Operation of a 25 KWth Calcium Looping Pilot-plant with High Oxygen Concentrations in the Calciner
Summary
This manuscript describes a procedure for operating a calcium looping pilot-plant for post-combustion carbon capture with high oxygen concentrations in the calciner in order to reduce or eliminate the flue gas recycle.
Abstract
Calcium looping (CaL) is a post-combustion CO2 capture technology that is suitable for retrofitting existing power plants. The CaL process uses limestone as a cheap and readily available CO2 sorbent. While the technology has been widely studied, there are a few available options that could be applied to make it more economically viable. One of these is to increase the oxygen concentration in the calciner to reduce or eliminate the amount of recycled gas (CO2, H2O and impurities); therefore, decreasing or removing the energy necessary to heat the recycled gas stream. Moreover, there is a resulting increase in the energy input due to the change in the combustion intensity; this energy is used to enable the endothermic calcination reaction to occur in the absence of recycled flue gases. This paper presents the operation and first results of a CaL pilot plant with 100% oxygen combustion of natural gas in the calciner. The gas coming into the carbonator was a simulated flue gas from a coal-fired power plant or cement industry. Several limestone particle size distributions are also tested to further explore the effect of this parameter on the overall performance of this operating mode. The configuration of the reactor system, the operating procedures, and the results are described in detail in this paper. The reactor showed good hydrodynamic stability and stable CO2 capture, with capture efficiencies of up to 70% with a gas mixture simulating the flue gas of a coal-fired power plant.
Introduction
CO2 emissions and the resulting global warming are critical environmental issues that have attracted a large amount of research in the past years. Carbon capture and storage (CCS) has been acknowledged as a potential technology for reducing CO2 emissions to the atmosphere1,2. The most challenging part of the CCS chain is the capture of CO2, which is also the most costly stage3. In consequence, there has been a focus on developing new technologies for CO2 capture from power plants and other industrial facilities.
CaL as a post-combustion CO2 capture technology, was first proposed by Shimizu et al.4 CO2 is captured by a CaO-based sorbent at 600-700 °C in a reactor called a carbonator, and released by subsequent calcination at 850-950 °C (in a calciner) according to Eq. (1), to produce a high-purity CO2 stream suitable for sequestration5,6. The CaL cycle utilises fluidized beds, which represent an optimal configuration for this process, since they allow for large amounts of solids to be circulated easily from one reactor to the other4,5,6,7,8.
CaO (s) + CO2 (g) ⇔ CaCO3 (s) ΔH25 °C = -178.2 kJ/mol (1)
This concept has been demonstrated at pilot scale by various groups and with different configurations and scales, such as the 0.2 MWth pilot in Stuttgart, the 1 MWth pilot in Darmstadt, the 1.7 MWth pilot in La Pereda and the 1.9 MWth unit in Taiwan9,10,11,12,13,14,15,16. Although this process has been proven, there are still possibilities for increasing its thermal efficiency, such as by modifying the standard operating conditions and changes in the design of the reactor configuration.
The use of heat pipes between the combustor and calciner has been studied instead of oxy-combusting fuel in the calciner. The results for the CO2 capture performance are comparable with those of a conventional CaL pilot-plant, however, this process has higher plant efficiencies and lower CO2 avoidance costs17. Martínez et al.18 investigated the heat integration possibilities in order to preheat the solid material entering the calciner and to reduce the heat needed in the calciner. The results showed 9% reduction in the coal consumption when compared with that of the standard case. Other studied possibilities for heat integration have also considered internal and external integration options19.
One of the main problems of the CaL cycle from the economic point of view is to supply the energy needed in the calciner by means of fuel combustion20. Increasing the oxygen concentration in the calciner's inlet is proposed in order to reduce or even avoid the need of CO2 recycle to the calciner. This alternative reduces the capital costs (reduced size of calciner and air separation units (ASU)), which can significantly improve the competitiveness of this process. The drastic change in the combustion conditions can be attained by exploiting the endothermic calcination reaction and the large CaO/CaCO3 flow circulating from the carbonator operating at lower temperatures (neither advantage is available with the oxy-combustion technology).
This work aims to develop a standard operating procedure for running a CaL pilot plant with a Circulating Fluidized Bed (CFB) carbonator and a Bubbling Fluidized Bed (BFB) calciner with 100% O2 concentration in the calciner's inlet. Several experimental campaigns have been run during the commissioning of the pilot plant to ensure proper operation as the oxygen concentration increased. Also, three limestone particle size distributions (100-200 µm; 200-300 µm; 300-400 µm) were studied to investigate how this parameter affects the elutriation of particles and capture efficiency in this operating mode.
