. Bar graphs are presented as the mean ± SEM.
are sample sizes for each data set. The number of degrees of freedom is conservatively estimated using the smaller of the two sample sizes for each comparison. Bar graphs are presented as the mean ± SEM.
7. Splenic T-lymphocyte Proliferation Assay
- Measure T-cell proliferation using an MTS- or MTT-based colorimetric plate reader proliferation assay.
NOTE: The assay is based on MTS tetrazolium compound (Owen's reagent) reduction to soluble formazan product, likely by NADPH or NADH produced in metabolically active cells by dehydrogenase enzymes. - Count purified T cells with a hemocytometer and re-suspend them in complete RPMI-1640 at a final density of 1 x 106 cell/ml.
NOTE: 1 mM DTT can be added to the cells at this stage. - Activate the cells with PMA and ionomycin along with test drugs, if required, and mix gently (similar to step 4).
- Plate 100 µl of the cells in each well of a 96-well cell culture treated plate and incubate at 37 °C with 5% CO2 for 48 hr. Add 100 µl of RPMI-1640 media without cells into one well to obtain a reading of the background absorbance.
- Add 20 µl of MTS-based reagent to each well and incubate at 37 °C with 5% CO2 for 4 hr.
- Take absorbance measurements at 490 nm using a plate reader. The absorbance measurements correspond to the number of metabolically active cells in each well.
NOTE: Subtract background levels if needed. The background absorbance values depend upon the type of culture medium, serum, external pH, and duration of MTS reagent exposure to light. MTS-based reagents are light-sensitive, and exposure to light for several hours may result in higher background absorbance values.
Representative Results
We used this assay to compare lymphocyte blast formation during stimulation with PMA, a phorbol ester, and ionomycin, a calcium ionophore. Figure 1A shows the frequency distribution of diameters of resting and pharmacologically activated splenic T cells. Treatment of cells with PMA/ionomycin for ~2 days resulted in a significant shift in the median of the distribution towards bigger diameters (see, for example reference4). The number of cells with smaller diameters was accordingly reduced. In order to ascertain that our device reported the correct diameters, we performed two calibrations with polystyrene beads of 6 µm and 8 µm diameters (see Materials Table). Figures 1B and 1C show readings from activated T cells superimposed with an 8 µm standard and resting T cells superimposed with a 6 µm standard. Figure 1D shows the control bead sizes reported by the manufacturer plotted against the median diameters measured with our automated cell counter. It appeared that the 6 µm size was slightly overestimated in our measurements, probably due to the measurement threshold of the instrument having a lower limit of 5 µm. However, the standard deviation of the bead diameters calculated from the cell counter measurements was in agreement with the standard deviation reported by the manufacturer. We conclude from these experiments that this device can be used reliably to compare resting and mitogen-stimulated mouse T-cell sizes and that the device can accurately measure the actual cell diameters.
We then proceeded to test the dependence of the mean diameter increase on PMA concentration and found that, at a fixed ionomycin concentration of 250 nM, the PMA effect was not significantly changed by elevating its concentration from 2 to 250 ng/ml (Figure 2A). Using the MTS-based assay, we measured T-cell proliferation at 50 and 250 ng/ml PMA and found no appreciable difference (Figure 2C), in agreement with our cell diameter data. The calcineurin inhibitor drugs cyclosporine A (300 nM) and FK506 (1 nM) suppressed both blastogenesis (Figure 2B) and proliferation (Figure 2C). Inhibition was not complete in both cases, however. Measurements performed 12 hr after PMA/ionomycin addition showed a 7.2% and 6.8% increase in diameter for 50 ng/ml and 250 ng/ml PMA, respectively (Figure 2D). Size increases at these two concentrations were not significantly different, as was the case for 48 hr stimulation (compare to Figure 2A).
The cell diameter data collected from resting and activated T cells after 48 hr of PMA/ionomycin stimulation are summarized in Table 1. The mean surface area and volume of resting ICR mouse splenic T cells were 151.4 µm2 and 1.9 x 10-4 nl, respectively. The mean surface area and volume of activated T cells were 300.6 µm2 and 5.9 x 10-4 nl, respectively. The overall average diameter increase for all activated cells was 40.92% (Table 1).
