Partial Optic Nerve Transection in Rats: A Model Established with a New Operative Approach to Assess Secondary Degeneration of Retinal Ganglion Cells
Summary
Secondary degeneration of retinal ganglion cells (RGCs) occurs commonly in glaucoma. This study describes an innovative operative approach for partial optic nerve transection. The use of this space-saving operative approach extends the model’s application range, and allows exploration of secondary injury mechanisms in RGCs in a new way.
Abstract
Previous studies have shown that the secondary degeneration of retinal ganglion cells (RGCs) occurs commonly in glaucoma. Partial optic nerve transection is considered a useful and reproducible model. Compared with other optic nerve injury models used commonly for assessing secondary degeneration, e.g. complete optic nerve transection and optic nerve crush models, the partial optic nerve transection model is superior as it distinguishes primary from secondary degeneration in situ. Therefore, it serves as an excellent tool for evaluating secondary degeneration. This study describes a novel operative approach of partial optic nerve transection by directly accessing the area of the retrobulbar optic nerve through the orbital lateral wall of the eyeball. Moreover, we present a newly designed, low cost surgical instrument to assist with transection. As demonstrated by the representative results in distinguishing the boundary of primary and secondary injury areas, the new approach and instrument ensures high efficiency and stability of the model by providing adequate space for surgical operation. This in turn makes it easy to separate the meningeal sheath and ophthalmic vessels from the optic nerve before transection. An additional benefit is that this space-saving operative approach improves the investigators' ability to administer drugs, carriers, or selective RGC tracers to the stump of the partially transected optic nerve, allowing the exploration of mechanisms behind secondary injury in RGCs, in a new way.
Introduction
Secondary degeneration occurs commonly in the central nervous system (CNS) after traumatic injuries, and following acute and chronic neurodegenerative diseases.1,2,3,4,5 The death of neurons and glial cells as an early consequence of primary pathological events is termed primary degeneration, while secondary degeneration refers to the death of neurons and glial cells, which are not or only partially affected by primary damage. Secondary degeneration of RGCs is also believed to occur in glaucoma.6 Yoles et al.7 confirmed that secondary injury of RGCs occurs in the optic nerve injury model. They showed that neurons whose axons were not injured after an acute damage will eventually degenerate due to degenerative environment produced by the injury surrounding these axons. This secondary degeneration affects the neurons in a progressive fashion related to the severity of the damage inflicted. So far, the mechanisms underlying RGC damage in glaucoma remain unclear, especially those related to secondary injury, which results in insufficient clinical treatment.8,9,10 Therefore, it is necessary to explore the underlying mechanisms of secondary degeneration of RGCs during the development of glaucoma.11 Establishment of animal models of secondary injury that can quantitatively assess the size, distribution, and mechanism of secondary degeneration of RGCs attracts increasing attention from scientists studying secondary injury of RGCs.
To clarify this issue, a rat PONT model was established by Levkovitch-Verbin et al.12 to evaluate axonal injury induced degeneration and death of RGCs. This model is believed to constitute a good tool for exploring the mechanisms of secondary degeneration and identifying the potential neuroprotective agents. The instrument used to generate this model of secondary injury is a diamond knife with a scale to transect quantitatively by setting the depth of abscission through the dial knob, in order to complete a quantitative transection of the optic nerve. The surgical path approaches from the eyeball upward or temporal conjunctiva. During the operative process, the retina and optic nerves might be affected by the force of forceps, which in turn may cause primary injury. More importantly, due to the limited space of the exposed optic nerve, it is difficult to separate the meningeal sheath before incision. Therefore, it is possible to damage the ophthalmic vessels during partial optic nerve transection, which results in retinal ischemia and the failure of the model. In addition, the diamond knife is costly, and each use decreases the tip sharpness. This might in turn affect the depth and effect of modeling.
The model of secondary degeneration of RGCs described in this study was obtained through a new operative approach from the lateral orbital wall of the eyeball. This novel operative approach directly accesses the retrobulbar optic nerve encircled by the orbital muscle cone, avoiding primary injury to the eyeball and the optic nerve when pulling downwards or towards the nasal lateral side of the eyeball. This also increases the space of surgical operation during model establishment, and enables meningeal sheath isolation before partially transecting the optic nerve. It is important to notice that the inadvertent involvement and injury of ophthalmic vessels can lead to the failure of the model. Furthermore, the model enables a follow-up assessment of the transfected cells, drugs and reagents on the stump of the partially transected optic nerve. The self-designed surgical instrument is cheap and can be used multiple times, thereby reducing the cost of modeling. The secondary injury model of RGCs established by this method was shown to have good reproducibility and stability.
Protocol
Procedures involving animal subjects were approved by the institutional animal care and use committee (IACUC) of the Capital Medical University. All surgical instruments and solutions were sterilized prior to surgery to limit microbial infection.
NOTE: The surgical protocol included five parts, namely anesthesia, surgical approach, optic nerve evaluation, closing, and recovery. To assist with partial quantitative transection of the optic nerve, a new, low cost surgical instrument was designed. All procedures were carried out following ethical rules and the surgical technique.