Protocol
1. Material Preparation
- Sieve the limestone (~50 kg of raw material) to the desired particle size distribution (300-400 µm or another distribution depending on the experiment) using a mechanical shaker. Put the sieved material in pots next to the calciner for feeding during the test.
- Prepare the material in batches to be introduced into the reactor. The batches are generally 0.5 L or 1 L (1 L of limestone is roughly 1.5 kg), but this can vary depending on the operating parameters.
2. Start-up Procedure
CAUTION: Extremely high temperatures are achieved here. Suitable PPE such as gloves, eye glasses, laboratory coat and safety shoes are required.
- Heat-up of Reactors
- Start the low flow of N2 in the carbonator (60 L/min) and calciner (20 L/min) as well as the loop-seals (10 L/min) in the rotameters.
- Turn on the carbonator transformers manually. Set the temperature of all electrical preheaters of the carbonator at 600 °C.
- Start acquiring data (for gas temperatures and pressures, use the recording button in the software). The data include temperatures, pressures and gas composition of both reactors. In Figure 1 and Figure 2, screenshots of the data acquisition system are shown.
- Turn on the calciner gas preheaters. Turn on the heater around the calciner to 600 °C measured inside the BFB via a thermocouple.
NOTE: Data such as temperature, pressure and gas composition are already being acquired as stated in step 2.1.3. - Put 3 L of the sieved limestone into the BFB in the calciner. First open the top valve, introduce the material in the down-pipe and close the top valve, then open the bottom valve so that the material flows into the reactor.
- Heat the material in the BFB to above 650 °C (by the electrical heater around the calciner).
NOTE: This usually takes ~1 h, during this time check the data acquisition and the pressures in the fluidized beds.
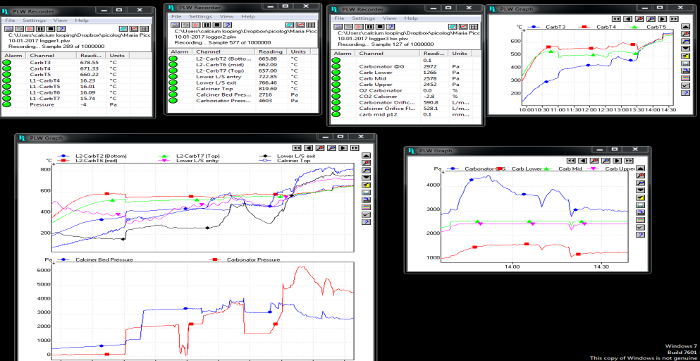
Figure 1: Screenshot of temperature and pressure data acquisition for both reactors. Please click here to view a larger version of this figure.
Figure 2: Screenshot of temperature data acquisition for the preheating system. Please click here to view a larger version of this figure.
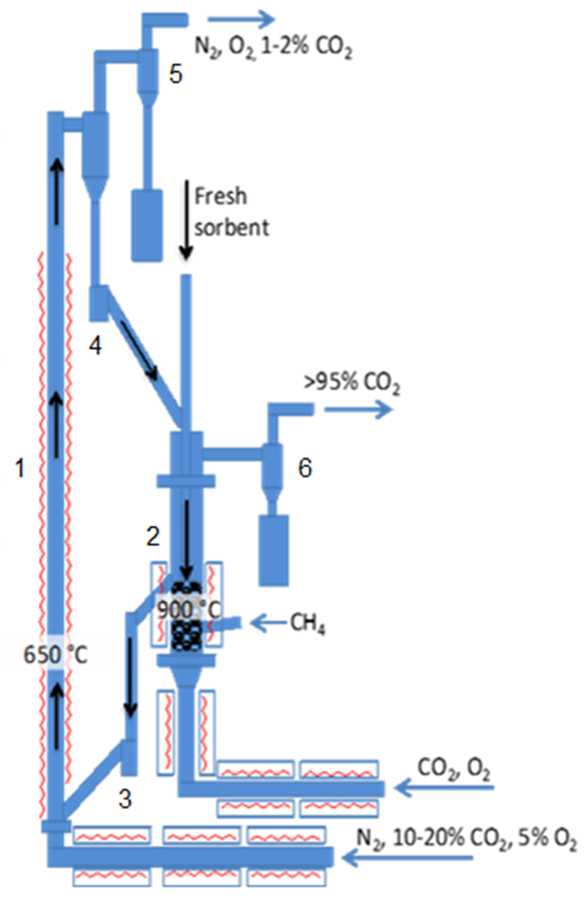
Figure 3: Schematic of the 25 kWth CaL (CFB carbonator and BFB calciner). 1: carbonator; 2: calciner; 3: lower loop-seal; 4: upper loop-seal; 5: carbonator cyclone; 6: calciner cyclone. Please click here to view a larger version of this figure.
- Start combustion in calciner
- Increase the oxygen concentration in the calciner from 0 to 40% vol, making sure that the concentration is stable before starting the combustion.
