Visualization of Cellular Electrical Activity in Zebrafish Early Embryos and Tumors
Summary
Here, we show the process of creating a cellular electric voltage reporter zebrafish line to visualize embryonic development, movement, and fish tumor cells in vivo.
Abstract
Bioelectricity, endogenous electrical signaling mediated by ion channels and pumps located on the cell membrane, plays important roles in signaling processes of excitable neuronal and muscular cells and many other biological processes, such as embryonic developmental patterning. However, there is a need for in vivo electrical activity monitoring in vertebrate embryogenesis. The advances of genetically encoded fluorescent voltage indicators (GEVIs) have made it possible to provide a solution for this challenge. Here, we describe how to create a transgenic voltage indicator zebrafish using the established voltage indicator, ASAP1 (Accelerated Sensor of Action Potentials 1), as an example. The Tol2 kit and a ubiquitous zebrafish promoter, ubi, were chosen in this study. We also explain the processes of Gateway site-specific cloning, Tol2 transposon-based zebrafish transgenesis, and the imaging process for early-stage fish embryos and fish tumors using regular epifluorescent microscopes. Using this fish line, we found that there are cellular electric voltage changes during zebrafish embryogenesis, and fish larval movement. Furthermore, it was observed that in a few zebrafish malignant peripheral nerve sheath tumors, the tumor cells were generally polarized compared to the surrounding normal tissues.
Introduction
Bioelectricity refers to endogenous electrical signaling mediated by ion channels and pumps located on the cell membrane1. Ionic exchanges across the cellular membrane, and the coupled electrical potential and current changes, are essential for signaling processes of excitable neuronal and muscular cells. In addition, bioelectricity and ion gradients have a variety of other important biological functions including energy storage, biosynthesis, and metabolite transportation. Bioelectrical signaling was also discovered as a regulator of embryonic pattern formation, such as body axes, the cell cycle, and cell differentiation1. Thus, it is critical for understanding many human congenital diseases that result from the mis-regulation of this type of signaling. Although patch clamp has been widely used for recording single cells, it is still far from ideal for the simultaneous monitoring of multiple cells during embryonic development in vivo. Furthermore, voltage sensitive small molecules are also not ideal for in vivo applications due to their specificities, sensitivities, and toxicities.
The creation of a variety of genetically encoded fluorescent voltage indicators (GEVIs) offers a new mechanism to overcome this issue, and allows for easy application to study embryonic development, even though they were originally intended for monitoring neural cells2,3. One of the currently available GEVIs is the Accelerated Sensor of Action Potentials 1 (ASAP1)4. It is composed of an extracellular loop of a voltage-sensing domain of voltage sensitive phosphatase and a circularly permuted green fluorescent protein. Therefore, ASAP1 allows visualization of cellular electric potential changes (polarization: bright green; depolarization: dark green). ASAP1 has 2 ms on-and-off kinetics, and can track subthreshold potential change4. Thus, this genetic tool allows for a new level of efficacy in real-time bioelectric monitoring in live cells. Further understanding of the roles of bioelectricity in embryonic development and many human diseases, such as cancer, will shed new light on the underlying mechanisms, which is critical for disease treatment and prevention.
Zebrafish have been proven a powerful animal model to study developmental biology and human diseases including cancer5,6. They share 70% orthologous genes with humans, and they have similar vertebrate biology7. Zebrafish provide relatively easy care, a large clutch size of eggs, tractable genetics, easy transgenesis, and transparent external embryonic development, which make them a superior system for in vivo imaging5,6. With a large source of mutant fish lines already present and a fully sequenced genome, zebrafish will provide a relatively unlimited range of scientific discovery.
To investigate the in vivo real-time electrical activity of cells, we take advantage of the zebrafish model system and ASAP1. In this paper, we describe how to incorporate the fluorescent voltage biosensor ASAP1 into the zebrafish genome using Tol2 transposon transgenesis, and visualize cellular electrical activity during embryonic development, fish larval movement, and in live tumor.
Protocol
The zebrafish are housed in an AAALAC-approved animal facility, and all experiments were carried out according to the protocols approved by the Purdue Animal Care and Use Committee (PACUC).
1. Tol2 Transposon Plasmid Construct Preparation
NOTE: Tol2, a transposon that was discovered in medaka fish, has widely been used in the zebrafish research community8,9. It has been successfully adopted to the Gateway site-specific recombination-based cloning system and known as the Tol2 kit10. The Tol2 kit allows for a more convenient way of creating customized expression constructs, while also increasing the efficiency of transgenesis. Thus, it was an easy decision to take advantage of this system, and create a ubiquitous ASAP1 expression zebrafish line using a validated ubiquitin promoter to drive ASAP111.
- Creating a middle entry ASAP1 construct: pDONR221-ASAP1
- Acquire the genetically encoded voltage sensor ASAP1 construct, pcDNA3.1/Puro-CAG-ASAP1 (Plasmid#52519), from Addgene. To amplify the ASAP1 coding region, set up a PCR using the customized primers (attB1-ASAP1F and attB2-ASAP1R) flanked with attB sequences at the 5' end of the primers (Figure 1). Phusion DNA polymerase was chosen for its high efficiency, and PCR conditions were optimized based on the previously published protocol12.