We also tested other chemical compounds reported to be immunosuppressive: CsA, FK506, rapamycin, FTY720, and TRAM-34. In Figure 3, cell size measurements obtained in resting and activated T cells in the absence and presence of these drugs are shown. CsA and FK506, which target the calcineurin-dependent nuclear factor of activated T cells (NFAT)27,28, were the most potent, inhibiting the blastogenic response by nearly 72% (Figure 3A and 3B). Interestingly, with increased PMA concentrations, the potency of CsA and FK506 dropped slightly, even though there was no additional cell diameter increase in the absence of these drugs (Figure 2A and 2B). Rapamycin, an immunosuppressant that inhibits mammalian target of rapamycin (mTOR)29,30, had a moderate but statistically significant effect (Figure 3A, 3B and 3C). FTY720 is a sphingosine 1-phosphate receptor agonist that inhibits lymphocyte egress into circulation31 and was recently found to inhibit TRPM7, a cation channel highly expressed in T lymphocytes32. Both FTY720 and rapamycin had a comparable effect on blastogenesis, reducing it from the average of a 52% increase in diameter to approximately 34% (Figure 3A and 3B). TRAM-34 is a blocker of the calcium-activated potassium channel KCa3.133. Tested at 700 nM, TRAM-34 was ineffective in murine T cells, in accordance with a recent human T-cell proliferation study; its potency may depend on the nature and strength of mitogenic stimulation34 (Figure 3A and 3B). 700 nM TRAM-34 was effective in blocking KCa3.1 channels in our patch clamp electrophysiology experiments (data not shown). This comparison was made between resting and drug-exposed cells in multiple trials within the same experiment at 48 hr. When rapamycin and CsA were used together, blastogenesis was completely inhibited (Figure 3C).
Activation of murine T cells with anti-CD3/anti-CD28-coated magnetic beads for 72 hr produced a 27% increase in the average diameter, which was reduced to 5% in the presence of CsA (Figure 3D). Human mononuclear cells upon PMA/ionomycin activation for 48 hr increased in diameter by 33%, and activation in the presence of CsA decreased the response to 23% (Figure 4).
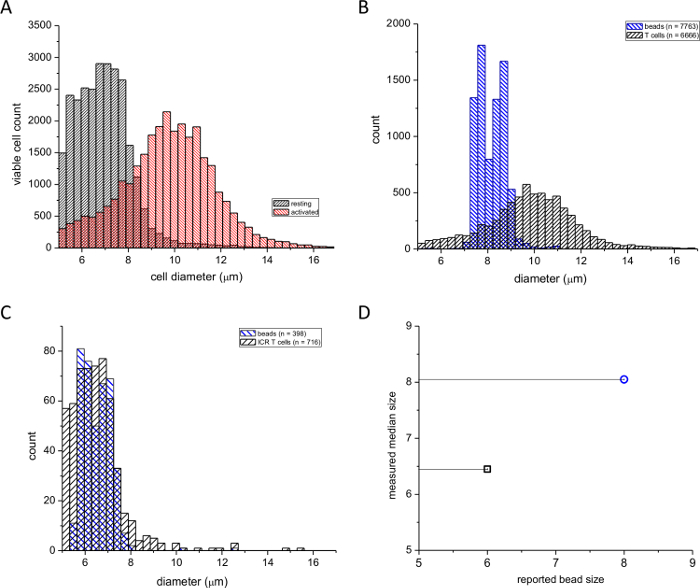
Figure 1: Frequency distributions of murine splenic T-cell diameters. (A) Black columns indicate resting and red columns indicate PMA/ionomycin-activated (48 hr) cells. (B) Distributions of activated splenic T cells superimposed on an 8 µm particle size calibration standard. (C) Distributions of resting T cells superimposed on a 6 µm calibration standard. The numbers of cells and beads used are indicated in boxes. (D) Median diameter measured by the cell counter plotted against the bead diameter reported by the manufacturer. Please click here to view a larger version of this figure.