- Start the stoichiometric flow of natural gas manually using a rotameter making sure that the combustion is stable.
NOTE: The natural gas flow should be increased carefully. Check that the data show an appropriate level of combustion reaction. - Increase the oxygen concentration in the calciner in 20% vol increments by adjusting the natural gas flow rotameter to ensure stoichiometric combustion.
NOTE: This process should be performed with extreme care. If any suspicion arises that the combustion is not occurring as expected from the preliminary calculations then stop the flow of natural gas and switch the oxygen flow to nitrogen for safe operation. Identify the source of this discrepancy. The overall duration of this process is about 1 h. - Achieve 100% oxygen concentration natural gas combustion.
NOTE: The temperature and gas composition data should be carefully followed throughout all the testing, but especially when the combustion is taking place in 100% oxygen. - Add limestone in 0.5 L increments until there is 7 L in the fluidized bed. Calcine all the material in the fluidized bed of the calciner (the estimated calcination temperature is 800-850 °C for the batch present in the calciner and the calciner temperature for the following batches).
- Increase the flow of N2 in the carbonator to start the circulation. Check the circulation view port regularly to ensure proper circulation.
- Calcine all the available limestone circulating in the rig before starting the CO2 capture.
3. Stable Operation
- Manually switch the carbonation gas from N2 to 15% vol CO2 using the rotameter, which allows the calcined limestone to begin capturing CO2.
- Adjust the flows in the calciner manually using the rotameters to achieve a stable 930-950 °C temperature in the calciner by regulating the flow of natural gas (NG) and oxygen (within the optimal fluidization regime). The O2 flow is usually 100% with enough bed material, but it is adjusted throughout the experiment.
- When the material starts to decline in activity (above 5% CO2 concentration at the exit of the carbonator, which is acquired continuously by the software as described in step 2.1.3), add more limestone.
4. Shut-down Procedure
- Manually turn off the natural gas flow using the rotameter and decrease the oxygen flow, and switch the gases in both reactors to N2. Turn off all the heaters (calciner and carbonator).
- Allow the temperature of the inventory of the rig to decrease (normally overnight), and empty the reactors when they are at room temperature.
- Weigh the extracted solids and perform a standard sieve analysis. Characterize the material: porosimetry, composition (X-ray fluorescence spectrometry, XRF)21,22 and microscopic structure (scanning electron microscopy, SEM).
Representative Results
The experimental set-up is shown in Figure 3. The plant comprises two interconnected fluidized-beds. Namely, the carbonator is a CFB with 4.3 m height and 0.1 m internal diameter (ID); while the calciner is a BFB with 1.2 m height and 0.165 m ID. The solid transport from one reactor to the other is controlled by two loop-seals fluidized with nitrogen. Both reactors are fed a mixture of gas through a preheating line, and both are electrically heated; moreover, the calciner is fed with natural gas in order to produce by combustion the heat needed for the endothermic calcination and to heat the circulating sorbent. The carbonator distributor plate has 8 nozzles, each of them with twenty 2 mm holes, while the calciner has 20 nozzles with six 1 mm holes each.
The results from three different experiments are discussed in this section. These tests provide an overview with regards to running the pilot plant from air (~20% vol O2) to 100% vol O2 at the calciner inlet. This work also explores the results of using different particle size distributions in this operational mode to see if this parameter has an impact on the overall performance of the system. The limestone used in this study has a minimum content of 98.25% CaCO3.
Experiment 1: Flue gas (15% vol CO2) with limestone (200-300 µm) 30% vol O2
This first test with the limestone fraction 200-300 µm was performed to test the rig with limestone circulating between the two reactors within the rig as a starting point for performance optimization. During this test, a capture efficiency of 45% was achieved (Figure 4). This capture efficiency, Ecarb, was calculated using the following formula23: where FCO2 is the molar flow rate of CO2 entering the carbonator and Fcarb is the molar flow rate of CO2 leaving the carbonator.
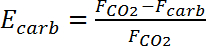 (2)
(2)
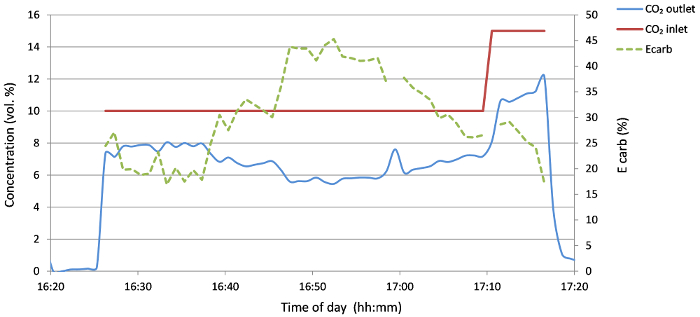
Figure 4: Concentration of CO2 at the carbonator inlet and outlet and the capture efficiency (Ecarb) for 200-300 µm limestone with 30% O2. Please click here to view a larger version of this figure.