- Load the 50 µL PCR products into a 1% TAE gel using a regular pipette with 200 µL tips, and perform electrophoresis at 160 V in a horizontal gel tank for about 30 min.
- Check the gel under a UV transilluminator (353 nm), excise out the desired band using a blade/scalpel under a UV transilluminator as previously published13,14, and put the DNA containing gel sample into a clean 1.5 mL microcentrifuge tube.
- Perform gel purification for the ASAP1 PCR products. Recover the DNA in the excised gel using a commercial DNA gel purification kit following manufacturer's instruction, and elute the DNA into 20 µL of water. Take 1 µL as a sample, and measure the DNA concentration using a spectrophotometer. Flow the software instructions using water as a blank control15.
- Take 100 ng of purified PCR product and mix it with 150 ng of pDONR221 plasmid in 10 µL of TE buffer (10 mM Tris, 1 mM EDTA pH 8.0)16. Add 2 µL of BP Clonase II into the reaction and incubate the reaction at room temperature overnight.
- On the second day, add 1 µL of proteinase K into the reaction and incubate the reaction at 37 °C for 30 min.
- Perform transformation. Transfer the reaction and mix it with 50 µL of Top10 competent E. coli cells and incubate the cells on ice for 30 min. Then, transfer the reaction tube into a 42 °C water bath for 1 min. Immediately remove the tube and incubate on ice for 2 min. Next, put the tube into a 37 °C shaker and incubate it for 1 h.
- Take the tube out and plate the cells onto a kanamycin LB plate. Next, incubate the plate overnight (16-18 h) at 37 °C.
- Pick single and well-separated colonies and inoculate them into 14 mL cell culture tubes with 3 mL of LB medium. Culture them overnight at 37 °C in a shaker with a rotation speed of 250 rpm (rotation per minute).
- Perform miniprep using a commercial miniprep kit following its instruction manual17.
- Sequence 3-4 plasmids with Sanger sequencing to identify positive pDONR221-ASAP1 clones using M13F and M13R sequencing primers.
- Creating the Tol2 construct for microinjection: pDestTol2-ubi-ASAP1
- Choose the sequencing verified pDONR221-ASAP1 clone and measure its DNA concentration using a spectrophotometer following the software instruction using water as a blank control15.
- Take 100 µg of pDONR221-ASAP1 and mix it with 100 µg of Tol2 kit 5-end plasmid (pENTR5'_ubi, Addgene #27320), 100 µg of p3E-polyA (Tol2 kit #302) and 150 µg of pDestTol2pA2 (Tol2 kit #394). Adjust total volume to 8 µL using TE buffer (10 mM Tris, 1 mM EDTA, pH 8.0) in a 1.5 mL microcentrifuge tube and mix well by a 2-5 s brief vortex. Then, add 2 µL of LR Clonase II plus, and incubate the reaction at room temperature overnight.
- On the second day, add 1 µL of proteinase K into the reaction using a 10 µL pipette, and incubate the reaction at 37 °C for 30 min.
- Perform transformation and identify positive clones as described above (steps 1.1.7-11).
- Measure the DNA concentration of sequencing verified pDestTol2-ubi-ASAP1 clone (Figure 1B) using a spectrophotometer15. Usually the concentration is around 200ng/µL.
2. Prepare Tol2 Transposase mRNA and Injection Solution
- Streak E. coli glycerol stock of pCS2FA-transposase plasmid (Tol2 kit #396) onto a LB plate (with 100 µg/mL ampicillin) using a sterilized inoculation loop. Incubate the plate at 37 °C in an incubator overnight. The next day, pick a single colony and inoculate it into 3 mL of LB (100 µg/mL ampicillin) using a sterilized 10 µL pipette tip. Culture it overnight at 37 °C in a shaker with a rotation speed of 250 rpm.
- Perform miniprep on the E. coli culture using a commercial miniprep kit following its instruction manual. Elute plasmid DNA into 30-50 µL of TE buffer by 1-minute centrifuge at 14,000 rpm, and measure its DNA concentration with a spectrophotometer.
- Linearize 1-2 µg of plasmid with Not I endonuclease and purify the DNA with a DNA cleaning kit following its instruction manual after Not I digestion. Elute the DNA into 5 µL of water by centrifuging at 14,000 rpm for 1 minute. The expected concentration is about 200-300 ng/µL.
- Perform in vitro transcription with Not I linearized pCS2FA-transposase as a DNA template using a commercial SP6 transcription kit.
- Once the reaction is finished, purify Tol2 transposase mRNA using a commercial RNA cleaning kit following the manufacturer's instructions. Finally, elute mRNA into 20 µL RNAase-free water and measure the RNA concentration in a spectrophotometer. The expected concentration is about 1-3 µg/µL. Samples can be stored in a -80 °C freezer if needed.
- Prepare the microinjection solution by mixing 20 ng/µL pDestTol2-ubi-ASAP1 and Tol2 transposase mRNA (100 ng/µL) in a microcentrifuge tube by pipetting. To prevent nucleic acid degradation caused by repeated thawing and refreezing, aliquot 6 µL per tube and store it in a -80 °C freezer for future use.
