Computational Analysis of the Caenorhabditis elegans Germline to Study the Distribution of Nuclei, Proteins, and the Cytoskeleton
Summary
We present an automated method for three-dimensional reconstruction of the Caenorhabditis elegans germline. Our method determines the number and position of each nucleus within the germline and analyses germline protein distribution and cytoskeletal structure.
Abstract
The Caenorhabditis elegans (C. elegans) germline is used to study several biologically important processes including stem cell development, apoptosis, and chromosome dynamics. While the germline is an excellent model, the analysis is often two dimensional due to the time and labor required for three-dimensional analysis. Major readouts in such studies are the number/position of nuclei and protein distribution within the germline. Here, we present a method to perform automated analysis of the germline using confocal microscopy and computational approaches to determine the number and position of nuclei in each region of the germline. Our method also analyzes germline protein distribution that enables the three-dimensional examination of protein expression in different genetic backgrounds. Further, our study shows variations in cytoskeletal architecture in distinct regions of the germline that may accommodate specific spatial developmental requirements. Finally, our method enables automated counting of the sperm in the spermatheca of each germline. Taken together, our method enables rapid and reproducible phenotypic analysis of the C. elegans germline.
Introduction
The conservation of signaling pathways with mammals makes C. elegans an excellent model to study multiple biological processes1,2. In our lab, we use the C. elegans germline to study stem cell development, apoptosis, and gene expression. While the germline is a three-dimensional structure, many studies are two dimensional due to the time-consuming and labor-intensive nature of three-dimensional analysis. It is highly likely that two-dimensional analysis may misrepresent in vivo events in the germline. The C. elegans adult hermaphrodite has two germline arms, each of which houses a somatic distal tip cell (DTC) that maintains distal germ cells in an undifferentiated state3,4. These germ cells begin to differentiate as they move away from the DTC, escaping its influence, and become oocytes and sperm as they reach the proximal end of the germline. During this process, germ cell nuclei undergo mitosis, before transitioning to meiosis5,6. Sperm production is completed by larval stage 4 (L4) of the development, after which oocytes are produced during the adulthood. The sperm are stored in the spermatheca where they fertilize oocytes to generate embryos.
There are multiple genetic and environmental factors that can influence germline development in C. elegans resulting in changes in the number of nuclei, number of apoptotic events, chromosome dynamics, and protein expression and/or localization7,8,9,10,11. The analysis of these events requires the identification of each stage of differentiation based on nuclear morphology and distribution. To accurately analyze these parameters manually with a large sample size is labor-intensive and time-consuming. To circumvent these drawbacks and to enable the consistency of analysis, we developed an automated method for three-dimensional examination of the C. elegans germline for nuclei counting, nuclei distribution, protein expression, and cytoskeletal structure. By combining confocal microscopy with three-dimensional rendering, we generated size and shape parameters for the identification of each stage of germ cell differentiation. Further, this method enables counting of germ cell nuclei and sperm plus scoring of chromosome number in each oocyte.
One crucial structure in the germline is the cytoskeleton, which provides stability to the germline compartment, aids cytoplasmic streaming and protection to germline nuclei12. Using computational rendering, we performed three-dimensional reconstruction of the germline cytoskeleton and identified distinct cytoskeletal features within the germline. Here, we describe a step-by-step protocol to illustrate how computational analysis combined with confocal imaging enables comprehensive analysis of the C. elegans germline.
We propose a rapid method for the three-dimensional analysis of C. elegans germline (Figure 1). Using three-dimensional analysis, it is possible to study the three-dimensional distribution of germline nuclei (Figure 2 and Figure 3), automated counting of cells (Figure 2), reconstruction of the germline cytoskeleton (Figure 3), distribution of proteins (Figure 4), and scoring the number of sperm in the spermatheca and chromosomes in oocytes (Figure 5). The method not only enables easy and accurate quantification of the germline but identifies physiologically relevant phenotypes.
Protocol
1. Preparation and Worm Husbandry
Note: Refer Table of Materials for all product information.
- OP50 Escherichia coli culture: culture OP50 bacteria in Lysogeny broth (LB) (1% tryptone, 0.5% yeast, 0.5% NaCl, pH 7.0) overnight at 37 °C without antibiotics.
- Seed 400 µL of OP50 bacteria to nematode growth media (NGM) plates (1.5 g of NaCl, 8.5 g of agar, 1.25 g of peptone, 1 mL of 1 M CaCl2, 1 mL of 5 mg/mL cholesterol in ethanol, 1 mL of 1 M MgSO4, and 25 mL of 1 M KPO4 buffer) and air dry the bacteria for 48 h.