1. Surgical Technique
- Carry out experiments using the aseptic technique; protocols for animal use should be institution specific.
- Sterilize instruments and materials (solutions, test substances, tracers, needles, etc.) coming into contact with living tissues in order to prevent infection and adverse impacts on animal welfare as well as potential negative impacts on the study.
2. Anesthesia
- Anesthetize SD rats using a veterinary isoflurane vaporizer system. Use medical grade oxygen at a rate of 1 L/min to vaporize the isoflurane gas. Place the animal in the attached anesthesia box and run isoflurane at a concentration of 4.5% until slow breathing and animal sedation.
- Switch the gas flow to the gas mask attachment and place the animal on the operation table. Lower the isoflurane concentration to 2% and monitor anesthesia. Larger animals (>300 g) may require a higher concentration of isoflurane. Monitor anesthesia during surgery, with isoflurane dosage adjusted accordingly. Constantly evaluate the depth and rate of breathing, and perform toe pinch evaluation every 5 min, to ensure the absence of deep pain.
- Once the surgery is completed, turn off the isoflurane and allow the animal to breath oxygen for several minutes prior to removal from the operation table. Maintain body temperature by covering the animal with a surgical blanket and/or using a regulated heating blanket during surgery.
3. Self-designed Surgical Assistant Instrument (SSAI)
- Use a SSAI made of stainless steel, and mainly comprises a hand-held pole (length, 100 mm; diameter, 9 mm) and a grooved head (Figure 1). The grooved surface of the grooved head is semi-circular, with vertical depth, width, and length of 200 µm, 500 µm, and 1,000 µm, respectively. Between the two parts, there is a joint section with a length of 50 mm. The grooved head's edge is 300 µm wide.
NOTE: The grooved surface enables the stabilization of the ventral optic nerve for transection. The dorsal optic nerve is exposed to the exterior of the groove when the ventral side of the optic nerve lays within it; meanwhile, the dorsal optic nerve exposed to the grooved edge can be transected when vertical cutting is performed. The ventral optic nerve laid within the grooved surface is protected by the grooved head's edge. - Achieve primary injury in RGCs corresponding to quantitatively transected optic nerve axons (dorsal side), while the secondary injury would be performed in RGCs corresponding to untransected optic nerve axons (ventral side) without direct damage.
4. Surgical Approach
- Place the right side of the rat upwards on the surgical table with head facing the surgeon. Adjust the right orbit in the center of the surgical field of view. Then, clean the incision area several times along the lateral canthus to the external acoustic foramen of the right orbital skin, applying 0.5% chlorhexidine in 75% ethanol. Remove the fur between the lateral canthus to the external acoustic foramen using iris scissors.
- Make a skin incision using iris scissors along the lateral canthus to the external acoustic foramen with a length of 0.5 to 1 cm. Then, pinch the fascia and pull upward to create a triangular wedge with 0.12mm toothed forceps. Insert the lower blade of Vannas spring scissors into the incision area and cut open the fascia in the same shear direction.
- Cut the fascia with Vannas spring scissors and expose the orbital vein. Use sharp-serrated forceps to clamp the sides of the orbital vein and blunt open both sides of the incision. Place the orbital vein in the surgical direction of the orbital muscle surface, which is positioned to facilitate follow-up operation and to avoid surgery related bleeding of the orbital vein.
- Use iris scissors to cut the right lateral canthus apart along the incision line to fully expose the field of vision for blunt dissection of orbital muscles during follow up.
- Continue to clamp the folder of the subfascial orbital muscle, and blunt separate vertically in the direction of skin and fascia incision. Separate along sides gradually to reach the orbital depth, until appearance of the orbital adipose tissue.
- After exposure of the orbital adipose tissue, change the rat head direction from facing the surgeon vertically to the right side of the surgeon. Meanwhile, keep the area constantly moist using surgical or cotton swabs containing sterile PBS. This procedure ensures a clear vision of the surgical field, while keeping the tissues moist and soft.
5. Accessing the Optic Nerve
- Cut off orbital adipose tissues covering the orbital muscle cone around the optic nerve in the orbital cavity. This ensures a better exposure of the appropriate surgical approach. Keep resection of the fat tissue limited to avoid sustained bleeding.
- Cut the fat tissue, exposing the lateral rectus. Clamp the lateral rectus outward, and then cut it with Vannas spring scissors. If the fat tissue remains under the lateral rectus, pull up the fat overlying the optic nerve using 0.12 mm toothed forceps and cut it with Vannas spring scissors.
NOTE: At this time, the tissue sheath around the optical nerve should be visible. - Continue to separate the tissue sheath along the direction of the optic nerve in the orbital depth, until total exposure of the optic nerve. Keep the area clean by using surgical swabs to clean small amounts of blood that arise from tissue removal.
NOTE: Now, the optic nerve should be visible. - In order to access it, remove the meningeal sheath that surrounds the nerve without damaging the ophthalmic artery. Gently rotate the sheath to examine the vascular pattern of the dura at high magnification under an operating microscope.
- Identify an area devoid of blood vessels, and perform a longitudinal cut on the dura.13 Rip the sheath parallel to the direction of the optic nerve with a 26G needle tip or knifepoint of sapphire surgical probe carefully, avoiding damage to the vasculature with lateral cuts.
