Detecting the Water-soluble Chloride Distribution of Cement Paste in a High-precision Way
Summary
A protocol for obtaining a water-soluble chloride profile by using a high precision milling method is presented.
Abstract
To improve the accuracy of the chloride distribution along the depth of cement paste under cyclic wet-dry conditions, a new method is proposed to obtain a high-precision chloride profile. Firstly, paste specimens are molded, cured, and exposed to cyclic wet-dry conditions. Then, powder samples at different specimen depths are grinded when the exposure age is reached. Finally, the water-soluble chloride content is detected using a silver nitrate titration method, and chloride profiles are plotted. The key to improving the accuracy of the chloride distribution along the depth is to exclude the error in the powderization, which is the most critical step for testing the distribution of chloride. Based on the above concept, the grinding method in this protocol can be used to grind powder samples automatically layer by layer from the surface inward, and it should be noted that a very thin grinding thickness (less than 0.5 mm) with a minimum error less than 0.04 mm can be obtained. The chloride profile obtained by this method better reflects the chloride distribution in specimens, which helps researchers to capture the distribution features that are often overlooked. Furthermore, this method can be applied to studies in the field of cement-based materials, which require high chloride distribution accuracy.
Introduction
The chloride induced corrosion of reinforcing steel is one of the major causes jeopardizing the service life of reinforced concrete structures exposed to an aggressive environment (e.g., marine environment or deicing salts environment). The chloride distribution can be used for investigations of the chloride penetration rate, the amount of steel corrosion, and predictions of the service life. Therefore, a precise chloride distribution is of great significance for the durability research of concrete structures.
Mechanisms or combined actions of multi-mechanisms are responsible for chloride transport in concrete under specific environments1. In submerged parts of marine structures, pure diffusion is the only mechanism driving chloride ingress2, which causes the chloride content to decrease with increasing depth. Concrete is in a non-saturated state3 when subjected to a wetting-drying environment such as a marine tidal zone or a deicing salt environment. In such conditions, the process of chloride ingress becomes very complicated and both diffusion and capillary suction operate in chloride transport4. Thus, the chloride distribution under wetting-drying conditions is probably more complicated than in a submerged condition. Therefore, the chloride distribution under cyclic wetting-drying conditions needs to be studied more precisely.
The chloride distribution in cement-based materials is usually represented by a chloride profile. The accuracy of a chloride profile mainly depends on two aspects: the accuracy of chloride content and the accuracy of chloride distribution along the depth. Regarding the chloride content test, the basic principle is based on the chemical reaction between (Cl–) and (Ag+)5,6, though different standards require different specific operations. The exact chloride content can be acquired as long as specific operations are followed. However, the accuracy of the chloride distribution along the depth relies mainly on the accuracy of the sampling position. The methods already known for obtaining power samples at different depths of specimen are an electric drill, a normal grinding machine, and a profile grinder. Unfortunately, they all share a disadvantage as the accuracy is low when the grinding thickness or sampling interval is small. Thus, the requirement of investigating chloride distribution in the surface layer of specimens under cyclic wetting-drying condition is not met. Therefore, a new method that can allow a smaller sampling interval (e.g., less than 0.5 mm) and reduce error to the minimum (e.g., less than 0.05 mm) is needed.
The detailed protocol here offers a more accurate way to get a chloride profile by improving the precision of chloride distribution along the depth.
Protocol
Caution: Several of the chemicals, such as silver nitrate, potassium chromate, and concentrated sulfuric acid, used in the testing process are acutely toxic and corrosive. Please adopt appropriate safety measures while using them, including the wear of safety glasses, gloves, lab coat, etc.
1. Preparation of Paste Specimens
- Preparation of the mold
- Use a brush to clean up a mold of size 70 mm × 70 mm × 70 mm, making sure that the inner surfaces of the mold are impurity-free.
- Brush mold-release diesel oil evenly on the inner surfaces of the mold using another brush.
- Molding Paste Specimen
- Use an electronic balance to weigh 1,000 g of water into a 1,500 mL plastic cup and 2,000 g of cement into a 3,000 mL plastic basin.
- Add 1,000 g water and 2,000 g of cement into a 5 L mixing pot sequentially. The water to cement ratio is 0.5.
- Put the mixing pot on the base of a cement paste mixer and fasten it after raising it to the stirring position.