- Pick worms on seeded NGM plates and incubate at 15 °C or 20 °C.
- For the analysis of the adult hermaphrodite germline, pick well-fed L4 worms to the bacterial lawn and incubate overnight at 20 °C.
- For scoring sperm number in hermaphrodites, incubate L4 worms at 20 °C for 12 h until the worms reach the L4/adult molt. To synchronize the worms to the L4 stage, prepare eggs by picking adult worms with a lot of eggs in bleach solution (1 M NaOH and bleach in 1:1 ratio). Allow the eggs to hatch and pick L4 animals for the experiment.
- Prepare Teflon microscope slides by placing 25 µL of poly-L-lysine solution on the slide and spreading the solution well, using a pipette tip. After removing excess poly-L-lysine with paper towel, allow the slides to air dry and incubate the slides at 65 °C for 15 – 20 min.
2. Germline Dissection and Staining6,13
- Spot 10 µL of 0.01% tetramisole (anesthetic) on a 22 mm × 22 mm coverslip and pick one-day old adult worms into it.
- Dissect the worm at the tail as it becomes sedated using a syringe (5 mL) and a needle (24" x 1"), and ensure that the germline is not damaged.
NOTE: The dissection must be carried out before the worm becomes fully paralyzed so that the negative pressure in the worm and the worm movement aid the ejection of the germline. For scoring sperm, precautions must be taken not to damage the spermatheca by the needle during dissection. Avoid touching the germline with the needle except the dissection point after the spermatheca. If there is break in the germline compartment or any discharge from the germline is observed, the germline should not be used for analysis. - Place the inverted coverslip on a poly-L-lysine coated slide, keeping the dissected germline on the slide.
- Remove excess liquid under the coverslip by placing a paper tower at a corner of the coverslip, then place the slide in liquid nitrogen for 1 min. Quickly remove the coverslip using a needle with the germlines on the slide.
- Transfer the slides into -20 °C methanol for 30 – 60 s in a chamber, then incubate for 30 min in freshly prepared fixing solution (1x phosphate buffered saline (PBS) containing 0.08 M HEPES pH 6.9, 1.6 mM MgSO4, 0.8 mM ethylene glycol-bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid (EGTA), and 3.7% paraformaldehyde) at room temperature.
- Wash the slides by placing in a chamber containing 1x PBS, pH 7.4 with 0.1% Tween-20 for 10 min. Repeat this step once.
- Block the germlines with 30 µL of blocking solution (30% of goat serum prepared by adding 900 µL of goat serum in 1700 µL of distilled H2O and 300 µL of 10x PBS) in a humid chamber prepared by placing wet paper towel in a plastic box at room temperature for 30 min.
- Add 50 µL of REC-8 antibody solution prepared in 30% goat serum in a ratio 1:300 to each sample. Incubate overnight at 4°C.
- Wash the slides by placing in a chamber containing 1x PBS with 0.1% Tween-20 for 10 min. Repeat the step once and remove excess liquid by placing a paper towel at the corner of the slide.
- Prepare secondary antibody solution by diluting green fluorophore (488 nm emission wavelength) conjugated secondary antibody (1:1000), phalloidin (actin stain, 1:4000), and DAPI (DNA stain, 1:4000) in 30% normal goat serum.
- Add 50 µL of secondary antibody solution to the slide.
- Incubate for 1 h at room temperature.
- Wash the slides twice by placing in a chamber containing 1x PBS with 0.1% Tween-20 for 10 min. Wipe off excess liquid.
- Add 30 µL of fixing reagent onto the slide and place a 12 mm2 x 1.5 µm coverslip on top and dry the slides at 4 °C overnight.
3. Confocal Microscopy
- Turn on the confocal microscope, lasers, resonant scanner, and confocal microscope software. Place the slides with stained germlines on the slide holder above the 63X objective.
- Locate a germline, focus on the top of the germline and mark it using confocal microscope software. Similarly focus on the bottom of the germline and mark it to establish the total germline thickness.
- Image the entire germline by defining the thickness of each slice up to 0.5 µm. Mark the complete germline and start acquisition. Scan each slice 8 times and average the values to improve image quality. The resonant scanner will permit fast scanning to avoid bleaching during imaging. If the microscope does not have a resonant scanner, reduce the number of scans to four to avoid bleaching.