NOTE: The only leftover covering the nerve should be the arachnoid membrane, which is very thin and transparent.
- Identify an area devoid of blood vessels, and perform a longitudinal cut on the dura.13 Rip the sheath parallel to the direction of the optic nerve with a 26G needle tip or knifepoint of sapphire surgical probe carefully, avoiding damage to the vasculature with lateral cuts.
- Similar to step 5.2, rip the arachnoid membrane gently with a 26G needle tip or knifepoint of sapphire surgical probe, parallel to the direction of the optic nerve.13
- Lay the optic nerve within the instrument groove softly and carefully, resulting in the dorsal optic nerve being slightly higher than the grooved head edge. At this time, transect the dorsal optic nerve above the edge of the grooved head platform with a 26G needle tip or knifepoint of sapphire surgical probe to complete partial optic nerve transection.
6. Closing and Recovery
- Move the instrument a little deeper towards the vertical direction of the optic nerve to free the latter. Then, remove the grooved head of the instrument gently. Try not to scratch the ocular muscles or other tissues to avoid extra damage. The stump of partial optic nerve transection can be observed.
- Replace the lateral rectus, fascia and other surrounding tissues of the eye to their original positions. Then, suture the muscle and the skin layers of the orbit in sequence. If bleeding persists, gently fill with a medical cotton ball before closing the wound, and maintain this for a period of time. Apply antibiotic ointment to the wound to prevent infection.
- Turn off the isoflurane source and allow the animal to breath oxygen for several minutes. In the process of rat resuscitation, prepare thermal insulation with a heated mat, or cover the cage surface with dry padding. Cover the animals with blankets, to ensure rat airway patency during the recovery process.
- House animals individually after surgery. Administer post-surgical analgesics according to the guidelines provided by institutional animal care authorities. Carefully monitor animals after surgery.
Representative Results
To verify success regarding the establishment of a secondary injury model with the new operative approach using SSAI (Figure 2A), RGCs were retrogradely labeled immediately after model establishment. The purpose of this procedure was to label RGCs retrogradely by injecting a neural tracer dye (3% fluorophore (e.g., Fluorogold) in sterile phosphate buffer saline) into the superior colliculus (Figure 2B). This approach yields reproducible labeling of viable RGCs with little variation.14,15,16,17,18 The dye will be retrogradely taken up by RGCs in the retina and constitutes a marker for live RGCs, with the axons not transected in the right eye. Meanwhile, RGCs corresponding to the partially transected optic nerve axons in the right eye could not be labeled with tracer dye (Figure 2C). As a control eye, the left eye without operation, RGCs along the optic nerve of the retina were all labeled with the fluorescent gold dye in a retrograde manner from the superior colliculus (Figure 2D).
Seven days after partial optic nerve transection and retrograde labeling of RGCs, the retinas were harvested, fixed, flattened, and mounted. The labeled RGCs were imaged under a fluorescent microscope in defined regions of the retina. The results of fluorescence-labeled RGCs with or without partial optic nerve transection are shown in Figure 3. Only the RGCs in the right retina corresponding to the untransected portion of the optic nerve were labeled with fluorescent gold, and a clear boundary of unlabeled and labeled RGCs could be visualized (Figure 3A, Figure 3B), demonstrating the partial transaction of the optic nerve. As a control eye, all RGCs of the left eye retina showed fluorescence (Figure 3C, Figure 3D).
To assess whether the vasculature around the optic nerve head and the ophthalmic artery that supplies blood to the entoretina were injured and affected during the operation, the right eye fundus was imaged before and after surgery. The images showed blood supply to the right eye (operative eye) before and 1 hour after operation. Blood in the arteries was adequate. No obstruction of the vein was observed. These findings indicated that there was no damage to the blood supply system during the operation (Figure 4A, Figure 4B). Therefore, the secondary degeneration model of RGCs was successfully established.