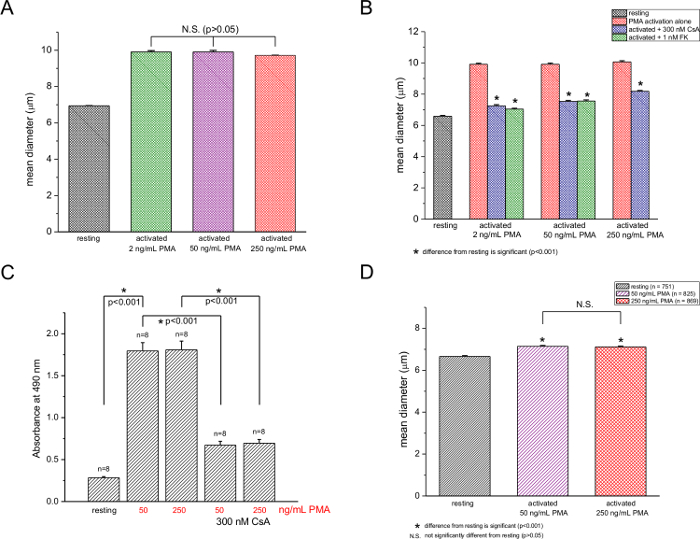
Figure 2: Dependence of murine T-cell activation on PMA concentration. (A) Mean diameters of T cells either untreated or treated with 2, 50, and 250 ng/ml PMA at a fixed ionomycin concentration (250 nM). (B) Cell counter measurements of resting and activated (2, 50, 250 ng/ml PMA) T cells in the absence and presence of 300 nM CsA or 1 nM FK506. (C) MTS proliferation assay at 50 and 250 ng/ml PMA with 250 nM ionomycin in the absence and presence of 300 nM CsA. Significant differences (p < 0.001) are indicated with asterisks. n is the total number of trials. Data are from cells isolated from 3 mice. (D) Cell diameters of resting and activated T cells (250 ng/ml PMA and 250 nM ionomycin) 12 hr after activation in the absence and presence of 300 nM CsA. Please click here to view a larger version of this figure.
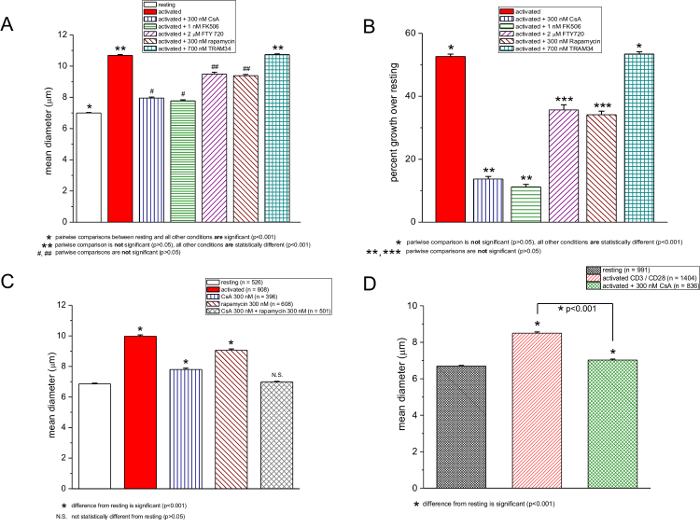
Figure 3: Effects of CsA, FK506, FTY720, rapamycin, and TRAM-34 on cell size. (A and C) Average diameters of resting and PMA/ionomycin-activated murine T cells in the absence and presence of drugs. Concentrations are shown in the box. (B) Data from A expressed as the percent increase over the resting T-cell diameter. Lymphocyte activation was performed with 250 ng/ml PMA and 250 nM ionomycin, and the measurements were taken after 48 hr. (D) Cell counter measurements of resting and anti-CD3/CD28-activated T cells, 72 hr after activation in the absence and presence of 300 nM CsA. Please click here to view a larger version of this figure.