4. Post Imaging Analysis of Nuclei Number and Distribution
Note: Refer Supplementary Figure 1 for screenshots of the software tools and buttons used.
- Import the image to Imaris 8.4.1 or later versions of the software.
- Define mitotic region, transition zone, meiotic region, oocyte region, and spermatheca of the germline by identifying the nuclear morphology in each region.
- Use the surface function button (Supplementary Figure 1A) of the software to select the region of interest.
- Cancel automatic surface creation wizard and manually draw the region of interest at the first and last slice of the image from the three-dimensional stack.
- Click Create surface.
NOTE: The software will automatically develop the stacks between first and last stack. - Mask all channels except DAPI by clicking Mask channel button (Supplementary Figure 1B-C).
- Define nuclear size parameters using the spot function button (Supplementary Figure 1A). For the mitotic region, define an XY diameter of 2 µm while leaving Z diameter undefined. For the transition zone, define an XY diameter of 2 µm and Z diameter as 1.5 µm. (Supplementary Figure 1D).
NOTE: Troubleshoot the diameters according to the magnification and specifications of the microscope. For the current protocol, the objective used was 63X and provided an extra 1.70 times magnification during imaging. If the objective changes, for instance 40X, the diameters should be changed accordingly. Troubleshooting should be performed with wildtype germlines to establish the correct parameters. The mitotic region of a wild type germline arm has approximately 250 nuclei. The diameter should be modified to achieve this value. Use at least 15 germlines to determine the optimal value for the diameter. A similar approach should be used for calibrating the transition zone (150 – 170 nuclei) and meiotic region (600 – 700 nuclei). - Limit the detection of spots by defining the minimum threshold (Supplementary Figure 1E). Use DAPI stained wild type germlines to establish minimum threshold by increasing the minimum three-dimensional rendering threshold until the first spot appears outside the germline.
- Save the image and obtain the number of nuclei from the table generated automatically by the software. Export the images as TIF files.
5. Post Imaging Analysis for Scoring Sperm and Chromosome Number
- Perform three-dimensional rendering by selecting the spermatheca as the region of interest. To detect each sperm, define the spot diameter between 0.75 – 1.0 µm for X and Y- axis, while Z diameter remains undefined. Use the spots function button as shown in Supplementary Figure 1.
- Subtract the background by ticking background subtract box and using background correction values calculated by the software (Supplementary Figure 1D).
NOTE: Imaris software picks high intensity points first, therefore the effect of background is minimal as long as there is significant difference between the region of interest and the background. A second method is to define the background correction manually, where appropriate values can be given for the background. Manual background correction will be appropriate if the intensity of independent samples needs comparison. - Adjust the three-dimensional rendering threshold to detect all DAPI stained sperm in the spermatheca (Supplementary Figure 1E).
- For chromosome counting, define the spot diameter <0.75 µm for X and Y- axis before developing three-dimensional models.
- Save the image and export as TIF file.
6. Post Imaging Analysis for Cytoskeletal Reconstruction of the Germline
- Identify the region of interest in the germline and mask all channels except phalloidin by clicking Mask channels button.
- Define a surface detail of 0.25 µm by using the surface function button (Supplementary Figure 1).
Note: This means every 0.25 µm will be rendered into three-dimensional models. Surface detail can be constant for any region of the germline where a three-dimensional surface model will be created for every 0.25 µm. However, for the oocyte region, surface detail can be increased to 0.35 – 0.5 µm due the presence of well-defined actin fibers in this region. - Subtract the background by ticking background subtract box and using background correction values calculated by the software (Supplementary Figure 1D).
- Adjust three-dimensional rendering threshold depending on the structure requiring analysis (Supplementary Figure 1E).
- Save the developed three-dimensional image and export as a TIF file.
Representative Results
Figure 1 indicates the time required for three-dimensional germline analysis. L4 hermaphrodites incubated at 20 °C were dissected to isolate germlines and stained with DAPI, phalloidin, and antibodies against germline proteins. Germlines are imaged using confocal microscopy. Staining and confocal microscopy requires approximately 24 h. Computational analysis for the complete germline requires 10 – 15 min to count the number and position of nuclei, identify the protein distribution, analyze the cytoskeletal structure and score the number of sperm. Complete manual analysis requires over 2 h per germline and is subject to operator influence, therefore, our automated high-throughput method reduces the analysis time and enhances the reproducibility.