The inferior capture efficiency during this experimental run was caused mainly by the insufficient supply of heat to calcine all the limestone residing in the BFB. This caused a decrease of the CaO/CaCO3 ratio in the carbonator feed. Another probable reason was the deactivation and carry-over of the lime particles from the calciner, which reduced the total bed inventory and the amount of sorbent present in the system. After the experiment, a material balance of the inventory of the reactor was performed (Table 1). A shift towards smaller fractions can be observed, because of the attrition of the material in both fluidized beds.
| Fraction | Limestone in | Calciner+Carbonator | Cyclone Calciner | Cyclone Carbonator |
| Total mass (g) | 9100 | 5000 | 500 | 20 |
| 250-300 µm | 22% | 24% | 2% | 0% |
| 212-250 µm | 47% | 41% | 6% | 18% |
| 150-212 µm | 28% | 34% | 24% | 18% |
| 0-150 µm | 3% | 1% | 69% | 65% |
Table 1: Weight balance of the material inputs and outputs for 200-300 µm limestone with 30% O2.
Experiment 2: Flue gas (15% CO2) with limestone (100-200 µm) 100% O2
In this test, the main aim was to use of smaller limestone particles in order to investigate their possible beneficial effect on the system performance. The secondary aim was to provide more heat to the calcination process in the BFB calciner by combusting the natural gas in highly concentrated oxygen, ideally up to 100% at the inlet.
In this experiment, we successfully tested the possibility of using pure O2 at the inlet of the calciner, which offers the possibility of completely eliminating the recycle of off-gas needed for a standard oxy-fuel process. This is made possible by the heat consumption in the form of a circulating fluidized bed material and the continuous calcination reaction.
The use of smaller particles did not have a beneficial effect on the carbonation process, most likely because of the higher contact area between particles and the gas. However, there is some controversy in this matter as smaller particles have shown decreased reactivity due to higher content of impurities24. Almost all the added limestone that was < 150 µm was very quickly elutriated from the calciner to the downstream cyclone. Therefore, it was very difficult to maintain the necessary inventory of lime in the rig needed to achieve higher capture efficiency. The results of the capture efficiency are in Figure 5.
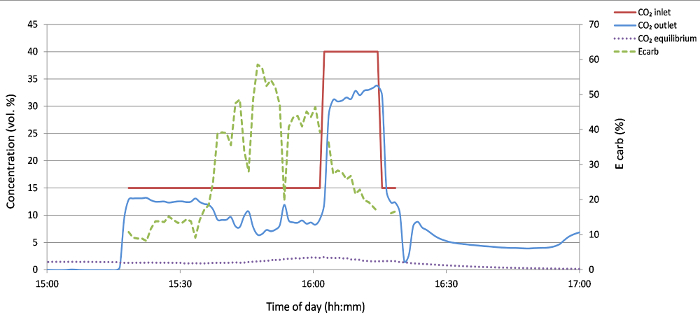
Figure 5: CO2 concentration at the carbonator inlet and outlet and the corresponding capture efficiency (Ecarb) for 100-200 µm limestone with 100% O2. Please click here to view a larger version of this figure.
The balance of the inputs and outputs of the solids was performed after the test (Table 2), and it was discovered that most of the material introduced into the rig during the experiment ended up in the calciner cyclone. This was probably the main cause of the low measured capture efficiency during this test.
| Fraction | Limestone | Carbonator | Calciner | Cyclone calciner | Cyclone carbonator |
| Total mass (g) | 19000 | 1200 | 2700 | 8700 | 360 |
| >212-212 µm | 0% | 21% | 2% | 0% | 39% |
| 212-150 µm | 18% | 39% | 73% | 5% | 42% |
| 150-125 µm | 40% | 22% | 13% | 32% | 10% |
| 125-63 µm | 41% | 18% | 12% | 60% | 9% |
| 0-63 µm | 2% | 0% | 0% | 3% | 1% |
Table 2: Balance of recovered material and its sieve analysis for 100-200 µm limestone with 100% O2.
To conclude, we successfully tested the use of pure O2 at the inlet of the calciner in order to completely eliminate the recycle of the off-gas needed for a standard oxy-fuel process. This is possible due to the heat sink provided in the form of circulating fluidized bed material and the continuous calcination reaction. However, the small particle size distribution (100-200 µm) was not beneficial for the capture process, due to the elutriation of the particles. It was extremely difficult to maintain the bed inventory needed to obtain a higher capture efficiency. Therefore, we decided to investigate the use of bigger particles in the next experimental campaign.