- Mix at 65 rpm for 90 s.
- Let the mixture sit for 30 s. During this period, scrape off the paste on the inner wall of pot using a scraper knife and mix it into the rest of the pot. Mix at 130 rpm for 60 s.
- Remove the mixing pot from the mixer. Pour the well-mixed cement paste into the mold. Shovel the cement paste with a scraper knife and vibrate the mold for several seconds to compact the paste on a vibrating table.
- After filling up the mold, seal the mold surface with cling film to prevent moisture evaporation. Let sit for 24 h in the molding at 23 ± 2 °C.
- Curing
- Remove the hardened paste specimens from the mold.
- Place hardened paste specimens in a curing room at 23 ± 2 °C and 95% relative humidity for 60 d.
- Cutting
- Take specimens out of the curing room after 60 d.
- Fix the specimen on a high precision cutting machine and cut off 20 mm from the mold-free surface. The processed specimens of size 70 mm × 70 mm × 50 mm will be subjected to chloride ingression exposure afterwards.
- Sealing with epoxy resin
- Taking the cut-surface as the exposure surface, seal the other five surfaces of the specimen (70 mm × 70 mm × 50 mm) with epoxy resin using a brush. The volume of epoxy resin used for each specimen is about 30 mL. Harden the epoxy resin through air exposure for 24 h.
NOTE: Prepare specimens for chloride ingression exposure after the epoxy resin has hardened.
- Taking the cut-surface as the exposure surface, seal the other five surfaces of the specimen (70 mm × 70 mm × 50 mm) with epoxy resin using a brush. The volume of epoxy resin used for each specimen is about 30 mL. Harden the epoxy resin through air exposure for 24 h.
2. Cyclic Wetting and Drying
- Add 0.35 kg of NaCI and 9.65 kg of deionized water into a 15 L plastic bucket for a NaCI solution with a mass ratio of 3.5%.
- With the exposure surface facing down, place specimens in a plastic box (50 cm × 30 cm × 20 cm) with two braces laid on the bottom. The brace can be stainless steel bars or plastic grid plates. The presence of the brace allows the exposure surface to take about 1.0 cm of space to the bottom of the box.
- Pour the 3.5% NaCI solution slowly into the plastic box and stop when the liquid level is about 1.0 cm above the exposure surface. Seal the box with a plastic film (about 0.25 mm thickness) to prevent concentration changes caused by evaporation. Then, put the plastic box in a room with constant temperature of 23 ± 2 °C and humidity of 65 ± 2%.
- Starting from the time of pouring the NaCl solution, soak specimens for 24 h for the wetting process.
- Take specimens out of solution after wetting for 24 h, wipe off the residual solution with a towel gently, and put them in the same constant temperature and humidity room for 6 d, which is the drying process.
- After drying for 6 d, put specimens back into the NaCI solution again.
- Repeat steps 2.5 and 2.6 (7 d for one wetting-drying cycle) for a total of 12 cycles.
3. Grinding Powder Samples
- After 12 wet-dry cycles, fix the specimen on the base of high precision computer numerical controlled (CNC) grinding machine, which was converted from a milling machine by replacing the original cutter with a titanium alloy cutter.
- Place powder-collecting paper around the specimen on the base of a grinding machine before starting to grind.
- Start the high precision CNC grinding machine and wait until the system is loaded.
- Press the "ZERO" button and the subsequent "X→0", "Y→0", "Z→0" buttons to make the operation desk and milling cutter return to the coordinate origin automatically.
- Press "Manual Data Input (MDI)", double click "PROGRAM", input "N3S1000" and click "INPUT", then press "CYCLE START" to start the main shaft. Note that "N3S1000" means the revolving speed of the shaft is being set at 1000 rpm.
- Load the main grinding program: Press "MDI", click "PROGRAM" repeatedly to find "PROGRAM (CATALOG)" page, and select the appropriate program. Next, click "EDIT", and press "INPUT" to load the main program.