3. Microinjection
- Set up 4-6 breeding tanks with at least 2 males and 2 females the afternoon before injection. These fish must not be fed in the afternoon. This step will reduce the amount of fish waste and save time to clean them out the next morning, while also helping to induce a breeding response.
- The following morning, remove the prepared injection solution (pDestTol2-ubi-ASAP1 construct and Tol2 mRNA) from the -80 °C freezer, and place it on ice.
- Pull the dividers in the fish breeding tanks and allow the fish to mate. In general, fish lay eggs within 20-30 minutes after pulling out the divider. If not, wait 1-2 hours longer. Some fish may not lay eggs at all. In this case, repeat this experiment for fish embryo collection.
- While waiting, make sure there are needles prepared with the tip broken at an angle creating a beveled edge with forceps, or by breaking on a delicate task wiper.
- Pull needles from capillary glass on a micropipette puller using the following parameters: heat 545; pull 60; velocity 80; time 250; pressure 500. Break the needle with forceps underneath a dissection scope (with eye-piece ruler for diameter estimation) by holding the forceps at an angle approximately 45°. Desired needle diameters can be variable depending on the microinjector settings, but smaller diameter is preferred for decreasing embryo mortality.
- Once the fish have laid eggs, collect them in a 10-cm diameter Petri dish and bring them to the dissection scope. Remove all abnormal embryos and fish waste.
- Pipet the fertilized embryos into the prepared 3% agarose injection mold. Remove excess water to help keep the embryos in place.
- Once all of the rows are filled with viable embryos, arrange them so that the single cells all face the same direction toward the needle, which is about a 45° angle horizontally. This will make injection much easier later.
- While wearing gloves, use a 20 µL loading pipet tip and remove 5 µL of the prepared construct from the tube on ice.
- Carefully insert the tip into the back end of the broken capillary tube all the way to where it begins to tapper, as to get the reagent as close to the tip. If there are still air bubbles, shake the needle, making sure to not break the tip.
- Insert the needle straight into the microinjection needle holder and carefully tighten until the needle stays in place. Adjust the angle to about 45°.
- Once the needle is prepared and attached, turn on the microscope and gas pressure tank. Commercial CO2 tanks are generally good for this purpose. The injection volume is adjusted by the holding and ejection pressure: approximate 0.5 psi for holding and 30 psi for ejection. Be sure to check that the solution comes out when pressing the pedal.
- Using a stage micrometer with a drop of mineral oil, adjust the volume and flow of the solution to ~150 µm in diameter (about 2 nL). Ensure that the back pressure will let a small amount drip out of the needle. If there is not enough back pressure, capillary action will cause liquid to enter the needle and destroy the mRNA.
- Once the needle is calibrated, begin injecting the construct into the single cell of the fertilized embryos.
NOTE: This takes a large amount of practice, patience, and finesse, due to the cell membrane being hard to pierce. It is important to inject the solution into the cell, not the yolk, for generating transgenic zebrafish. This is different from morpholino injection. It does not matter which side the needle enters the cell as long as the construct goes in the cell. The transgenesis will have a very low rate of success if injected into the yolk instead of the cell. Single-cell stage injection is also important, or somatic chimera fish will be created. This will reduce the chance of the transgene going into to the germ cells. - Use the edge of the gel notch to provide a backing that keeps the embryo in place and allows the needle to apply pressure without moving the embryo. Once the tip of the needle is in the single cell, press the pedal to release the desired amount of solution. Repeat this process for all of the embryos.
- When completed, transfer the injected embryos into a labeled dish by rinsing them out of the agarose notch with fish system water and a disposable 3.4 mL transfer pipette. Store the embryos in a 28.5 °C incubator to let them develop. Check back throughout the day removing dead fish embryos and replace water with 0.1% methylene blue in fish water.
- Around 6-8 hours after injection, take 10 individual injected fish embryos and prepare genomic DNA from them using the Hotshot method18.
- The following morning, use a dissection microscope with a fluorescence light source to sort out the embryos showing GFP in the non-yolk tissues. These fish embryos should contain the injected construct.
- Perform Tol2 excise assay to check the transposon activity as described previously19. If excised plasmid can be detected, keep the injected fish embryos and raise them. If no excised plasmid can be detected, repeat the Tol2 mRNA synthesis and microinjection process until achieving the positive results of the Tol2 excise assay.
4. Establish Transgenic ASAP1 fish, Tg(ubi:ASAP1)
- Raise the injected fish (F0 generation) to adulthood as described previously in the zebrafish book20. This usually takes about 4 months.
- Take a single adult F0 fish and cross it with a single opposite gender wildtype fish. Collect fish embryos after breeding later in the day. Keep the collected fish embryos in the 28.5 °C incubator in fish water with 0.1% methylene blue.
- On the third day, check the fish embryos underneath a fluorescent dissection microscope with a GFP filter. Sort out green fish embryos, if there are any, and raise them to adulthood as F1 generation transgenic fish, Tg(ubi:ASAP1).
NOTE: Mendelian ratio is not expected since most of the parental F0 fish are germ-line genetic chimeras. - Cross single F1 adult fish with wildtype fish and collect fish embryos. Sort out green fish embryos and raise them to adulthood as F2 generation fish.