Automated nuclei scoring reveals that a germline arm houses approximately 1,000 – 1,200 nuclei (Figure 2C), corresponding well with previously published manual scoring data1. To confirm the accuracy of scoring, multiple mutants and different conditions are used (Figure 2D-G). For instance, we compared rnp-8(tm435), cpb-3(bt17), and glp-1(e2141) mutant worms to wild type animals. Automated counting reproduced the known reduction of number of nuclei in cpb-3 and glp-1 mutant germlines14,15,16,17. A severe reduction in nuclei number was also observed in the glp-1(e2141) temperature-sensitive mutant when incubated at 25 °C. The distribution of nuclei is dependent on the stage of differentiation. The mitotic region, which contains approximately 250 nuclei in wild type, is tightly-packed with nuclei compared to the rest of the germline (Figure 3)1. The spacing between mitotic nuclei is minimal and nuclei are located throughout the mitotic region. However, as the nuclei move away from distal end, they appear more towards the circumference of the germline (Figure 3). The change in distribution starts at the transition zone and completes as the nuclei enter meiosis/pachytene.
The germline has specific cytoskeletal structures at different stages of differentiation. We visualized F-actin by staining the germline with phalloidin. The distribution of F-actin from the distal to proximal end of the germline was distinct at each region (Figure 3 and Figure 5). In general, there are two separate structures that appear as a 'cylinder within cylinder.' At the mitotic region, inner actin appears as a solid mass with a specific shape (Figure 3). As the germline reaches the transition zone, the inner actin assumes a more cylindrical structure and it becomes a hollow cylinder as it reaches late pachytene. The second layer of actin covers the inner layer giving shape to the germline. This 'cylinder within cylinder' actin structure disappears towards the oocyte region (Figure 5). At the oocyte region, actin appears as thick fibers. The oocytes are separated by an actin rachis, though it appears to be dynamic to allow the movement of oocytes to the spermatheca. Finally, actin in the spermatheca also forms thick bundles of actin fibers. However, the fibers appear to be packed closer than in the oocyte region. The cytoskeletal structure appears to complement the distribution patterns of nuclei (Figure 3). At the mitotic region, the nuclei appear to be placed around the inner actin structure. As they reach meiosis/pachytene, the nuclei organize between the two actin cylinders leaving the middle of the germline mostly devoid of nuclei (Figure 3). This could probably aid uninterrupted cytoplasmic streaming in the germline. Germlines were stained with an antibody against the REC-8 protein and analyzed by confocal microscopy. REC-8 is homogeneously distributed around early nuclei of the germline and is later cleaved and degraded during meiosis18. REC-8 distribution in three-dimensional cross-sectional analysis of the germline shows the distribution of protein in the mitotic region (Figure 4).
Three-dimensional rendering of the proximal end of the germline includes the spermatheca and first three oocytes distal to the spermatheca. Our analysis recognized an average of 151 sperm in each spermatheca (n = 18). This corresponds to the published literature, indicating the reliability of the method (Figure 5F)19. This analysis may be performed together with cytoskeletal or protein distribution studies. The C. elegans genome is distributed among six chromosomes. In fully developed wild type oocytes, these chromosomes are visible and can be counted. This chromosome separation can be used to study chromosome stability or other defects associated with oocyte development. By three-dimensional rendering, the number of chromosomes can be visualized, and if the chromosomes are separated properly, the number can be accurately obtained (Figure 5).
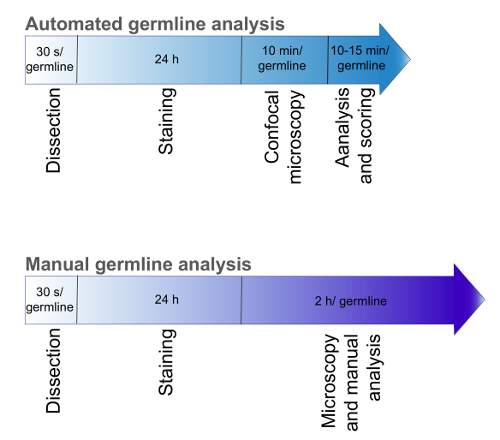
Figure 1. Timeline for automated analysis. Time comparison between manual and computational analysis of the C. elegans germline. Please click here to view a larger version of this figure.