NOTE: The main program can be personalized according to a specific grinding requirement, including settings of the total grinding depth, number of layers, thickness of each layer, and time interval between grinding two layers. For this protocol, the total grinding depth is 10 mm, and the number of layers is 20; the grinding thickness of each layer is 0.5 mm; and the time interval between grinding layers is 60 s. See the disclosure for details of program. - Set the milling cutter position: First, click "MANUAL OPERATION" and then "MAINSHAFT FORWARD" to rotate the main shaft. Next, click "HAND WHEEL" and manually adjust the cutter to the ready-to-grind position (usually on the one third of the front-left surface of specimen). Finally, click "SET" and "X", "INPUT", "Y", "INPUT", "Z", and "INPUT" buttons subsequently. Record the relative coordinates as the original space coordinates to finish cutter setting.
- Launch the main grinding program: Press "START", then click "AUTO" and "CYCLE START" buttons subsequently. The machine starts grinding automatically as pre-programmed.
NOTE: During the grinding process, powder will be accumulated on the collecting paper which is pre-spread on the base of machine. After grinding each layer, the milling cutter will rise away from the specimen and remain stationary in the X, Y, Z directions for 60 s as programmed. Collect a powder sample during the 60 s break and re-spread the collecting paper.
CAUTION: Though the milling cutter stops moving in the X, Y, Z directions, it is still rotating. Attention should be paid to avoid touching the milling cutter with any body parts to avoid injury. Besides, it is necessary to use a mask and gloves due to the dust generated during grinding process. - Shut down the machine after finishing grinding.
4. Detecting Chloride Content7
- Preparation of solution
- Potassium chromate solution
- Dissolve 5 g of potassium chromate in 20 mL of deionized water. Add 10 mg of silver nitrate. Shake well and let sit for 24 h.
CAUTION: Potassium chromate and silver nitrate are toxic. It is necessary to wear rubber gloves and a mask while using them. - Filter the solution into a conical flask with a funnel and filter paper and move it into a 100 mL volumetric flask. Add deionized water until reaching the 100 mL calibration mark. The potassium chromate indicator of 5% concentration is obtained.
- Dissolve 5 g of potassium chromate in 20 mL of deionized water. Add 10 mg of silver nitrate. Shake well and let sit for 24 h.
- Prepare 0.5% phenolphthalein solution: Dissolve 0.5 g of phenolphthalein in 75 mL of ethyl alcohol. Add 25 mL of deionized water and shake well.
- Prepare dilute sulfuric acid: Dissolve 5 mL of concentrated sulfuric acid (98.3%) in 100 mL of deionized water.
CAUTION: Concentrated sulfuric acid is very corrosive. It is necessary to wear rubber gloves, a mask, and goggles while using it. - NaCI standard solution
- Heat about 2 g of pure sodium chloride under 200 – 300 °C in a crucible with an electric furnace, and stir with a glass rod during the process until there are no sounds of salt cracking (devoid of water).
- After cooling in a desiccator, take 1.169 g of NaCI crystals, dissolve in a 1,000 mL volumetric flask with 100 mL deionized water. Add deionized water until reaching the 1,000 mL calibration mark. An NaCl standard solution of 0.02 M is obtained. Calculate the concentration of the NaCl standard solution with the equation below:
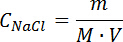
where, CNaCl is the standard concentration of NaCl solution, mol/L; V is the volume of solution, L; M is the molar mass of NaCl, 58.45 g/mol; m is the mass of NaCl, g.
- Silver nitrate solution
- Dissolve 1.7 g of silver nitrate in 100 mL of deionized water with a 200 mL breaker. Move it into a 1,000 mL brown volumetric flask and add deionized water up to the 1,000 mL calibration mark.
- Pipette three 10 mL (V1) units of 0.02 M standard NaCI solution into three conical flasks. Add 10 drops of potassium chromate indicator in each with a dropper.
- Titrate the solution from step 4.1.5.2 with the prepared silver nitration from step 4.1.5.1. Stop when the solution becomes reddish and the red color does not fade away. Record the volume of consumed silver nitration solution (V2). Calculate the standard concentration of silver nitration solution with the equation below, and take the mean value of three testing results.
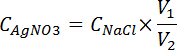
Where, CAgNO3 is the concentration of silver nitrate solution, mol/L; V1 is the volume of NaCl solution, 10 mL; V2 is the volume of silver nitrate solution consumed, mL.
- Potassium chromate solution
- Water-soluble chloride content
- Grind every sample obtained in section 3 in a mortar until it can be sifted through an 80 µm sieve. Heat and dry the sifted samples at 105 °C for 2 h in an oven.