NOTE: Green and non-green fish embryos should be close to 1:1 if there is a single transgene. - To view electric potential changes in tumor like malignant peripheral nerve sheath tumors (MPNST), cross the F2 generation Tg(ubi:ASAP1) fish with rpl35hi258/wt fish. It is known that visible tumors start to be found in the 6-8 month old adults21,22.
5. Imaging
- To image zebrafish embryos, take multiple F2 generation founder fish and cross them with wildtype fish in individual pairs. Collect fish embryos at different desired developmental stages according to the zebrafish staging guide23.
- For the early stages of fish embryos, peel and remove chorions of the embryos carefully using a pair of forceps under a dissection scope in a 10-cm diameter Petri dish with fish system water.
- Transfer a few fish embryos onto a concaved glass slide with 3% methylcellulose using a 3.4 mL disposable transfer pipette. Adjust the embryos to the desired positions to view the cellular GFP activity using a needle underneath a fluorescent dissection scope.
- For the moving stages of fish embryos (older than 12 somite stage), use 0.05% tricaine mesylate to anesthetize the fish embryos before transferring them to slides. Briefly, fish embryos were emerged into 0.05% tricaine mesylate in fish water until they stopped swimming and loss body balance. Also, add a drop of 0.05% tricaine mesylate with the methylcellulose on the slide.
- For less than 12 somite stage fish embryos, use an epi-fluorescence compound microscope with a compatible camera and software for imaging. For older than 12 somite stage fish embryos, use a fluorescence dissection microscope.
- To image tumor cell voltage, first identify the fish with MPNST tumors. Then, anesthetize the fish with 0.05% tricaine mesylate. For whole mount imaging, put the fish into a 10-cm diameter Petri dish. To view the tumor cell electrical activity, fish tumors may be dissected out after whole mount imaging.
Representative Results
In a successful injection, more than 50% injected fish embryos will display some degree of green fluorescence in the somatic cells, and most of them will be positive by Tol2 transposon excise assay (Figure 2). After 2-4 generations of out-cross with wildtype fish (until the fluorescent fish reach 50%, the expected Mendelian ratio), the transgenic fish were used for the imaging experiment to track cell membrane potentials during embryonic development. First, membrane potential changes were examined throughout the cell cycle during zebrafish early embryonic developmental stages. It was observed that the cells hyperpolarized before the cleavage furrow formation (Figure 3A-3C, and Supplementary Video 1). Moreover, different tissues showed a variety of membrane potentials in 1-3 day old fish embryos. (Figure 3D-3G). For example, the somites and notochord are generally hyperpolarized, compared to the adjacent tissues/organs. Once the zebrafish embryos were able to move, we were also able to detect the neuromuscular electrical activities (Figure 4, Supplementary Video 2). As bioelectric properties of cancer cells could be altered, we took advantage of this ASAP1 reporter fish, and crossed it with an rpL35 gene mutant, which is prone to spontaneous malignant peripheral nerve sheath tumors21,24,25. Although only a few fish tumors were examined, due to the long potential growth period for the fish tumor mutant, it was noticeable that there were voltage differences between tumors and surrounding tissues in live tumor-bearing zebrafish (Figure 5). Thus, these representative results demonstrated the successful generation of a cellular electric reporter fish line, and its potential application to developmental and cellular biology.
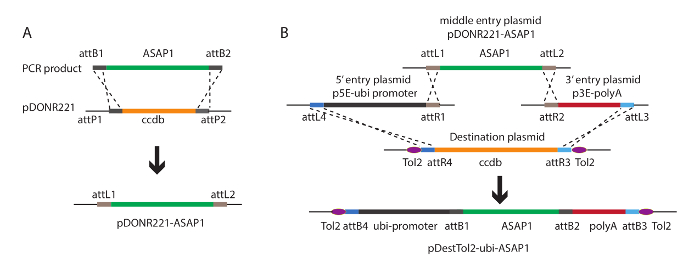
Figure 1: Illustration of the Tol2 transposon-based plasmid construction.
(A) BP recombination was used for ASAP1 sub-cloning into the pDONR221 middle entry vector. attB sequences were added to the 5-end of the primers for ASAP1. (B) Diagram for Tol2 transposon-based construct assembling based on LR recombination. Purple oval shape shows Tol2 inverted repeats. The dashed lines indicate homologous recombination. Please click here to view a larger version of this figure.
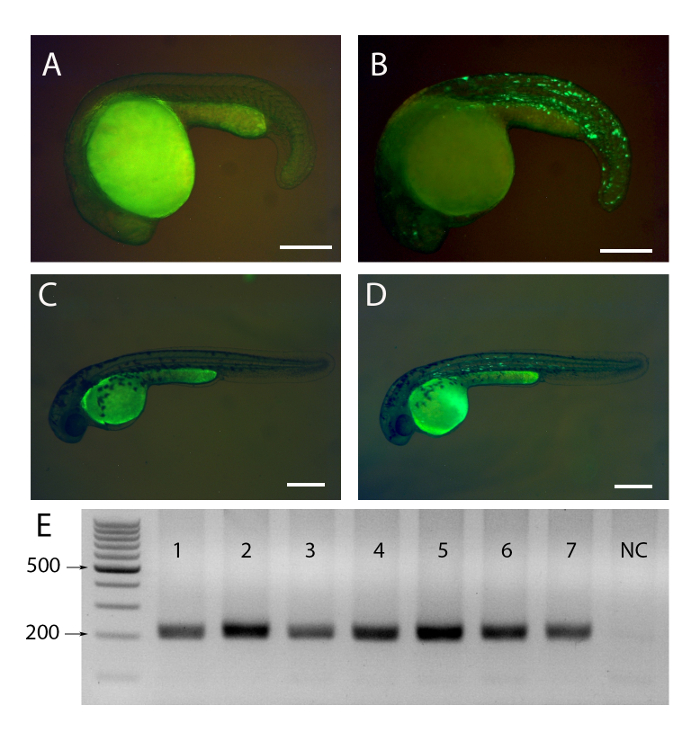
Figure 2: Typical results of injected embryos by epifluorescence and Tol2-excise assay.