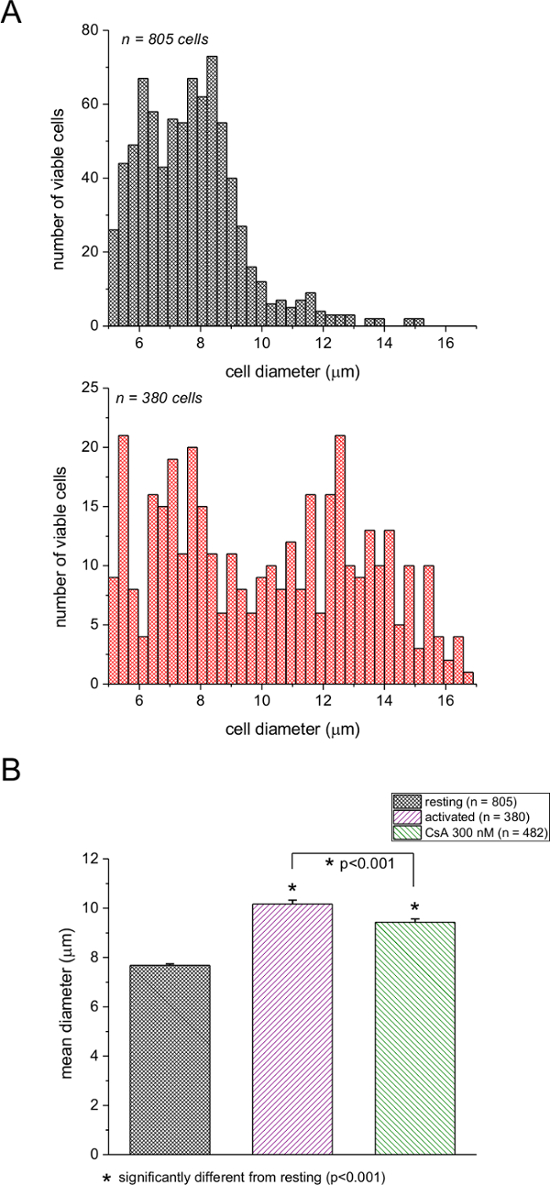
Figure 4: Blastogenesis in human PBMCs upon mitogen stimulation. (A) Black columns indicate resting and red columns indicate activated PBMCs. (B) Mean diameters of resting and activated PBMCs in the absence and presence of 300 nM CsA. Cells were activated with 250 ng/ml PMA and 250 nM ionomycin, and the measurements were taken after 48 hr of activation. Please click here to view a larger version of this figure.
| Column1 | Resting | Activated | CsA 100 nM | CsA 200 nM |
| Average Diameter | 6.94 µm | 9.78 µm | 8.19 µm | 7.92 µm |
| Average Increase in Diameter | 40.92% | 17.88% | 14.09% | |
| Average Surface Area | 151.37 µm² | 300.61 µm² | 210.56 µm² | 197.22 µm² |
| Average Increase in Surface Area | 98.59% | 39.00% | 30.16% |
Table 1: Summary of the average cell diameter and relative increases in surface area. Measurements were performed 48 hr after activation with 250 ng/ml PMA and 250 nM ionomycin.
Discussion
Here, we describe a technique for the rapid detection and quantification of T-cell blastogenic transformation using an automated cell counter. Under our conditions (250 ng/ml PMA and 250 nM ionomycin stimulation), the cell surface area was increased two-fold and the volume three-fold after 48 hr of activation. The assay is sufficiently sensitive to detect blasting during the first 12 hr of activation, where the cell volume increased only 1.25-fold compared to resting (Figure 2D). A more physiologically relevant mechanism of T-cell activation using anti-CD3 and anti-CD28 antibody-coated magnetic beads produced a significant blastogenic response, with an average 2.3-fold increase in volume (72 hr after activation, Figure 3D). Human mononuclear cells showed a 2.6-fold change in volume upon PMA/ionomycin stimulation for 48 hr (Figure 4). The ICR mouse splenic T cell average diameter and volume were determined to be 6.9 µm and 1.9 x 10-4 nl, respectively. Human PBMCs (from one healthy donor) had an average diameter of 7.7 µm and an average volume of 2.7 x 10-4 nl. Using this protocol, we also tested increasing concentrations of PMA for their ability to activate T cells. These single-cell results were consistent with proliferation assays performed at similar PMA concentrations.
Several compounds reported to affect cell proliferation were tested: FTY72031,32; the immunosuppressants cyclosporine A, FK506, and rapamycin27,28; and TRAM-34, a blocker of calcium-activated KCa3.1 channels33,35. We found that, at the tested concentrations, the most potent inhibitors of blastogenesis were CsA and FK506. Rapamycin had a smaller but statistically significant suppressive effect on blastogenesis. TRAM-34, on the other hand, did not affect blastogenesis.
CsA suppressed both blastogenesis and proliferation, but not completely (Figure 2B and 2C), demonstrating that automated cell counter measurement is a good correlate of drug efficiency in T-cell proliferation. In the presence of rapamycin together with CsA, blastogenesis was completely inhibited, suggesting that NFAT- and mTOR-mediated pathways in combination can fully account for T-cell enlargement upon mitogenic stimulation with PMA/ionomycin (Figure 3C).