- Take 2 g of each dried sample, put them in 100 mL plastic bottles, add 50 mL (V3) of deionized water with a 50 mL measuring pipette, and seal the bottles with caps. Shake the bottles violently to guarantee that the sample and deionized water are well-mixed.
- Fasten plastic bottles on an automatic vibrator and vibrate for 24 h to dissolve the water-soluble chloride from samples.
- Filter the solution in bottles after vibrating for 24 h with funnels and filter paper. Pipette two 10 mL (V4) units of filtrated solution from every bottle into two conical flasks.
- Add two drops of phenolphthalein solution to each conical flask to make the solution present purplish red. Neutralize the solution to colorless with dilute sulfuric acid.
- Add 10 drops of potassium chromate indicator into the colorless solution and titrate immediately with silver nitrate solution. Shake the conical flask manually during titrating to make sure that the chloride ions react with silver nitrate quickly and completely. Stop titrating when the solution becomes reddish and the red color does not fade away. Record the volume of consumed silver nitrate solution (V5).
- Calculate the content of water-soluble chloride with the equation below and take the mean value of the two testing results.
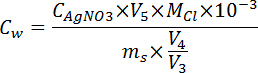
where, Cw is the water-soluble chloride content in the paste sample, % cement; ms is the mass of powder sample, 2 g; V3 is the volume of deionized water used to dissolve sample, 50 mL; V4 is the volume of filtrated solution extracted at each nitration, 10 mL; V5 is the volume of silver nitrate solution consumed at each nitration, mL; Mcl is the molar mass of Cl, 35.5 g/mol. - Plot water-soluble chloride profiles after getting the chloride content at different depths of paste specimens.
5. Accuracy Test of Grinding Thickness
- Set five grinding thicknesses: 1.0 mm, 0.5 mm, 0.2 mm, 0.1 mm, and 0.05 mm in the main grinding program. Grind with each thickness five times.
- Measure the thickness of specimen before grinding (H1) and after grinding (H2) with a vernier caliper, and calculate the practical grinding thickness with the equation below. Do not take the specimen down from the machine during measuring to guarantee the reliability of measurement.
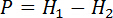
Where, P is the practical grinding thickness, mm; H1 is the thickness of specimen before grinding, mm; H2 is the thickness of specimen after grinding, mm.
Representative Results
The original data and statistical results about the accuracy of grinding thickness are collected (Table 1)8. Mean and error are used to reflect the accuracy and standard deviation (SD) is used to reflect the consistency of this method.
The water-soluble chloride content of testing interval 0.5 mm (Figure 1) and 2.0 mm (Figure 2) and the corresponding diffusion coefficient D are collected using the equation below, called the "error function" of Fick's second law. The law of change of chloride content along the depth is used to reflect chloride distribution features, and D is used to evaluate the penetration rate of chloride.
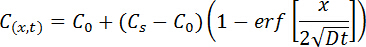
Where, x is the distance from the exposed surface; t is the exposure time; C(x,t) is the chloride content at a depth of x and time of t; Cs is the surface chloride content; D is the chloride diffusion coefficient; C0 is the initial chloride content.
| S (mm) | H1 (mm) | H2 (mm) | P (mm) | Error (mm) | Mean (mm) | SD (mm) |
| 1 | 18.78 | 17.82 | 0.96 | 0.04 | ||
| 17.82 | 16.82 | 1 | 0 | |||
| 16.82 | 15.83 | 0.99 | 0.01 | 0.998 | 0.026 | |
| 15.83 | 14.83 | 1 | 0 | |||
| 14.83 | 13.79 | 1.04 | -0.04 | |||
| 0.5 | 25.09 | 24.55 | 0.46 | 0.04 | ||
| 24.55 | 24.07 | 0.48 | 0.02 | |||
| 24.07 | 23.59 | 0.48 | 0.02 | 0.482 | 0.019 | |
| 23.59 | 23.11 | 0.48 | 0.02 | |||
| 23.11 | 22.6 | 0.51 | -0.01 | |||
| 0.2 | 19.24 | 19.01 | 0.23 | -0.03 | ||
| 19.01 | 18.8 | 0.21 | -0.01 | |||
| 18.8 | 18.62 | 0.18 | 0.02 | 0.208 | 0.02 | |
| 18.62 | 18.43 | 0.19 | 0.01 | |||
| 18.43 | 18.2 | 0.23 | -0.03 | |||
| 0.1 | 17.66 | 17.57 | 0.09 | 0.01 | ||
| 17.57 | 17.46 | 0.11 | -0.01 | |||
| 17.46 | 17.34 | 0.12 | -0.02 | 0.1 | 0.026 | |
| 17.34 | 17.26 | 0.08 | 0.02 | |||
| 17.26 | 17.16 | 0.1 | 0 | |||
| 0.05 | 16.26 | 16.19 | 0.07 | -0.02 | ||
| 16.19 | 16.14 | 0.05 | 0 | |||
| 16.14 | 16.07 | 0.07 | -0.02 | 0.056 | 0.012 | |
| 16.07 | 16.03 | 0.04 | 0.01 | |||
| 16.03 | 15.98 | 0.05 | 0 |
Table 1: The original data and statistical results about the accuracy of grinding thickness.