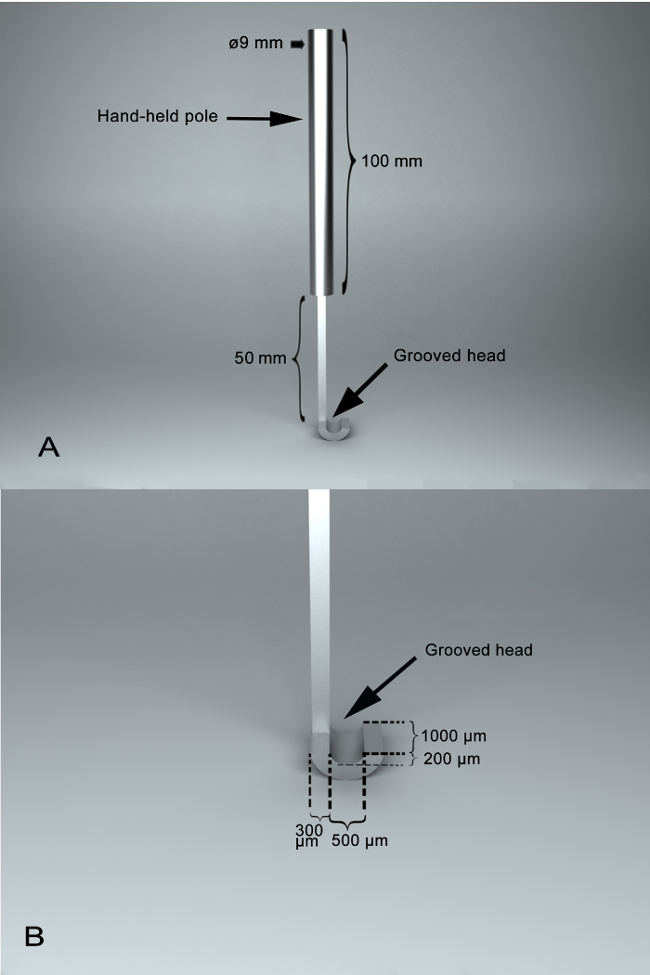
Figure 1: Photographs of self-designed surgical assistant instrument, SSAI. (A) A panoramic view of the surgical instrument,with two main parts, including a hand-held pole and a grooved head. Between them, there is a joint section with a length of 50 mm. The length of the hand-held pole is 100 mm, and the diameter of the hand-held pole is 9 mm. (B) A feature of the SSAI groove. The grooved surface of the grooved head is semi-circular, which enables the optic nerve to lay within it, being stabilized for transection. The grooved surface is with a vertical depth of 200 µm and a width of 500 µm and a length of 1,000 µm. The width of edge of the grooved head is 300 µm. Please click here to view a larger version of this figure.
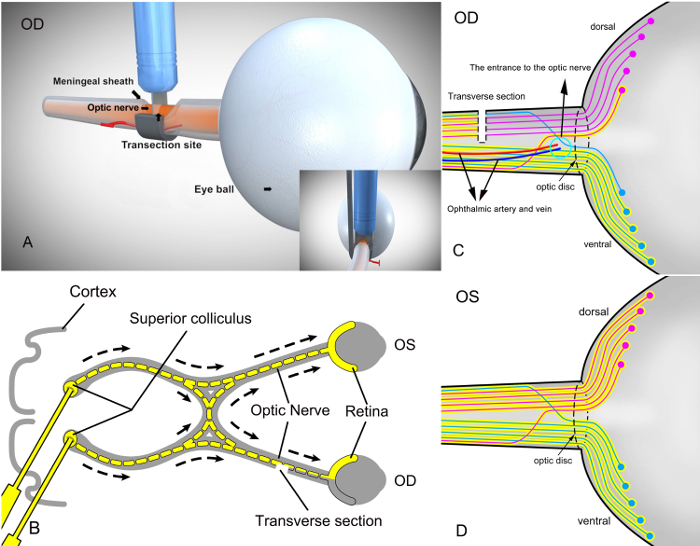
Figure 2: Schematic diagram of RGC labeling on the retina after partial transection of the right optic nerve with the self-designed surgical assistant instrument (SSAI) and retrogradely labeling the superior colliculus with the fluorophore. (A) The surgical view of partial right optic nerve transection in rats with the self-designed surgical assistant instrument (SSAI). (B) After modeling, RGCs were labeled retrogradely by injecting a neural tracer dye (yellow color, 3% fluorophore in sterile phosphate buffer saline) into the superior colliculus in the brain. Since the axons of RGCs reside in the superior colliculus, the tracer dye is taken up by RGCs retrogradely and constitutes a marker for live cells. The transverse section in the figure represents a cross-section of the optic nerve. OD, operated eye; OS, control eye without operation. In (C), only the RGCs corresponding to the untransected portion of the optic nerve were labeled with fluorophore. Blue represents unseparated ventral optic nerves and the corresponding RGCs on the retina; red reflects partially transected dorsal optic nerves and the corresponding RGCs on the retina. (D) The retinal RGCs of the left eye (control eye) without the surgical procedure were all labeled by the dye. Dorsal and ventral optic nerves were all labeled by the dye as well. Please click here to view a larger version of this figure.