Experiment 3: Flue gas (15% CO2) with limestone (300-400 µm) 100% O2
During this trial, the performance of the fraction 300-400 µm was tested so as to reduce the high material losses from the fluidized bed of the calciner as seen in previous runs. This was expected to enable retention of the necessary lime/limestone inventory needed for its efficient circulation and carbon capture. Due to use of an appropriate amount of sorbent circulating in the rig and sufficient heat provided by the combustion of natural gas in pure oxygen (releasing 18 kW), a stable capture efficiency of ~ 70% was achieved for more than 3 h; this is a very good result when considering the relatively short carbonator reactor tube and the consequent short contact time between the sorbent and CO2. The concentration of CO2 at the outlet of the carbonator was maintained below 5% vol, and fresh limestone (in 0.5 L batches) was added to the calciner when the concentration of CO2 at the outlet of the carbonator exceeded this value. A stable experimental run was achieved with optimized conditions.
The process started with the standard procedure; i.e., first the reactor was heated up to 700 °C, then 2.9 L of limestone was added into the calciner and heated up. The temperatures and gas concentrations in the calciner are shown in Figure 6. Note that the numbers below correspond to those steps in Figure 6. 1) The air flow was replaced by a flow mixture of 40% O2 and 60% N2 and the combustion of natural gas in the fluidized bed was initiated (9.1 kW). The limestone in the fluidized bed was heated above 800 °C and 3 more batches of limestone (1 L) were added to the calciner. 2) While the limestone was calcining in the fluidized bed, the circulation of the lime/limestone was started by flowing preheated N2 through the carbonator (at a velocity of 2.5 m/s at 650 °C). An additional 0.9 L limestone was added and 3) a fresh O2 cylinder was connected to the inlet of the calciner. 4) After reconnecting the oxygen, the combustion was initiated again, this time in an inlet O2 concentration of 70% (and 30% N2), which led to a consumption of 14 kW of natural gas to reach an O2 concentration at the outlet of ~ 5% (in wet gas). 5) Pure O2 was introduced at the calciner inlet, which led to the heat release of 18 kW into the calciner, and 6) the carbonation was initiated in the carbonator by injecting 15% of CO2. The efficiency of the carbonation (Figure 7) was the highest yet on this reactor design (~ 70%). 7) The gas velocity flowing through the BFB of the calciner had to be lowered to 0.30 m/s (required by the desired temperature) to maintain the temperature of about 930 °C generated by the combustion of natural gas in pure O2 (while maintaining the O2 concentration in the off-gas to an industrially acceptable level below 5% vol).
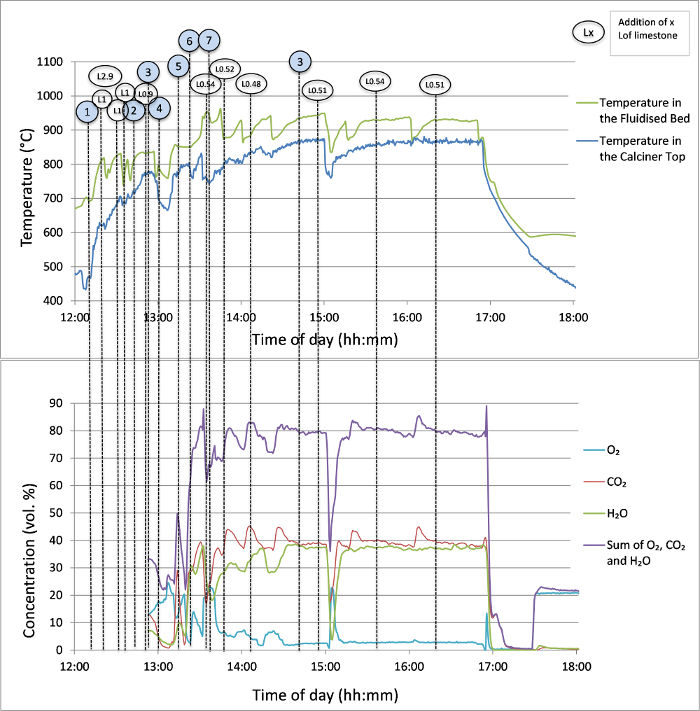
Figure 6: Temperature of the BFB of the calciner and the temperature and composition of the off-gas at its exit. Please click here to view a larger version of this figure.