S is the set grinding thickness, and P is the practical grinding thickness. Error is the difference between S and P. The mean is the average value of five practical grinded thicknesses, and the SD is the standard deviation.
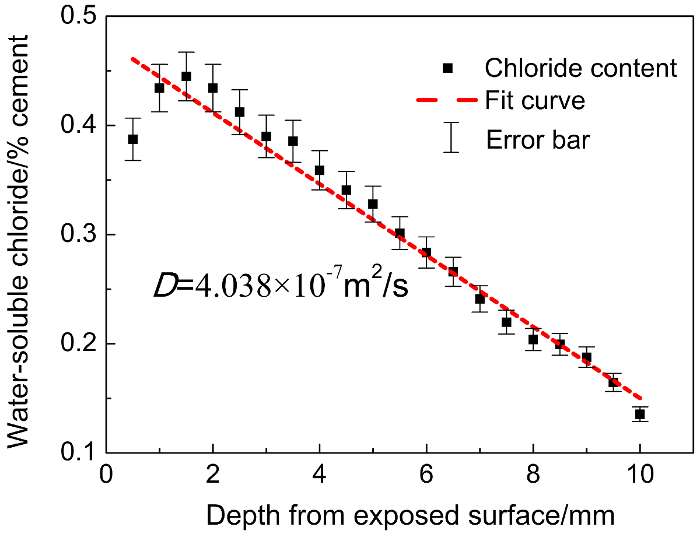
Figure 1: Water-soluble chloride profile of paste specimens with testing interval of 0.5 mm.
After exposure for 12 weeks under cyclic wetting-drying conditions, 20 powder samples are obtained within a depth of 10 mm through grinding every 0.5 mm in section 3. The 20 powder samples are first partly used for testing the chloride content in each. 20 data points (black) are obtained and presented in Figure 1. The red line is the fitting line of those data with the "error function" of Fick's second law, D is the chloride diffusion coefficient, and the error bars depict the error or uncertainty of the data. Please click here to view a larger version of this figure.
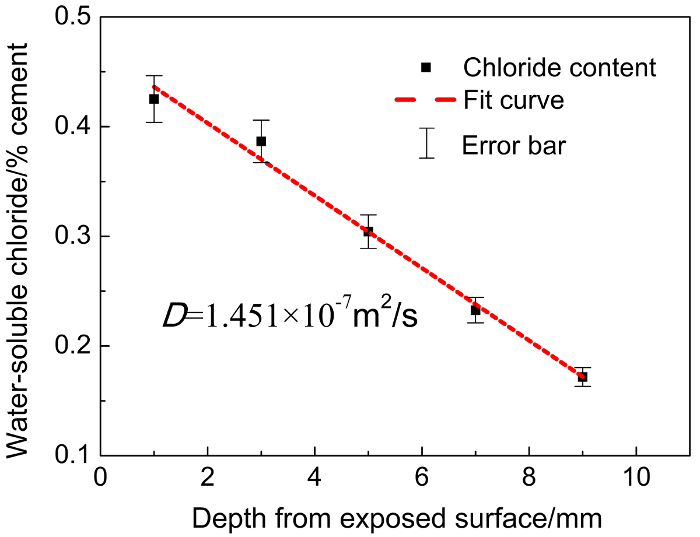
Figure 2: Water-soluble chloride profiles of paste specimens with testing interval of 2.0 mm.