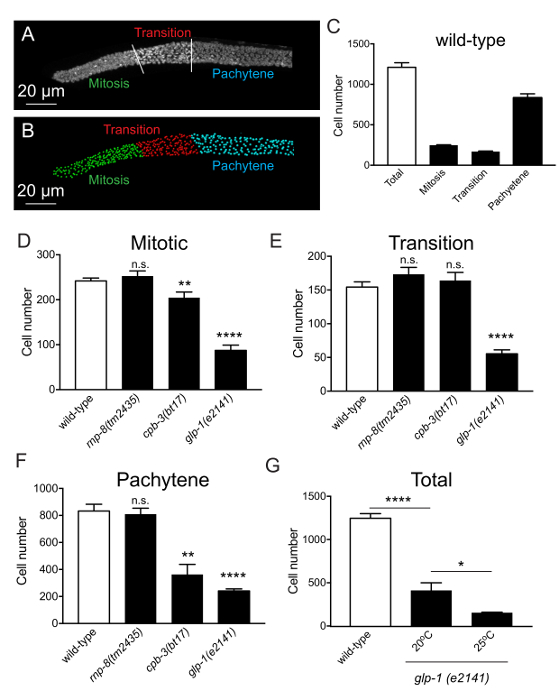
Figure 2. Nuclei distribution in the germline. (A) DAPI stained germline showing mitotic, transition, and pachytene/meiotic regions. (B) Computational three-dimensional model of the germline. (C) Scoring for the total number of nuclei in wild type germlines. (D-F) Scoring for the number of nuclei at mitotic, transition, and meiotic regions of wild type, rnp-8, cpb-3, and glp-1 mutant germlines. (G) Comparison in the total number of nuclei in the glp-1 mutant at 20 °C and 25 °C. Error bar represents standard error. Student's test. ****n <0.0001, ***n <0.001, **n <0.01, *n <0.05. Please click here to view a larger version of this figure.
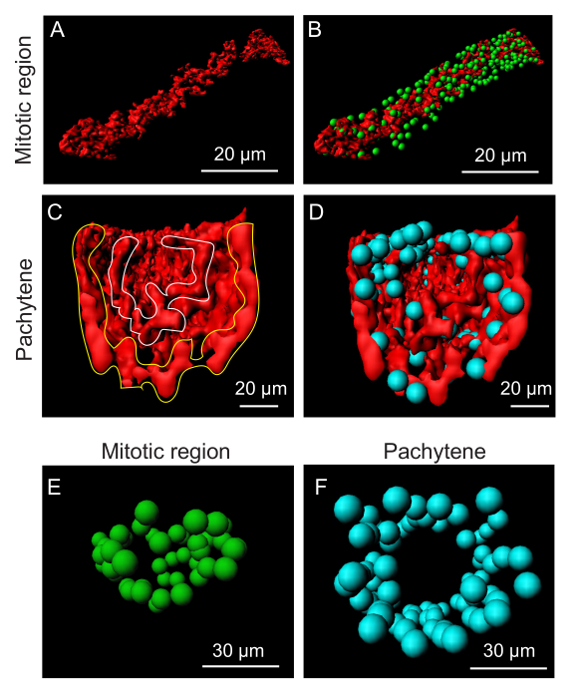
Figure 3. Cytoskeletal structure of the germline. (A-B) Organization of the inner actin (red) cytoskeleton of the germline at the mitotic region. The inner actin has a diffuse structure compared to outer actin at the mitotic region. (C-D) At the pachytene region, the cytoskeleton assumes a cylindrical structure by forming two cylinders between which the nuclei (blue) are organized. (E-F) Organization of germline nuclei in the mitotic and pachytene regions. The distribution of nuclei is more towards the circumference of the 'germtube' at the pachytene region compared to mitotic region (green) and middle of the germline become devoid of nuclei. Please click here to view a larger version of this figure.
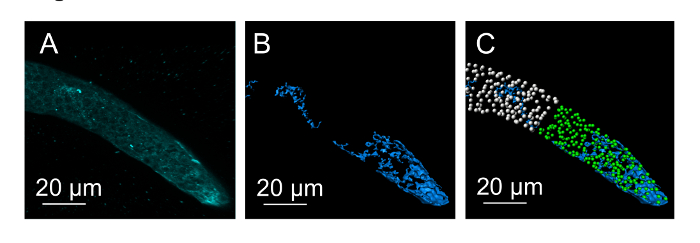
Figure 4. REC-8 expression in the mitotic region. (A) Micrograph showing the distal end of a REC-8-stained germline. (B-C) Three-dimensional rendering of REC-8 staining (shown in blue) and distribution of protein between the mitotic nuclei (green). REC-8 staining is less abundant proximal to the mitotic region (marked by white dots representing nuclei). Please click here to view a larger version of this figure.