(A) Non-positive 1dpf fish embryo. (B) Successfully injected 1dpf fish embryo. GFP spots are evident in the trunk. (C) Non-positive 2dpf fish embryo. (D) Successfully injected 2dpf fish embryo. GFP spots are evident in the trunk. (E) A representative result of Tol2 excise assay. Lane 1-7 PCR were amplified from 7 randomly selected fish embryos 8 hours after injection. The last one is a negative control (NC) without any genomic DNA. Scale bar = 250 μm. Please click here to view a larger version of this figure.
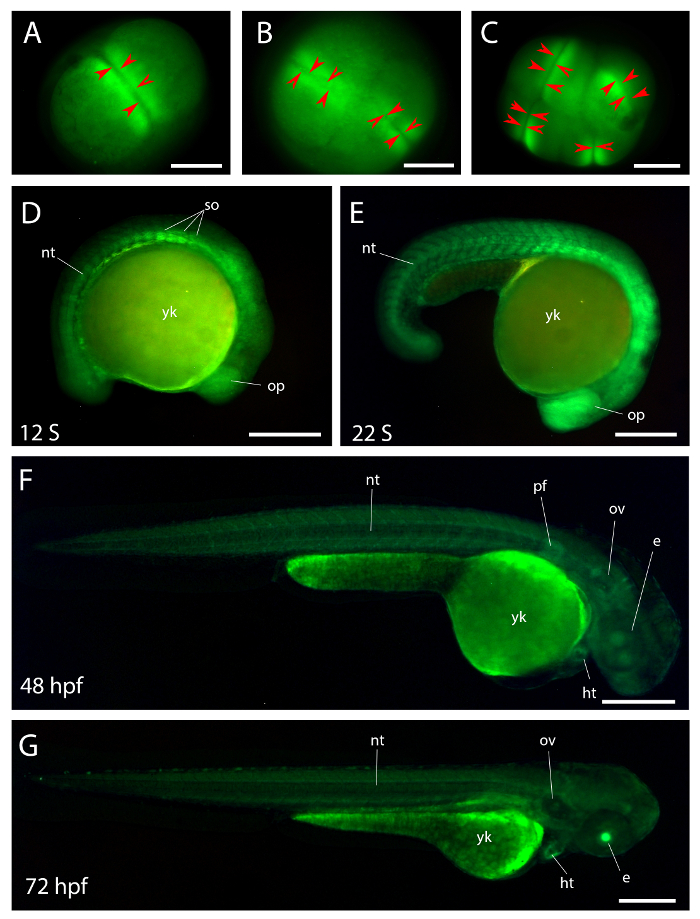
Figure 3: Dynamic voltage changes during zebrafish embryo development.
(A-C) Differential cellular voltage polarity during mitosis in the fish embryos. (A) 2-cell stage zebrafish embryo. (B) 4-cell stage embryo. (C) 8-cell stage embryo. The red arrow heads indicate the positions of the cleavage furrows in the panels (A–C). The changes are also evident in the corresponding movie (Supplementary Video 1). The region around the cleavage furrow is more polarized compared the rest of the cell. (D-G) Dynamic electric voltage changes in the different early stages of zebrafish embryos. (D) 12-somite stage. (E) 22-somite stage. (F) 48 hours post fertilization. (G) 72 hours post fertilization. e, eye; ht, heart; nt, notochord; op, optic vesicle; ov, otic vesicle; pf, pectoral fin; so, somite; yk, yolk. Scale bar = 250 μm. Please click here to view a larger version of this figure.
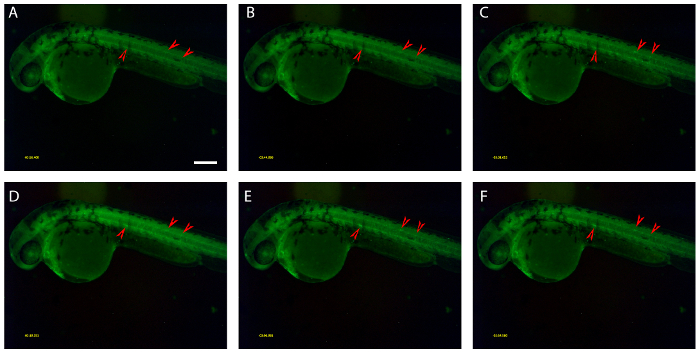
Figure 4: Electrical voltage changes of the fish body during fish embryo movement.