For the rest of the 20 samples, every four sequential layers (with the same amount taken from each) (1~4, 5~8, 9~12, 13~16, and 17~20) are well blended, and the chloride content in each is tested; that is, the testing interval is 2.0 mm. Five data points (black) of chloride content are thus obtained. The red line is the fitting line of those data with the "error function" of Fick's second law, D is the corresponding diffusion coefficient, and the error bars depict the potential error or uncertainty of each chloride value.
Note: The mass of sample used to determine chloride content is 2 g as introduced in 4.2.2. Please click here to view a larger version of this figure.
Discussion
The grinding error of the high precision CNC milling machine is controlled within 0.04 mm and the standard deviation is less than 0.03 mm (Table 1)8. It proves that this milling method has a high degree of accuracy and stability in the measurements of chloride contents as a function of depth, contributing to a better illustration of real chloride distribution in the specimens.
When the testing interval is 0.5 mm, with the depth from exposure surface increasing, there is a maximum point in the chloride content (Figure 1). When the testing interval is 2.0 mm, chloride content decreases monotonically (Figure 2). It can be observed that in the surface layer, the interval value selected can significantly affect the profiles. Lower intervals are recommended since they can capture and record more details. Moreover, with different testing intervals, the D obtained through fitting with the "error function" differs. The D values of interval 0.5 mm (4.038 × 10-7 m2/s, Figure 1) are more than twice the D values of interval 2.0 mm (1.451 × 10-7 m2/s, Figure 2),despite testing the same specimen. Obviously, when the interval of 2 mm is employed, due to the lack of important data and thus the poor description of chloride content evolution, the derived D value cannot be reliable.
To achieve small interval values, the grinding thickness should be lowered. The three most widely used grinding methods in this field at present cannot guarantee a small error when the grinding thickness is less than 0.5 mm. The first method (getting a powder sample with an electric drill) features substantial difficulties in controlling the position of drilling with manual operation,which results in an error value of more than 1.0 mm, even if the sampling interval is 5.0 mm9. The second method (a normal grinding machine10,11) is extensively applied in China. The calibration requirement for each layer is the defect of this machine, and the corundum disk with a diameter of 100 mm used for grinding tends to become deformed and damaged. The grinding thickness of this method is usually 2.0 mm and the error is more than 0.5 mm. The third method is a Profile Grinder. Though the error of this method is smaller than that of the first two methods, the smallest labeled grinding thickness is 2.0 mm. If a smaller grinding thickness is required, manual calibration is needed, which greatly reduces the method's accuracy. In contrast, the error of the method used here is less than 0.03 mm even when the grinding thickness is less than 0.2 mm (Table 1), which shows extremely high accuracy in the measurements of chloride content distribution as a function of depth.
However, this high-precision grinding method also has limitations. It requires manual work to collect powder, and dust is generated during grinding that can be inhaled. To improve this method, an automatic powder collecting device is being designed to complement the high precision CNC grinding machine. Hopefully, this new invention will be both healthy and labor-saving.
The grinding method used here achieves automatic grinding layer by layer starting from the surface of the specimen. It guarantees a minimum error even for small grinding thicknesses, which greatly improves the accuracy of chloride distribution as function of depth and is of great significance for chloride transport study. This method can also be utilized with other cement-based materials (e.g., mortar and concrete). Since mortar and concrete contain hard sand and gravel, the titanium alloy cutter should be replaced with harder materials (e.g., diamond). No other amendments are needed. In addition, the sample obtained with this method can also be used for the detection and measurement of other ions, such as 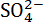 . In conclusion, this grinding method would be helpful in addressing durability issues of cement-based materials, for both research and field work.
. In conclusion, this grinding method would be helpful in addressing durability issues of cement-based materials, for both research and field work.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors appreciate the financial support from the National Basic Research Program of China (973 Program) under the contract No. 2015CB655105, the Natural Science Foundation the contract No. 51308262, and the Natural Science Foundation of Jiangsu Province under the contract No. BK20131012.