The dynamic range of some proliferation assays is limited (see Figure 2). Proliferation assays, such as the one we have used (Figure 2C), report a signal that includes contributions from apoptotic and necrotic cells. Automated diameter change measurements do not have that limitation, as the measurements pertain to viable cells only. Cell viability is assessed by the exclusion of trypan blue stain from healthy, viable cells. In size measurements, using forward light scatter in flow cytometry doublet cell discrimination can be problematic22. In the automated cell counter measurements, no doublet population was found (Figure 1). Also, the measurement was sufficiently precise to distinguish between the effects of 100 and 200 nM cyclosporine (Table 1) and to detect blastogenesis within 12 hr of mitogenic stimulation (Figure 2D).
This new assay is particularly useful for small numbers of samples. Up to 15 samples can be measured in 1 hr. However, the assay has its limitations, most of which can be mitigated. Even though it can effectively resolve small (<1 µm) difference in cell diameter, the machine model we used has a lower detection threshold of 5 µm. This limit can cause the overestimation of very small cell sizes, since it effectively excludes all cells with smaller diameters. However, newer models of the cell counter have detection thresholds of ~2 µm, which should alleviate this problem. If there is too much debris in the sample, the instrument treats them as viable cells. Additionally, air bubbles can occasionally get into the flow cell and cause distortion of the cell images captured by the machine. The distortion does not appear to affect the viability determination, but it can affect the measured diameters. Therefore, it is recommended that all images be checked by the experimenter for air bubbles before the data from each trial are accepted for analysis. Since the software only draws circles around the cells, diameters of non-spherical cells may be skewed towards the long axis, making the assay less suitable for non-spherical cells.
This assay is quick, taking approximately 4 min per sample, compared to proliferation assays, which require a few hours. Data collection is also rather simple using the software included with the device. The software can export measurement data to a spreadsheet for analysis. Finally, it is a single-cell, as opposed to a population, measurement; both blastogenesis and proliferation are measured; and it distinguishes between viable and dead cells simultaneously. It can be used to evaluate various mouse strains for their ability to mount an immune response. The assay can also be used successfully for the measurement of blastogenesis in T cells from human donors (Figure 4), and it can also be used in other cell types.
Cell volume increases are known to contribute to the regulation of metabolism: cell swelling stimulates glutamine-induced glycogen synthesis and lipogenesis in hepatocytes36,37. Neutrophils display migration-associated volume increases of 35-60%, while chemotactic agents induce 10-15% swelling38-40. The current assay can potentially be used to study these processes.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We thank Lucile Wrenshall for the use of the plate reader, Nancy Bigley for useful discussion, and Tom Brown for the gift of ICR mice. This work was funded by grant 1R01AI114804 from the National Institute of Allergy and Infectious Diseases and by WSU Faculty Development funds (to J.A.K.). J.N.G. was supported in part by the National Institute of General Medical Sciences grant R25GM090122.
Materials
| RPMI-1640 | Lonza | BW12-702F | |
| 40 μm nylon cell strainers | Thermo Fisher Scientific | 22363547 | |
| nylon wool fiber columns | Polysciences, Inc. | 21759-1 | |
| 50 ml conical tubes | The Lab Depot | TLD431697 | |
| 6-well cell culture treated plates | USA Scientific | CC7682-7506 | |
| 96-well cell culture treated plates | Thermo Fisher Scientific | 130188 | |
| Dimethyl sulfoxide (DMSO) | Fisher BioReagents | BP231-100 | |
| CellTiter 96 AQueous One Solution Cell Proliferation Assay | Promega | G3582 | MTS based assay |
| Cyclosporine A | Sigma-Aldrich | 30024 | |
| FK506 | Cayman Chemical Company | 104987-11-3 | |
| Rapamycin | Santa Cruz Biotechnology | sc-3504 | |
| TRAM-34 | Sigma-Aldrich | T6700 | |
| FTY720 | Sigma-Aldrich | SML0700 | |
| Dynabeads Mouse T-Activator CD3/CD28 | Gibco | 11456D | |
| Phorbol 12-myristate 13-acetate (PMA) | Acros Organics | 356150010 | |
| Ionomycin calcium salt | Sigma-Aldrich | I0634 | |
| Penicillin-Streptomycin | MP Biomedicals | ICN1670049 | 100 x stock |
| Dulbecco’s phosphate buffered saline (DPBS) | HyClone | SH30378.02 | 10 x stock |
| 1,4-dithiothreitol (DTT) | Research Products International | 12/3/3483 | reducing agent |
| 8 µm micro particle size standard | Sigma-Aldrich | 84192-5ML-F | Actual 8.02 µm |
| 6 µm micro particle size standard | Sigma-Aldrich | 89756-5ML-F | Actual 6.084 µm |
| Single magnetic separation stand for 1.5 – 2 mL tube | V&P Scientific, Inc. | VP772F5 | |
| Cell culture incubator | Forma Scientific | 3110 | |
| Synergy H1 hybrid reader | Bio Tek | BTH1M | |
| Vi-CELL cell viability analyzer | Beckman Coulter | 731050 |
References
- Weiss, A., Samelson, L. E., Paul, W. E. T-lymphocyte activation. Fundamental Immunology. , 321-364 (2003).