2-day old fish embryos show the neuro-muscular electric activities during movement. (A) – (F) Sequential imaging of the same fish embryo. Color density changes are corresponding to the electric signaling transduction. The interval time between two consecutive images is about 12.4 milliseconds. The red arrows indicate the positions that voltage changed during the imaging period. The changes are also evident in the corresponding movie (Supplementary Video 2). All the panels are in the same scale. Scale bar = 250 μm. Please click here to view a larger version of this figure.
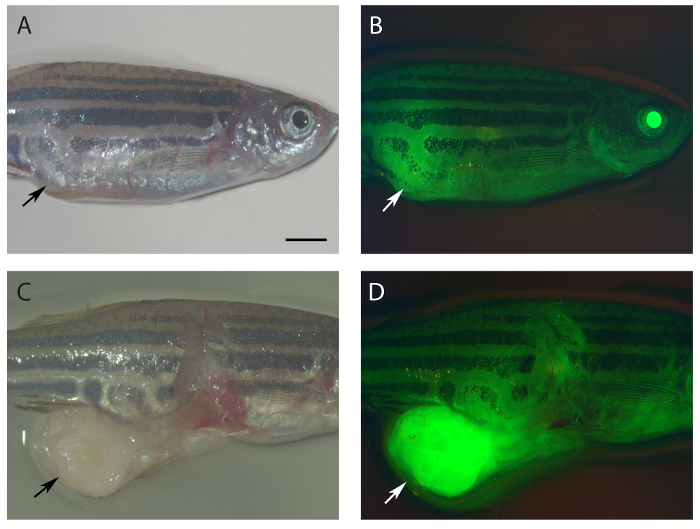
Figure 5: Tumor cells tend to be more polarized.
A 10-month old fish (rpL35hi258/wt; Tg(ubi:ASAP1) developed a malignant peripheral nerve sheath tumor in the abdomen. (A) & (C) Bright field image. (B) & (D) Image with GFP channel. (A) & (B) Intact fish. (C) & (D) Abdomen tumor was dissected out. Tumor cells are more polarized (brighter green) compared to surrounding tissues (dark green). Arrow heads show the tumors. All the panels are in the same scale. Scale bar = 25 mm. Please click here to view a larger version of this figure.
Supplementary Video 1. Epifluorescent imaging of electrical signaling during cleavage stages in Tg(ubi: ASAP1) fish embryo. This movie was recorded from the view of animal pole. The ASAP1 fluorescence is associated with the formation of cell cleavage furrow, a temporary structure during cell division. Please click here to download this file.
Supplementary Video 2. Epifluorescent imaging of electrical signaling during 2-day old Tg(ubi: ASAP1) fish embryo movement. The move was recorded from the lateral view of the 2-day old fish embryo after anesthetization. The ASAP1 fluorescence alterations are evident in neuromuscular tissue during the moving process. Please click here to download this file.
Discussion
Although the cellular and tissue level electrical activities during embryonic development and human disease were discovered a long time ago, the in vivo dynamic electrical changes and their biological roles still remain largely unknown. One of the major challenges is to visualize and quantify the electrical changes. Patch clamp technology is a break-through for tracking single cells, but its application to vertebrate embryos is limited because they are composed of many cells. The current chemical voltage dyes are also not ideal due to their sensitivities, specificities, and toxicities. The recent efforts on the invention of GEVIs provide us a new path to visualize cellular electric activities in vivo and in real time. Here, we showed the process of creating a zebrafish electric reporter line, Tg(ubi:ASAP1).
Using this reporter fish line, we show cellular electrical activities that can be monitored in zebrafish embryos. The electric voltage change is highly related to the cell cycle during early embryonic development. We have observed that hyperpolarization happens before the formation of the cleavage furrow/cell division (Figure 3). This is in contrast to the current knowledge that depolarization happens before cell division26. Thus, more details of cell membrane voltage changes during the cell cycle of other animal and human cells, and whether this is related to tissue context, require further studies. Related studies are currently underway in our laboratory. Moreover, we have verified that ASAP1 is able to track physiological voltage changes in the neural-muscular system (Figure 4), in which the alteration is relatively fast compared to the changes during cell cycles.
It was also demonstrated that this reporter can also be used to visualize zebrafish tumors (Figure 5). It was interesting to find tumor cells were generally more polarized compared to the surrounding normal tissues. However, whether this is a general phenomenon for all malignant tissues requires further investigation, due to the limitation of tumor samples and fish tumor types in this study. Future investigations on cell membrane polarization and voltage quantification on other types of tumors and human cancer cells will be informative for better understanding its roles during tumorigenesis.