Materials
| Cement | Jiangnan Xiaoyetian | P.II. 52.5 | |
| Potassium chromate, 99.7% | Tianjin Kemiou | HG391887 | Toxic |
| Ethyl alcohol | Sinopharm | XK10009257 | |
| Silver nitrate, 99.8% | Sinopharm | 7761888 | Toxic |
| Phenolphthalein, 99.5% | Tianjin Fuchen | XK1301100017 | |
| Concentrated sulfuric acid, 98.3% | Shanghai Lingfeng | XK1301100085008 | Highly corrosive |
| Sodium chloride, 99.7% | Xilong Scientific | XK1320100153 | |
| Diesel oil | China Petroleum | 0# | |
| Epoxy resin | Yifeng Chemical | E44-6101 | |
| Deionized water | Beijing Liyuan | PUW-10N | |
| CNC Milling meachine | Foshan Xiandao Digital Technology | C31E | |
| Cement paste mixer | Wuxi Construction and Engineering | NJ160 | |
| High precision cutting machine | Buehler | 2215 | |
| Mixing spot | Wuxi Construction and Engineering | JJ-5 | |
| Scraper knife | Jinzheng Building Materials | CD-3 | |
| Cling film | Miao Jie | 65300 | |
| Mold (70mm×70mm×70mm) | Jingluda | ABS707 | |
| Plastic box | Fangao Household | 32797 | |
| Stainless steel brace | An Feng | 316L | |
| Paper | Deli | A4 | |
| Oven | Shanghai Huatai | DHG-9070A | |
| Automatic vibrator | Lichen | HY-4 | |
| Vibrating table | Jianyi | GZ-75 | |
| plastic film | Miao Jie | 65303 | |
| Vernier caliper | Links | 601-01 | |
| Electronic balance | Setra | BL-4100F | |
| Plastic bottle | Lining Plastic | 454 | |
| Brush | Huoniu | 3# | |
| Mask | UVEX | 3220 | |
| Gloves | Ammex | TLFGWC | |
| Plastic cup | Maineng | MN4613 | |
| Desiccator | Shenfei | GZ300 | |
| Filter paper | Hangzhou Wohua | 9614051 | |
| Dropper | Huaou | 1630 | |
| Breaker | Huaou | 1101 | |
| Funnel | Huaou | 1504 | |
| Measuring cylinder | Huaou | 1601 | |
| volumetric flash | Huaou | 1621 | |
| Conical flash | Huaou | 1121 | |
| Pipette | Huaou | 1633 | |
| Burette | Huaou | 1462 | |
| Mortar | Huaou | YBMM254 | |
| 80µm sieve | Shanghai Dongxing | KJ-80 | |
| Crucible | Oamay | GYGG | |
| Electric furnace | Tyler | SX-B06 |
References
- Byang, H. O., Jang, S. Y. Effects of material and environmental parameters on chloride penetration profiles in concrete structures. Cem. Concr. Res. 37 (1), 47-53 (2007).
- Mehta, P. K. . Concrete: structure, properties and materials. , 105-169 (1986).
- Khelidj, A., Loukili, A., Bastian, G. Experimental study of the hydro-chemical coupling inside maturing concretes: effect on various types of shrinkage. Mater. Struct. 31 (9), 588-594 (1998).
- Nielsen, E. P., Geiker, M. R. Chloride diffusion in partially saturated cementitious material. Cem. Concr. Res. 33 (1), 133-138 (2003).
- He, F., Shi, C., Yuan, Q., Chen, C., Zheng, K. AgNO3-based colorimetric methods for measurement of chloride penetration in concrete. Constr. Build. Mater. 26 (1), 1-8 (2012).
- Collepardi, M., Turriziani, R., Marcialis, A. Penetration of chloride ions into cement pastes and in concretes. J. Am. Ceram. Soc. 55 (10), 534-535 (1972).
- . . JTJ 270-98. Testing Code of Concrete for Port and Waterwog Engineering. , 202-207 (1998).
- Chang, H., Mu, S., Xie, D., Wang, P. Influence of pore structure and moisture distribution on chloride “maximum phenomenon” in surface layer of specimens exposed to cyclic drying-wetting condition. Constr. Build. Mater. 131 (1), 16-30 (2017).
- Lu, C., Gao, Y., Cui, Z., Liu, R. Experimental Analysis of Chloride Penetration into Concrete Subjected to Drying-Wetting Cycles. J. Mater. Civil. Eng. 27 (12), 1-10 (2015).
- Xu, K. . Properties of Chloride Ions Transportation in Concrete under Different Drying-wetting Cycles. , (2012).
- Zhao, T., Fan, H., Cao, W., Wang, P. Concrete powder grinding machine. China patent. , (2012).