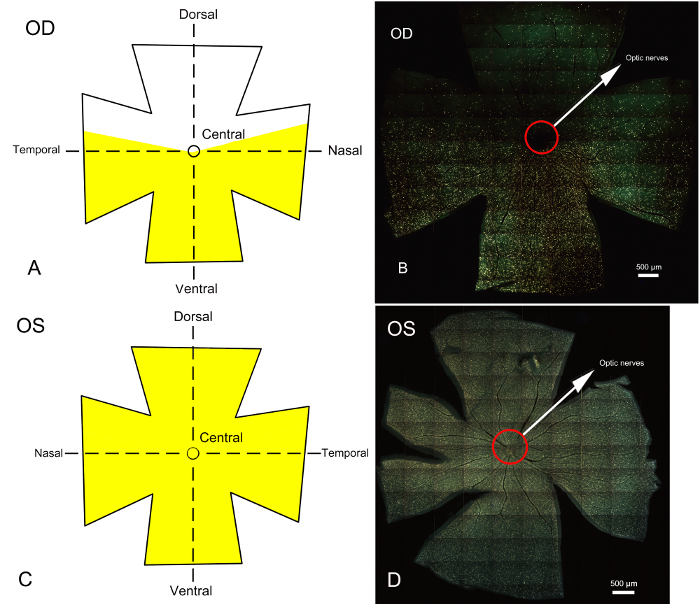
Figure 3: Whole retina epifluorescence micrographs of fluorophore labeled RGCs 7 days after establishing the partial optic nerve transection model and retrograde labeling of the superior colliculus. The corresponding schematic diagrams of Fluorogold stained RGC areas on the retina are also presented. (A) and (C) represent the schematic diagrams of RGCs in the retina of the right (operative) and left (control) eyes after labeling with fluorophore, respectively. Yellow indicates the area labeled with the fluorescent gold dye. The retina is divided into dorsal, ventral and central parts. (B) and (D) represent whole retinal epifluorescence micrographs obtained under a fluorescence microscope; yellow represents the area of RGCs labeled with fluorophore. In the surgical eye (right eye) shown in B, the unlabeled region represents the area of RGCs corresponding to the optic nerve which is partially transected, mainly on the dorsal side of the retina. The region labeled by the fluorescent gold dye is the area of RGCs corresponding to the optic nerve which is not transected and mainly concentrated in the central and ventral sides of the retina. The boundary between the areas of unlabeled and labeled RGCs is clear. Primary degeneration of RGC bodies would be limited to the dorsal retina, and all loss of RGC bodies in the central and ventral retinas could be attributed to secondary degeneration. (D) Whole retinal photomicrograph of the left eye after labeling of RGCs with fluorophore. The RGCs of the left control eye without surgical procedure were totally stained by fluorophore. Scale bars = 500 µm. Please click here to view a larger version of this figure.
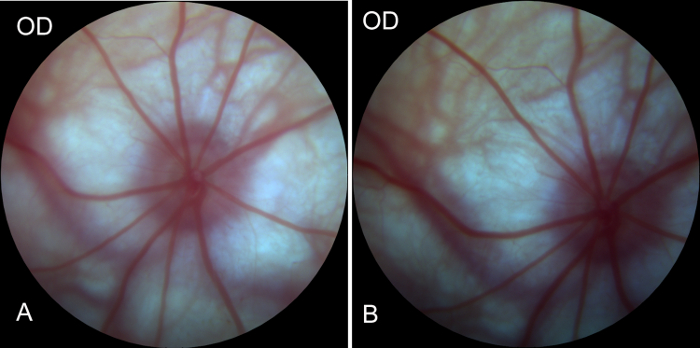
Figure 4: Preoperative and postoperative images of the fundus of the right eye obtained by Fundus camera. (A) Image of the fundus before right eye surgery in rats, showing good blood supply of the fundus, arterial filling, and no venous return or obstruction, indicating a good retinal blood supply system. (B) Image of the fundus of the right eye 1 hour after surgery. Compared with the preoperative image of the fundus, no significant changes were observed in retinal blood supply, indicating that the blood supply system of the eyeball was not affected during the process of modeling. Please click here to view a larger version of this figure.
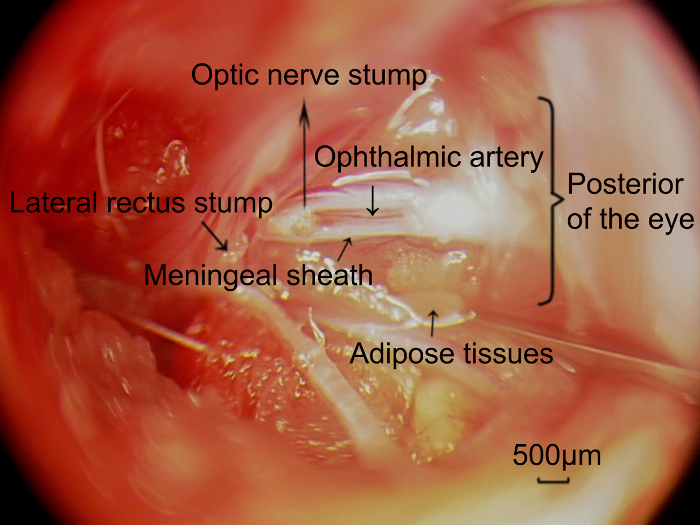
Figure 5: Photographs of the retrobulbar optic nerve and the ophthalmic artery located on the meningeal sheath, captured through the surgical approach. After completely removing the targeted length of the optic nerve, the ophthalmic artery (arrowhead) concomitant with the meningeal sheath of the optic nerve was exposed, and parallel to the optic nerve. Scale bars = 500 µm. Please click here to view a larger version of this figure.