In this protocol, we chose a ubiquitous promoter to drive ASAP1 expression to track all the cells in fish embryos. Tissue or organ specific promoters could be another option if only a certain cell/tissue type is preferred. The ASAP1 voltage sensor is a relatively well characterized biosensor, and it is composed of a voltage sensitive domain of sea squirt voltage-sensitive phosphatase (S3-S4 loop) and a circular permutation of GFP (default is low fluorescence). It was reported to be expressed on the outside cellular membrane in human neuron cells and mouse brain slices4,27,28. The brightness of the sensor is dominantly determined by the conformational positions of the S3-S4 loop and GFP. The rapid green fluorescence change was unlikely caused by protein concentration, due to the speed of the brightness changes and protein synthesis. However, the transgene, ASAP1, may have altered expression in tumor cells, due to the nature of genomic instability. In addition to ASAP1, other GEVIs, such as archaerhodopsin-based voltage indicators (QuasAr1 and QuasAr2), may also be a good complementary option, since they use a completely different mechanism and they also have a high sensitivity and speed 29. In addition, their emission is in the red color range. This makes them particularly complimentary to the green ASAP1, if there is already another florescent protein in the same cell. For example, ASAP1 and QuasAr can be combined with Fucci zebrafish30 for studying the relationship between cell cycle and electric potential changes.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The research work reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under the Award Number R35GM124913, Purdue University PI4D incentive program, and PVM Internal Competitive Basic Research Funds Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agents. We thank Koichi Kawakami for the Tol2 construct, Michael Lin for the ASAP1 construct, and Leonard Zon for the ubi promoter construct through Addgene.
Materials
| 14mL cell culture tubes | VWR | 60818-725 | E.Coli culture |
| Agarose electrophoresis tank | Thermo Scientific | Owl B2 | DNA eletrophoresis |
| Agarose RA | Amresco | N605-500G | For making the injection gels |
| Attb1-ASAP1-F primer | IDT DNA | GGGGACAAGTTTGTACAAAAAAGCAGGCTTCACCATGGAGACGACTGTGAGGTATGAACA | ASAP1 coding region amplification for subcloning |
| Attb2-ASAP1-R primer | IDT DNA | GGGGACCACTTTGTACAAGAAAGCTGGGTCTTAGGTTACCACTTCAAGTTGTTTCTTCTGTGAAGCCA | ASAP1 coding region amplification for subcloning |
| Bright field dissection scope | Nikon | SMZ 745 | Dechorionation, microinjection, mounting |
| Color camera | Zeiss | AxioCam MRc | Fish embryo image recording |
| Concave slide | VWR | 48336-001 | For holding fish embryos during imaging process |
| Disposable transfer pipette 3.4 ml | Thermo Scientific | 13-711-9AM | Fish embryos and water transfer |
| Endonuclease enzyme, Not I | NEB | R0189L | For linearizing plasmid DNA |
| Epifuorescent compound scope | Zeiss | Axio Imager.A2 | Fish embryo imaging |
| Epifuorescent stereo dissection scope | Zeiss | Stereo Discovery.V12 | Fish embryo imaging |
| Fluorescent light source | Lumen dynamics | X-cite seris 120 | Light source for fluorescence microscopes |
| Forceps #5 | WPI | 500342 | Dechorionation and needle breaking |
| Gateway BP Clonase II Enzyme mix | Thermo Scientific | 11789020 | Gateway BP recombination cloning |
| Gateway LR Clonase II Plus enzyme | Thermo Scientific | 12538120 | Gateway LR recombination cloning |
| Gel DNA Recovery Kit | Zymo Research | D4002 | DNA gel purification |
| Loading tip | Eppendorf | 930001007 | For loading injection solution into capilary needles |
| Methylcellulose (1600cPs) | Alfa Aesar | 43146 | Fish embryo mounting |
| Methylene blue | Sigma-Aldrich | M9140 | Suppresses fungal outbreaks in Petri dishes |
| Microinjection mold | Adaptive Science Tools | TU-1 | To prepare agaorse mold tray for holding fish embryos during injection |
| Microinjector | WPI | Pneumatic Picopump PV820 | Microinjection injector |
| Micro-manipulator | WPI | Microinjector mm33 rechts | Microinjection operation |
| Micropipette puller | Sutter instrument | P-1000 | For preparing capillary needle |
| Mineral oil | Amresco | J217-500ml | For calibrating injection volume |
| mMESSAGE mMACHINE SP6 Transcription Kit | Thermo Scientific | AM1340 | mRNA in vitro transcription |
| Monocolor camera | Zeiss | AxioCam MRm | Fish embryo image recording |
| Plasmid Miniprep Kit | Zymo Research | D4020 | Prepare small amount of plasmid DNA |
| Plastic Petri dishes | VWR | 25384-088 | For holding fish or fish embryos during imaging process |
| RNA Clean & Concentrator-5 | Zymo Research | R1015 | mRNA cleaning after in vitro transcription |
| Spectrophotometer | Thermo Scientific | NanoDrop 2000 | For measuring DNA and RNA concentrations |
| Stage Micrometer | Am Scope | MR100 | Microinjection volume calibration |
| Thermocycler | Bio-Rad | T100 | DNA amplification for gene cloning |
| Thin wall glass capillaries | WPI | TW100F-4 | Raw glass for making cappilary needle |
| Tol2-exL1 primer | IDT DNA | GCACAACACCAGAAATGCCCTC | Tol2 excise assay |
| Tol2-exR primer | IDT DNA | ACCCTCACTAAAGGGAACAAAAG | Tol2 excise assay |
| TOP10 Chemically Competent E. coli | Thermo Scientific | C404006 | Used for transformation during gene cloning |
| Tricaine mesylate | Sigma-Aldrich | A5040 | For anesthetizing fish or fish embryos |
| UV trans-illuminator 302nm | UVP | M-20V | DNA visualization |
| Water bath | Thermo Scientific | 2853 | For transformation process of gene cloning |
References
- Levin, M. Molecular bioelectricity: how endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo. Molecular Biology of the Cell. 25 (24), 3835-3850 (2014).