The temperature in the fluidized bed was maintained constant during the test by regulating the flow rate of natural gas and consecutively adjusting the flow rate of O2 to achieve a calciner velocity of ~0.30 m/s. During the constant operation, the following energetic balance was observed: in the calciner, the oxygen was preheated to only 300 °C for safety reasons; hence, providing 0.5 kW in latent heat. Around 15.5 kW was released by the combustion of natural gas in the fluidized bed, but only 5.3 kW was needed for a continuous calcination (when no fresh material was added), 3.6 kW to cover the latent heat of the off-gas and ~7 kW for heating the circulating adsorbent and to cover the heat losses. In the carbonator, 3.2 kW was provided by preheating the gas (to 350 °C), and 5.4 kW was released by the carbonation process. Almost 5 kW was carried out of the carbonator as latent heat of the off-gas and 3.6 kW needed to be dissipated by heat losses to cover the heat released by the exothermic reaction while maintaining the temperature at 650 °C. When the carbonation process starts (and is effective), the temperature in the carbonator approaches 700 °C, which shifts the attainable thermodynamic equilibrium concentration of the outlet gas to higher values. This theoretical minimum concentration, dependent on the carbonator temperature, is depicted alongside the concentration measured during testing in Figure 7.
The carbonation process was initiated with ~6.7 L of limestone present in the rig. An additional 0.54 L at the beginning of the carbonation (13:45 in Figure 7) resulted in the decrease of residual CO2 in the gas below 5% vol, and this level was maintained throughout the test by additions of 0.5 L limestone batches (when the concentration of CO2 at the exit of carbonator exceeded 5%). The time intervals between the additions of fresh limestone to the calciner were 15, 20, 50, 45 and 50 min. Therefore, we concluded that a stable operation requires the addition of fresh sorbent corresponding to 0.5 L (750 g) of limestone approximately every 50 min, which is equivalent to a make-up ratio (F0/FCO2) of 6%, as described elsewhere25. The make-up ratio in these experiments was mainly influenced by the limestone (reactivity decay and elutriation). The value of this parameter was chosen based on the CO2 concentration at the carbonator outlet, i.e., adding more limestone when it reached a 5% vol CO2.
Based on the sieving analysis, we conclude that the original limestone fraction was retained mostly in the rig circulation, while particles mostly < 250 µm were carried over to the calciner cyclone. These particles resulted predominantly from the breakage/attrition of larger particles.
| Fraction | Limestone in | Carbonator | Calciner | Cyclone calciner | Cyclone Carbonator |
| Total mass (g) | 14,000 | 1,900 | 4,200 | 2,000 | 120 |
| >355 µm | 21% | 16% | 4% | 0% | 0% |
| 300-350 µm | 43% | 45% | 38% | 1% | 1% |
| 250-300 µm | 33% | 26% | 48% | 3% | 0% |
| 212-250 µm | 2% | 4% | 9% | 7% | 1% |
| 150-212 µm | 0% | 3% | 1% | 35% | 14% |
| 63-150 µm | 0% | 5% | 0% | 41% | 46% |
| 0-63 µm | 0% | 0% | 0% | 12% | 38% |
Table 3: Mass balance of solids and sieve analysis of the inputs (limestone) and outputs (other) for 300-400 µm limestone with 100% O2.
| Fraction | Limestone in | Carbonator | Calciner | Cyclone calciner | Cyclone Carbonator | Difference (out – in) |
| Total mol | 130 | 31 | 66 | 32 | 2 | 0 |
| >355 µm | 27 | 5 | 2 | 0 | 0 | -20 |
| 300-350 µm | 56 | 14 | 25 | 0 | 0 | -17 |
| 250-300 µm | 4 | 8 | 32 | 1 | 0 | -2.6 |
| 212-250 µm | 2 | 1 | 6 | 2 | 0 | 6.9 |
| 150-212 µm | 0 | 1 | 1 | 11 | 0 | 13 |
| 63-150 µm | 0 | 2 | 0 | 13 | 1 | 16 |
| 0-63 µm | 1 | 0 | 0 | 4 | 1 | 4.1 |
Table 4: Molar balance estimate (10% humidity of the raw limestone, 75% wt of the output in calcined state) for 300-400 µm limestone with 100% O2.
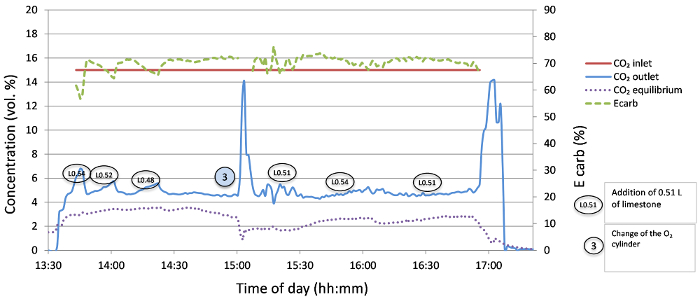
Figure 7: CO2 concentration at the carbonator inlet and outlet and the corresponding capture efficiency (Ecarb) for 300-400 µm limestone with 100% O2. Please click here to view a larger version of this figure.