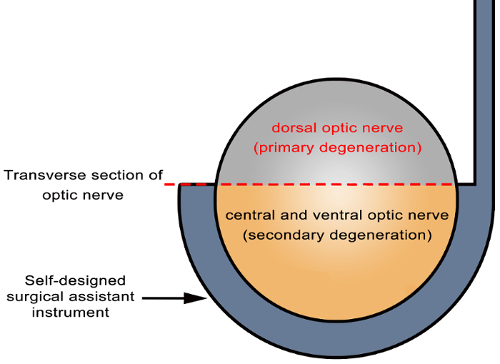
Figure 6: Schematic diagram of primary and secondary degeneration locations in the optic nerve. Partial incision of the optic nerve was achieved using the self-designed surgical assistant instrument (arrowhead). The axons in directly damaged sites (dorsal cutting site of the optic nerve in the transverse section in gray) undergo primary degeneration, while those in indirectly damaged sites (central and ventral areas of the optic nerve in the transverse section in yellow) undergo secondary degeneration. Please click here to view a larger version of this figure.
Discussion
Operational Procedure
There are some points worthy of notice in the process of model construction. In step 4.2, the surgical movement should be performed carefully to avoid damaging the vasculature above the subfascial muscle. Especially, when cutting the subcutaneous fascia in the outer lateral canthus, sharp-serrated forceps should be used to pull upward the subcutaneous fascia on the fascia surface vertically; the fascia should be cut with Vannas spring scissors to avoid damage to the orbital vein at the outer canthus, which can result in model failure by excessive bleeding. Step 4.3 has the advantage of potentially preventing bleeding when directly removing from blood vessels. In separating orbital muscles in step 4.5, the reason for picking sharp-serrated forceps but not Vannas spring scissors is to avoid continuous bleeding and hemorrhage. The muscles are bluntly separated on both sides in the perpendicular direction to the skin fascia incision; meanwhile, the deep muscles of the orbit are stretched outward and peripherally. This procedure will reveal deeper portions of the orbital cavity, providing a larger surgical window and allowing unimpeded access to tissues overlaying the optic nerve. In the above procedures, if bleeding occurs, pressure should be applied using sterile surgical or cotton swabs. Minor bleeding will stop after several seconds by this procedure. The purpose of step 4.6 is to facilitate follow-up operations to easily remove some fat and separate muscles in the orbit muscle cone to expose the optic nerve along the direction of the optic nerve in the orbital depth.
The most critical parts of the current protocol are Steps 5.1-5.6. It is important not to damage the vasculature around the optic nerve head. The optic nerve should be partially transected at least 1.5-2.0 mm from the back of the eye, to avoid any damage to the ophthalmic artery that penetrates the nerve within 1 mm of the eye and supplies blood to the inner retina. The purpose of cutting the lateral rectus is to achieve better exposure of the optic nerve as the lateral rectus is wide and obviously blocks the view of the optic nerve. Meanwhile, to avoid removing the ophthalmic artery that is associated with the meningeal sheath (Figure 5),it is necessary to separate and dissociate the dura around the optic nerve and examine the vascular pattern of the meningeal sheath, using forceps to gently rotate the sheath. In addition, an area devoid of blood vessels should be identified, allowing a longitudinal cut in the meningeal sheath. It is also necessary to maintain a small working distance from the back of the eye, to avoid the portion of the dura that is closely associated with the ophthalmic artery. The retina is normally transparent, and blood vessels can be clearly demarcated. In case of damaged retinal blood supply, the retina is degenerated, leading to a milky-white flocculent appearance. The vitreous chamber of the eye and the lens will typically become cloudy as well, with decreased eye size over time. In this study, preoperative and postoperative images of the fundus confirmed no damage to the fundus blood supply in the model after applying the above steps.
Furthermore, special care is required in several steps of this model. When using sharp-curved-serrated forceps or other surgical instruments to expose the optic nerve, the surgeon should avoid excessive force, as it may damage the optic nerve, eyeball or ophthalmic artery, resulting in primary injury and retinal ischemia. In addition, blood vessels surrounding the eye should not be damaged, to avoid sustained bleeding, which could lead to the failure of modeling. The SSAI used in this experiment requires delicate usage. When the optic nerve is placed within the instrument groove, the optic nerve and grooved surface need to be tightly fitted to ensure good consistency and repeatability of each animal model. With practice, the full surgical procedure can be completed within 15-20 minutes per eye, after the initial entry cuts have been made.
Wang et al.19 published a similar animal model of partial optic nerve transection established using an optic nerve quantitative amputator. The surgical procedures includes: 1) Cutting apart the outer canthus, suspending and fixing the palpebral superior; 2) Exploring the optic nerve, and transecting the superior portion of the optic nerve using the amputator; and 3) Suturing the conjunctiva and skin. Although the surgical procedure was relatively simple, the following problems were encountered during the operation. Although lateral canthus incision could expose certain space for operation, there was an inevitable need to constantly stretch the eyeball in order to expose the retrobulbar optic nerve sheath, especially when the surgeons desired to expose a longer retrobulbar optic nerve sheath to facilitate further sheath isolation; the force for stretching the eyeball was greater, which is likely to cause direct traction injury of the eyeball and optic nerve. No special attention was paid to blood vessels that might be cut together with the optic nerve sheath, and damage to blood vessels is likely to lead to failed model establishment. The principal procedures of secondary injury model described in this paper are: a new operative approach from the lateral orbital wall of the eyeball to directly access the retrobulbar optic nerve surrounded by the orbital muscle cone, avoiding primary injury to the eyeball and optic nerve, when pulling downwards or towards the nasal lateral side of the eyeball. This new operative approach increases the space of surgical operation during modeling, and enables easy isolation of the meningeal sheath, which is closely associated with the ophthalmic artery, before partial transection of the optic nerve. Partial optic nerve transection was performed with a self-designed surgical instrument, which is cost effective and reusable, reducing the overall cost of modeling. The rat's orbital structure is different from those of other mammals, with the orbit closest to the canthus and no bony structure, but covered with muscles. The surgical approach could reach the posterior part of the eyeball without the need of destroying the orbital bone and periosteum. Through strict preoperative disinfection and postoperative antibiotic prophylaxis, infection, inflammation and edema were greatly reduced.