- Storace, D., et al. Toward Better Genetically Encoded Sensors of Membrane Potential. Trends in Neuroscience. 39 (5), 277-289 (2016).
- Inagaki, S., Nagai, T. Current progress in genetically encoded voltage indicators for neural activity recording. Current Opinion in Chemical Biology. 33, 95-100 (2016).
- St-Pierre, F., et al. High-fidelity optical reporting of neuronal electrical activity with an ultrafast fluorescent voltage sensor. Nature Neuroscience. 17 (6), 884-889 (2014).
- Lieschke, G. J., Currie, P. D. Animal models of human disease: zebrafish swim into view. Nature Reviews Genetics. 8 (5), 353-367 (2007).
- Santoriello, C., Zon, L. I. Hooked! Modeling human disease in zebrafish. Journal of Clinical Investigation. 122 (7), 2337-2343 (2012).
- Howe, K., et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. , (2013).
- Kawakami, K., Shima, A., Kawakami, N. Identification of a functional transposase of the Tol2 element, an Ac-like element from the Japanese medaka fish, and its transposition in the zebrafish germ lineage. Proceedings of the National Academy of Science USA. 97 (21), 11403-11408 (2000).
- Urasaki, A., Asakawa, K., Kawakami, K. Efficient transposition of the Tol2 transposable element from a single-copy donor in zebrafish. Proceedings of the National Academy of Science USA. 105 (50), 19827-19832 (2008).
- Kwan, K. M., et al. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Developmental Dynamics. 236 (11), 3088-3099 (2007).
- Mosimann, C., et al. Ubiquitous transgene expression and Cre-based recombination driven by the ubiquitin promoter in zebrafish. Development. 138 (1), 169-177 (2011).
- Lorenz, T. C. Polymerase chain reaction: basic protocol plus troubleshooting and optimization strategies. Journal of Visualized Experiments. (63), e3998 (2012).
- Ordovas, J. M. Separation of small-size DNA fragments using agarose gel electrophoresis. Methods in Molecular Biology. 110, 35-42 (1998).
- Downey, N. Extraction of DNA from agarose gels. Methods Mol Biol. 235, 137-139 (2003).
- Desjardins, P., Conklin, D. NanoDrop microvolume quantitation of nucleic acids. Journal of Visualized Experiments. 45 (45), (2010).
- Green, M. R., Sambrook, J. . Molecular cloning : a laboratory manual. , (2012).
- Zhang, S., Cahalan, M. D. Purifying plasmid DNA from bacterial colonies using the QIAGEN Miniprep Kit. Journal of Visualized Experiments. (6), 247 (2007).
- Meeker, N. D., Hutchinson, S. A., Ho, L., Trede, N. S. Method for isolation of PCR-ready genomic DNA from zebrafish tissues. Biotechniques. 43 (5), (2007).
- Kawakami, K., Koga, A., Hori, H., Shima, A. Excision of the tol2 transposable element of the medaka fish, Oryzias latipes, in zebrafish, Danio rerio. Gene. 225 (1-2), 17-22 (1998).
- Westerfield, M. . The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio). , (2000).
- Amsterdam, A., et al. Many ribosomal protein genes are cancer genes in zebrafish. PLoS Biology. 2 (5), E139 (2004).
- Lai, K., et al. Many ribosomal protein mutations are associated with growth impairment and tumor predisposition in zebrafish. Developmental Dynamics. 238 (1), 76-85 (2009).
- Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B., Schilling, T. F. Stages of embryonic development of the zebrafish. Developmental Dynamics. 203 (3), 253-310 (1995).
- Zhang, G., et al. Comparative oncogenomic analysis of copy number alterations in human and zebrafish tumors enables cancer driver discovery. PLoS Genetics. 9 (8), e1003734 (2013).
- Zhang, G., et al. Highly aneuploid zebrafish malignant peripheral nerve sheath tumors have genetic alterations similar to human cancers. Proceedings of the National Academy of Science USA. 107 (39), 16940-16945 (2010).
- Urrego, D., Tomczak, A. P., Zahed, F., Stuhmer, W., Pardo, L. A. Potassium channels in cell cycle and cell proliferation. Philosophical Transactions of the Royal Society of London Series B. 369 (1638), 20130094 (2014).
- Yang, H. H., et al. Subcellular Imaging of Voltage and Calcium Signals Reveals Neural Processing In Vivo. Cell. 166 (1), 245-257 (2016).
- Chamberland, S., et al. Fast two-photon imaging of subcellular voltage dynamics in neuronal tissue with genetically encoded indicators. Elife. 6, (2017).
- Hochbaum, D. R., et al. All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nature Methods. 11 (8), 825-833 (2014).
- Sugiyama, M., et al. Illuminating cell-cycle progression in the developing zebrafish embryo. Proceedings of the National Academy of Science USA. 106 (49), 20812-20817 (2009).