Discussion
The operation of the calciner with an inlet of 100% vol oxygen is achievable, based on exploiting the endothermic nature of the calcination reaction, as well as the fact that the solids circulate between the two reactors at different temperatures. This operating mode aims to make the CaL process more economically promising by reducing capital and operating costs. As the recycle of flue gas (mainly CO2, water vapor and unreacted O2) is reduced or even eliminated, the heat consumed to preheat this stream is lower. Therefore, less oxygen is needed and a smaller ASU would be required. As the gas flow would be lower in this configuration, the size of the calciner would be smaller for the same fluidization velocity.
A standard methodology was developed to ensure the safe operation of the high-oxygen concentration in the calciner. The results showed a capture efficiency of up to 70% in some of the performed experiments. Also, different particle size distributions were used in this reactor configuration (carbonator as a CFB; calciner as a BFB). The distributions were: 100-200 µm; 200-300 µm; 300-400 µm. For the smallest distribution (100-200 µm), however, there were elutriation issues and the majority of the bed inventory was found in the calciner's cyclone catch-pot. The best results were achieved with the largest particle size distribution (300-400 µm): an Ecarb of ~70% was maintained throughout the experiment with a make-up ratio of about 6%.
This protocol was improved by minimizing the electrical heating provided to the gas and calciner when the natural gas is being combusted, in order to protect the tubes from electrical discharges caused by the heating elements. Also, it has been noted that the O2 concentration increase can be achieved more rapidly than in initial experimental campaigns, from 20% vol to 60% vol and 100% vol. It is important to highlight that the most critical steps in this process are the start of the combustion and the increase of the oxygen concentration, which can cause increased temperatures that will turn off the natural gas source if the temperature goes higher than 980 °C. Also, the material make-up is a concern as it can lower the temperature of the reactor and stop the combustion process, and therefore, it should be added in small batches.
With this methodology applied to this experimental rig, it is possible to test new synthetic materials, as well as materials improved via doping, thermal pre-treatment, chemical pre-treatment, etc.26 This protocol allows these new sorbents to be tested under realistic conditions providing a standard methodology for sorbent comparison. However, there are some challenges when applying this concept at larger scale, such as the use of coal in the calciner under these operating conditions. The use of solid fuels would increase the difficulty in calciner operation due to the high temperatures, which can lead to ash agglomeration and eventually defluidizing phenomena27. This needs further study in order to determine the feasibility of this protocol; however, the concept was proved successful at pilot-scale in this work using natural gas.
Another limitation arising from this study is the duration of the tests, with ~3 h of steady state operation per test; this is due to the heating process of the plant, which is a slow process. The average number of carbonation/calcination cycles experienced by a particle when circulating between reactors is not known. It is possible that the high oxygen concentration had a negative effect causing more sintering in the limestone particles. Further investigation of these challenges would help to assess the suitability of the protocol as a novel and feasible operating mode for CaL plants at a higher scale.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The research leading to these results has received funding from the European Community's Research Fund for Coal and Steel (RFCS) under grant agreement n° RFCR-CT-2014-00007. This work was funded by the UK Carbon Capture and Storage Research Centre (UKCCSRC) as part of Call 2 projects. UKCCSRC is supported by the Engineering and Physical Sciences Research Council (EPSRC) as part of the Research Council's UK Energy Programme, with additional funding from the Department of Business, Energy and Industrial Strategy (BEIS – formerly DECC). The authors would also like to thank Mr. Martin Roskilly for his enormous help throughout the course of this work.
Materials
| Longcal limestone | Loncliffe | Longcal SP52 | n/a |
| Mechanical Shacker | SWECO | LS24S544+C | Mechanical siever to separate particles |
| Oxygen | BOC | n/a | BOC cylinders |
| Nitrogen | BOC | n/a | BOC tank |
| Carbon dioxide | BOC | n/a | BOC tank |
| Natural gas | n/a | n/a | Taken from the line |
References
- Bernstein, L., Lee, A., Crookshank, S. Carbon dioxide capture and storage: a status report. Climate Policy. 6 (2), 241-246 (2011).
- Boot-Handford, M. E., et al. Carbon capture and storage update. Energy Environmental Science. 7 (1), 130-189 (2014).
- Herzog, H. J. Scaling up carbon dioxide capture and storage: from megatons to gigatons. Energy Economics. 33 (4), 597-604 (2011).
- Shimizu, T., Hirama, T., Hosoda, H., Kitano, K., Inagaki, M., Tejima, K. A twin fluid-bed reactor for removal of CO2 from combustion processes. Chemical Engineering Research and Design. 77 (1), 62-68 (1999).
- Blamey, J., Anthony, E. J., Wang, J., Fennell, P. S. The calcium looping cycle for large-scale CO2 capture. Progress in Energy and Combustion Science. 36 (2), 260-279 (2010).