Self-designed Surgical Assistant Instrument
The rat model of partial optic nerve transection was established using the self-designed surgical assistant instrument, whose main features are as follows. It can assist in partial quantitative transection of the optic nerve exposed to the grooved edge, also ensuring transection consistency among different animals. We tested and verified the repeatability of model establishment with SSAI. The maximum coefficient of variation was 1.85%, with an average value 0.67%±0.44%.20 These results indicate that the SSAI could be used to establish partial optic nerve transection models, with satisfactory reproducibility and uniformity.
The grooved surface's width and the design of the semi-circle of the groove's inner surface can have a more fixed effect on the optic nerve and make the grooved surface and optic nerve attach more tightly, also decreasing experimental errors and adverse reactions. The grooved edge allows a better protection of the optic nerve in the groove during the operation, which will not damage the optic nerve in the groove, regardless of the cutter's sharpness. Another advantage of the grooved edge is crush injury prevention during optic nerve transection.
It is appropriate for operating in deep and narrow space. Although the new operative approach has been expanded, the pathway remains deep, and the hand-held pole and joint section can be used to place the grooved head easily under the optic nerve sheath to perform follow-up operations. When the instrument is used for operation, a wide range of cutters can be used for transection, e.g. a 26 G needle tip. Even a sapphire surgical probe knife can be selected to avoid contusion and crush injuries caused by scissors. Groove surfaces can be made in different vertical depths to complete varying degrees of optic nerve cutting.
Compared with the amputator of Wang et al. the SSAI has a simpler structure. In addition, the cutting step is more convenient using the SSAI, with improved consistency and repeatability of the animal model. Finally, the range of tools applicable for cutting with the SSAI is also wider. In conclusion, SSAI, which makes quantitative and uniform incisions of the nerve, may serve as an effective instrument for establishing rat models for assessing optic nerve transection.
Characteristics of the Rat Partial Optic Nerve Transection Model
The partial optic nerve transection model is useful to assess secondary degeneration in RGCs. The potential merit of this model is the ability to separate primary from secondary degeneration accurately in situ, both in the optic nerve and retina. The central and ventral optic nerves were more susceptible to secondary injury after partial transection (about 1/3 to 1/2) of the dorsal optic nerve (Figure 6). In the retina, the regional location of primary and secondary injuries of RGCs should be based on the topography of the optic nerve corresponding to retinal RGCs after partial transection. If the entire retina of the rat is divided into dorsal (superior) and ventral (inferior) parts, secondary and primary injuries are present in both parts. However, based on the relationship between RGCs on the retina and the optic nerve axon, RGC death in the ventral retina should be mainly attributed to secondary injury (Figure 3).12,22,23 The advantages of this model include: simple and easy-to-operate instrument with standard procedures; no effect on ophthalmic vessels; good reproducibility and stability. This technique might be used to transfect RGCs from this space-saving operative approach by applying short interfering RNAs (siRNAs), plasmids, and viral vectors to the partial optic nerve stump; in addition, reagents could be placed on the partial optic nerve stump for the selective treatment or labeling of RGCs.
Overall, primary and secondary injuries of RGCs coexisted after partial optic nerve transection in this animal model, with a clear boundary in the retina between the two injury types. Although the association of optic nerve axons and RGC location on the retina needs further investigation for a more precise distinction, this space-saving operative approach extends the application range of the model and allows researchers to explore the mechanisms of secondary injury in RGCs in a new way.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Beijing Natural Science Foundation (7152038), the Fundamental Research Funds for the Central Universities of Central South University (2016zzts162) and the Science Research Foundation of Aier Eye Hospital Group (Grant No. AF156D11). Finally, Fancheng Yan thanks Yiping Xu for the invaluable support over the years.