- Gergely, P., Ernberg, I., Klein, G., Steinitz, M. Blastogenic response of purified human T-lymphocyte populations to Epstein-Barr virus (EBV). Clin Exp Immunol. 30 (3), 347-353 (1977).
- Sanderson, R. J., Rulon, K., Groeneboer, E. G., Talmage, D. W. The response of murine splenic lymphocytes to concanavalin A and to co-stimulator. J Immunol. 124 (1), 207-214 (1980).
- Decoursey, T. E., Chandy, K. G., Gupta, S., Cahalan, M. D. Mitogen induction of ion channels in murine T lymphocytes. J Gen Physiol. 89 (3), 405-420 (1987).
- Nibbering, P. H., Zomerdijk, T. P., Tilburg, A. J., Furth, R. V. Mean cell volume of human blood leucocytes and resident and activated murine macrophages. J Immunol Methods. 129 (1), 143-145 (1990).
- Segel, G. B., Cokelet, G. R., Lichtman, M. A. The measurement of lymphocyte volume: importance of reference particle deformability and counting solution tonicity. Blood. 57 (5), 894-899 (1981).
- Cooper, H. L., Braverman, R. Protein synthesis in resting and growth-stimulated human peripheral lymphocytes. Evidence for regulation by a non-messenger RNA. Exp Cell Res. 127 (2), 351-359 (1980).
- Cooper, H. L., Braverman, R. Close correlation between initiator methionyl-tRNA level and rate of protein synthesis during human lymphocyte growth cycle. J Biol Chem. 256 (14), 7461-7467 (1981).
- Teague, T. K., et al. Activation changes the spectrum but not the diversity of genes expressed by T cells. Proc Natl Acad Sci U S A. 96 (22), 12691-12696 (1999).
- Tzur, A., Kafri, R., Lebleu, V. S., Lahav, G., Kirschner, M. W. Cell growth and size homeostasis in proliferating animal cells. Science. 325 (5937), 167-171 (2009).
- Messele, T., et al. Nonradioactive techniques for measurement of in vitro T-cell proliferation: alternatives to the [3H]thymidine incorporation assay. Clin Diagn Lab Immunol. 7 (4), 687-692 (2000).
- Maghni, K., Nicolescu, O. M., Martin, J. G. Suitability of cell metabolic colorimetric assays for assessment of CD4+ T cell proliferation: comparison to 5-bromo-2-deoxyuridine (BrdU) ELISA. J Immunol Methods. 223 (2), 185-194 (1999).
- Weichert, H., Blechschmidt, I., Schröder, S., Ambrosius, H. The MTT-assay as a rapid test for cell proliferation and cell killing: application to human peripheral blood lymphocytes (PBL). Allerg Immunol (Leipz). 37 (3-4), 139-144 (1991).
- Huang, K. T., Chen, Y. H., Walker, A. M. Inaccuracies in MTS assays: major distorting effects of medium, serum albumin, and fatty acids. Biotechniques. 37 (3), 410-412 (2004).
- Rampersad, S. N. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors (Basel). 12 (9), 12347-12360 (2012).
- Quent, V. M., Loessner, D., Friis, T., Reichert, J. C., Hutmacher, D. W. Discrepancies between metabolic activity and DNA content as tool to assess cell proliferation in cancer research. J Cell Mol Med. 14 (4), 1003-1013 (2010).
- Augustine, N. H., Pasi, B. M., Hill, H. R. Comparison of ATP production in whole blood and lymphocyte proliferation in response to phytohemagglutinin. J Clin Lab Anal. 21 (5), 265-270 (2007).
- Sottong, P. R., Rosebrock, J. A., Britz, J. A., Kramer, T. R. Measurement of T-lymphocyte responses in whole-blood cultures using newly synthesized DNA and ATP. Clin Diagn Lab Immunol. 7 (2), 307-311 (2000).