- Masnadi, M. S., Grace, J. R., Bi, X. T., Ellis, N., Lim, C. J., Butler, J. W. Biomass/coal steam co-gasification integrated with in-situ CO2 capture. Energy. 83, 326-336 (2015).
- Abanades, J. C., Anthony, E. J., Lu, D. Y., Salvador, C., Alvarez, D. Capture of CO2 from combustion gases in a fluidized bed of CaO. AIChE Journal. 50 (7), 1614-1622 (2004).
- Hughes, R. W., Lu, D. Y., Anthony, E. J., Macchi, A. Design, process simulation and construction of an atmospheric dual fluidized bed combustion system for in situ CO2 capture using high-temperature sorbents. Fuel Processing Technology. 86 (14), 1523-1531 (2005).
- Lu, D. Y., Hughes, R. W., Anthony, E. J. Ca-based sorbent looping combustion for CO2 capture in pilot-scale dual fluidized beds. Fuel Processing Technology. 89 (12), 1386-1395 (2008).
- Hawthorne, C., et al. CO2 capture with CaO in a 200 kWth dual fluidized bed pilot plant. Energy Procedia. 4, 441-448 (2011).
- Sánchez-Biezma, A., et al. Postcombustion CO2 capture with CaO. Status of the technology and next steps towards large scale demonstration. Energy Procedia. 4, 852-859 (2011).
- Dieter, H., Hawthorne, C., Zieba, M., Scheffknecht, G. Progress in calcium looping post combustion CO2 capture: successful pilot scale demonstration. Energy Procedia. 37, 48-56 (2013).
- Arias, B., et al. Demonstration of steady state CO2 capture in a 1.7 MWth calcium looping pilot. International Journal of Greenhouse Gas Control. 18, 237-245 (2013).
- Ströhle, J., Junk, M., Kremer, J., Galloy, A., Epple, B. Carbonate looping experiments in a 1MWth pilot plant and model validation. Fuel. 127, 13-22 (2014).
- Bidwe, A. R., Hawthorne, C., Dieter, H., Dominguez, M. A., Zieba, M., Scheffknecht, G. Cold model hydrodynamic studies of a 200kWth dual fluidized bed pilot plant of calcium looping process for CO2 Capture. Powder Technology. 253, 116-128 (2014).
- Chang, M. H., et al. Design and experimental testing of a 1.9 MWth calcium looping pilot plant. Energy Procedia. 63, 2100-2108 (2014).
- Reitz, M., Junk, M., Ströhle, J., Epple, B. Design and operation of a 300kWth indirectly heated carbonate looping pilot plant. International Journal of Greenhouse Gas Control. 54, 272-281 (2016).
- Martínez, A., Lara, Y., Lisbona, P., Romeo, L. M. Energy penalty reduction in the calcium looping cycle. International Journal of Greenhouse Gas Control. 7, 74-81 (2012).
- Perejón, A., Romeo, L. M., Lara, Y., Lisbona, P., Martínez, A., Valverde, J. M. The calcium-looping technology for CO2 capture: on the important roles of energy integration and sorbent behavior. Appl Energy. 162, 787-807 (2016).
- Mantripragada, H. C., Rubin, E. S. Calcium looping cycle for CO2 capture: Performance, cost and feasibility analysis. Energy Procedia. 63, 2199-2206 (2014).
- . . ASTM C1271-99(2012), Standard Test Method for X-ray Spectrometric Analysis of Lime and Limestone. (2012), C1271-C1299 (2012).
- . . ASTM C25-11e2, Standard Test Methods for Chemical Analysis of Limestone, Quicklime, and Hydrated Lime. , C25-C11 (2011).
- Alonso, M., Rodríguez, N., Grasa, G., Abanades, J. C. Modelling of a fluidized bed carbonator reactor to capture CO2 from a combustion flue gas. Chem Eng Sci. 64 (5), 883-891 (2009).
- Manovic, V., Anthony, E. J. Parametric study on the CO2 capture capacity of CaO-based sorbents in looping cycles. Energy Fuels. 22 (3), 1851-1857 (2008).
- Duhoux, B., Mehrani, P., Lu, D. Y., Symonds, R. T., Anthony, E. J., Macchi, A. Combined Calcium Looping and Chemical Looping Combustion for Post-Combustion Carbon Dioxide Capture: Process Simulation and Sensitivity Analysis. Energy Technol. 4 (10), 1158-1170 (2016).
- Erans, M., Manovic, V., Anthony, E. J. Calcium looping sorbents for CO2 capture. Appl Energy. 180, 722-742 (2016).
- Basu, P. A study of agglomeration of coal-ash in fluidized beds. The Canadian Journal of Chemical Engineering. 60 (6), 791-795 (1982).