Materials
| Animal Aneathesia Ventilator System | MIDMARK | Matrx VMR | |
| Isoflurane | RWD Life Science Co. | R510-22 | |
| Surgical Microscope | Leica AG, Heerbrugg, Switzerland | M620 F20 | |
| Tobramycin Eye ointment | Alcon | H20110312 | |
| Fluorogold | Biotium | 80014 | |
| Iris scissors | 66vision Co. | 54026 | |
| Vannas spring scissor | 66vision Co. | 54137B | |
| Sharp-serrated forceps/0.12mm toothed forceps | 66vision Co. | 53329A | |
| Sharp-curved forceps | 66vision Co. | 53324A | |
| Sapphire surgical probe | 66vision Co. | 50205TA | |
| 26G needle tip | Shandong Weigao Group Medical Polymer Co. | 3151474 | |
| 10 μl Hamilton Syringe | Hamilton Co. | 80030 | |
| 5-0 non-absorbable suture | Johnson & Johnson International Co. | W580 | |
| Chlorhexidine | Sigma-Aldrich | 282227 | |
| Stereotaxie apparatus | RWD Life Science Co. | 68026 | |
| Retinal Imaging System | OptoProbe Ltd. | OPTO-RIS | |
| RetCamII wide field imaging system | Clarity Medical Systems,Inc. | RetCamII | |
| Fluorescence microscope | Leica Microsystems Inc. | DM6000 |
References
- Stoica, B. A., Faden, A. I. Cell death mechanisms and modulation in traumatic brain injury. Neurotherapeutics. 7 (1), 3-12 (2010).
- Hausmann, O. N. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 41 (7), 369-378 (2003).
- Oyinbo, C. A. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp (Wars). 71 (2), 281-299 (2011).
- Guimaraes, J. S., et al. Mechanisms of secondary degeneration in the central nervous system during acute neural disorders and white matter damage. Rev Neurol. 48 (6), 304-310 (2009).
- Stewart, S. S., Appel, S. H. Trophic factors in neurologic disease. Annu Rev Med. 39, 193-201 (1988).
- Brubaker, R. F. Delayed functional loss in glaucoma. LII Edward Jackson Memorial Lecture. Am J Ophthalmol. 121 (5), 473-483 (1996).
- Yoles, E., Schwartz, M. Degeneration of spared axons following partial white matter lesion: implications for optic nerve neuropathies. Exp Neurol. 153 (1), 1-7 (1998).
- Nickells, R. W. From ocular hypertension to ganglion cell death: a theoretical sequence of events leading to glaucoma. Can J Ophthalmol. 42 (2), 278-287 (2007).
- Doucette, L. P., Rasnitsyn, A., Seifi, M., Walter, M. A. The interactions of genes, age, and environment in glaucoma pathogenesis. Surv Ophthalmol. 60 (4), 310-326 (2015).
- Osborne, N. N., Melena, J., Chidlow, G., Wood, J. P. A hypothesis to explain ganglion cell death caused by vascular insults at the optic nerve head: possible implication for the treatment of glaucoma. Br J Ophthalmol. 85 (10), 1252-1259 (2001).
- Rokicki, W., Dorecka, M., Romaniuk, W. Retinal ganglion cells death in glaucoma–mechanism and potential treatment. Part II. Klin Oczna. 109 (7-9), 353-355 (2007).
- Levkovitch-Verbin, H., et al. A model to study differences between primary and secondary degeneration of retinal ganglion cells in rats by partial optic nerve transection. Invest Ophthalmol Vis Sci. 44 (8), 3388-3393 (2003).
- Magharious, M. M., D’Onofrio, P. M., Koeberle, P. D. Optic nerve transection: a model of adult neuron apoptosis in the central nervous system. J Vis Exp. (51), (2011).
- Yoles, E., et al. GM1 reduces injury-induced metabolic deficits and degeneration in the rat optic nerve. Invest Ophthalmol Vis Sci. 33 (13), 3586-3591 (1992).
- Fisher, J., et al. Vaccination for neuroprotection in the mouse optic nerve: implications for optic neuropathies. J Neurosci. 21 (1), 136-142 (2001).
- Levkovitch-Verbin, H., et al. RGC death in mice after optic nerve crush injury: oxidative stress and neuroprotection. Invest Ophthalmol Vis Sci. 41 (13), 4169-4174 (2000).
- Li, Y., et al. VEGF-B inhibits apoptosis via VEGFR-1-mediated suppression of the expression of BH3-only protein genes in mice and rats. J Clin Invest. 118 (3), 913-923 (2008).
- Tang, Z., et al. Survival effect of PDGF-CC rescues neurons from apoptosis in both brain and retina by regulating GSK3beta phosphorylation. J Exp Med. 207 (4), 867-880 (2010).
- Wang, X., Li, Y., He, Y., Liang, H. S., Liu, E. Z. A novel animal model of partial optic nerve transection established using an optic nerve quantitative amputator. PLoS One. 7 (9), e44360 (2012).
- Yan, F. C., Li, S. N., Liu, K. G., Lu, Q. J., Wang, N. L. The establishment of a rat partial optic nerve transection model and assessment of its reproducibility. Ophthalmology in China. 22 (1), 34-37 (2013).
- Fitzgerald, M., et al. Secondary degeneration of the optic nerve following partial transection: the benefits of lomerizine. Exp Neurol. 216 (1), 219-230 (2009).
- Fitzgerald, M., et al. Near infrared light reduces oxidative stress and preserves function in CNS tissue vulnerable to secondary degeneration following partial transection of the optic nerve. J Neurotrauma. 27 (11), 2107-2119 (2010).
- Li, H., et al. Lycium barbarum (wolfberry) reduces secondary degeneration and oxidative stress, and inhibits JNK pathway in retina after partial optic nerve transection. PLoS One. 8 (7), 68881 (2013).