- Wallace, P. K., Muirhead, K. A. Cell tracking 2007: a proliferation of probes and applications. Immunol Invest. 36 (5-6), 527-561 (2007).
- Quah, B. J., Warren, H. S., Parish, C. R. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat Protoc. 2 (9), 2049-2056 (2007).
- Teague, T. K., Munn, L., Zygourakis, K., Mcintyre, B. W. Analysis of lymphocyte activation and proliferation by video microscopy and digital imaging. Cytometry. 14 (7), 772-782 (1993).
- Böhmer, R. M., Bandala-Sanchez, E., Harrison, L. C. Forward light scatter is a simple measure of T-cell activation and proliferation but is not universally suited for doublet discrimination. Cytometry A. 79 (8), 646-652 (2011).
- Lee, J., Sadelain, M., Brentjens, R. Retroviral transduction of murine primary T lymphocytes. Methods Mol Biol. 506, 83-96 (2009).
- Gunzer, M., Weishaupt, C., Planelles, L., Grabbe, S. Two-step negative enrichment of CD4+ and CD8+ T cells from murine spleen via nylon wool adherence and an optimized antibody cocktail. J Immunol Methods. 258 (1-2), 55-63 (2001).
- Trickett, A., Kwan, Y. L. T cell stimulation and expansion using anti-CD3/CD28 beads. J Immunol Methods. 275 (1-2), 251-255 (2003).
- Pène, J., Rahmoun, M., Temmerman, S., Yssel, H. Use of anti-CD3/CD28 mAb coupled magnetic beads permitting subsequent phenotypic analysis of activated human T cells by indirect immunofluorescence. J Immunol Methods. 283 (1-2), 59-66 (2003).
- Sigal, N. H., Dumont, F. J. Cyclosporin A, FK-506, and rapamycin: pharmacologic probes of lymphocyte signal transduction. Annu Rev Immunol. 10 (1), 519-560 (1992).
- Fruman, D. A., Klee, C. B., Bierer, B. E., Burakoff, S. J. Calcineurin phosphatase activity in T lymphocytes is inhibited by FK 506 and cyclosporin A. Proc Natl Acad Sci U S A. 89 (9), 3686-3690 (1992).
- Pollizzi, K. N., Waickman, A. T., Patel, C. H., Sun, I. H., Powell, J. D. Cellular size as a means of tracking mTOR activity and cell fate of CD4+ T Cells upon antigen recognition. PLoS One. 10 (4), e0121710 (2015).
- Pollizzi, K. N., Powell, J. D. Regulation of T cells by mTOR: the known knowns and the known unknowns. Trends Immunol. 36 (1), 13-20 (2015).
- Mandala, S., et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 296 (5566), 346-349 (2002).
- Qin, X., et al. Sphingosine and FTY720 are potent inhibitors of the transient receptor potential melastatin 7 (TRPM7) channels. Br J Pharmacol. 168 (6), 1294-1312 (2013).
- Wulff, H., Kolski-Andreaco, A., Sankaranarayanan, A., Sabatier, J. M., Shakkottai, V. Modulators of small- and intermediate-conductance calcium-activated potassium channels and their therapeutic indications. Curr Med Chem. 14 (13), 1437-1457 (2007).
- Petho, Z., et al. The anti-proliferative effect of cation channel blockers in T lymphocytes depends on the strength of mitogenic stimulation. Immunol Lett. 171, 60-69 (2016).
- Wulff, H., et al. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc Natl Acad Sci USA. 97 (14), 8151-8156 (2000).
- Lang, F., et al. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 78 (1), 247-306 (1998).
- Hue, L. Control of liver carbohydrate and fatty acid metabolism by cell volume. Biochem Soc Trans. 22 (2), 505-508 (1994).
- O’Flaherty, J. T., Kreutzer, D. L., Ward, P. A. Neutrophil aggregation and swelling induced by chemotactic agents. J Immunol. 119 (1), 232-239 (1977).
- Hsu, L. S., Becker, E. L. Volume changes induced in rabbit polymorphonuclear leukocytes by chemotactic factor and cytochalasin B. Am J Pathol. 81 (1), 1-14 (1975).
- Rosengren, S., Henson, P. M., Worthen, G. S. Migration-associated volume changes in neutrophils facilitate the migratory process in vitro. Am J Physiol. 267, C1623-C1632 (1994).