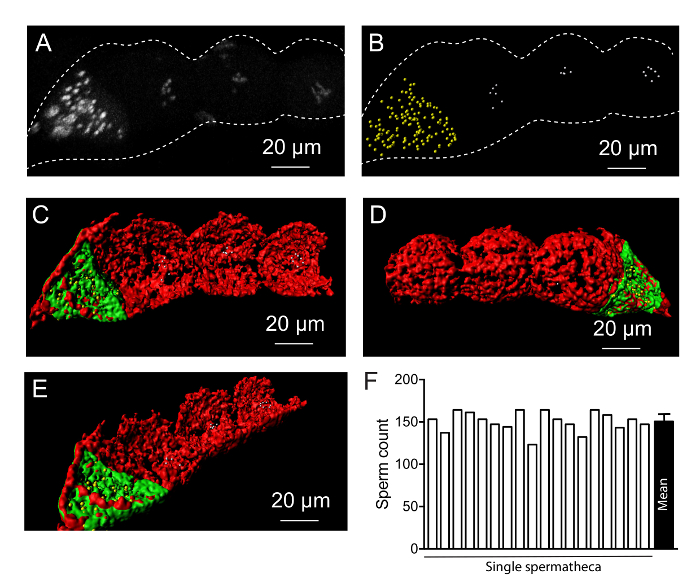
Figure 5. Germline structure at the proximal end. (A) DAPI staining of the germline at the proximal end. (B) Three-dimensional rendering of DAPI staining showing sperm in the spermatheca (yellow) and chromosomes in the oocytes (white). (C-E) Three-dimensional cytoskeletal structure at the proximal end of the germline. Red marks the complete proximal end and green marks the spermatheca. Cross sectional analysis shows that the cytoskeleton not only forms a filamentous structure of actin around oocytes, but also separates between oocytes. (F) Graph showing the number of sperm in each spermatheca and average obtained from multiple spermatheca (n = 18). Error bar represents standard error. Please click here to view a larger version of this figure.
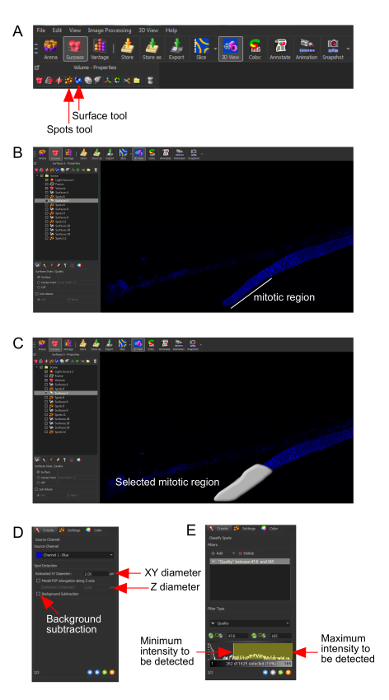
Supplementary Figure 1. Software tools description. (A) The spots and surface tools for detecting nuclei and protein staining. (B-C) The selection of three-dimensional region of interest using the surface tool. (D) Defining the XY-diameter, using spots tool, for nuclei detection. Depending on the region of interest and staining, the Z-axis diameter and background correction can be used. (E) Minimum and maximum intensity threshold detection. A similar approach can be used for surface function where instead of defining the diameter, and the surface details can be defined. Please click here to view a larger version of this figure.
Discussion
The goal of this protocol is to improve the accuracy and reduce the time required for germline analysis. After standard preparation of dissected germlines, a three-dimensional model of germline nuclei is prepared by computational rendering. While allowing the observation of germline nuclei distribution in space, three-dimensional rendering calculates the number of nuclei at specific regions of the germline. The critical aspect of our method is accurate definition of size and shape parameters of nuclei. This depends on clarity of staining and magnification used to image. We confirmed the accuracy of the method by comparing to published manually-analyzed data1. To further confirm the accuracy of our method, we used multiple mutants under different conditions and confirmed the results with published literature. It also confirmed the ability of the method to detect subtle and strong phenotypes – cpb-3 and glp-1 mutants, respectively. In addition, this method shows adaptability to changes in nuclei size and shape within the germline or even between strains. This property also enables the identification and scoring of smaller structures in the germline such as chromosomes and sperm. The method also allows further troubleshooting for size and shape parameters, if required. Our analysis was able to elucidate the distribution of nuclei in the germline and showed that nuclei in mitotic and pachytene regions are organized in different patterns. By applying three-dimensional rendering to REC-8 and DAPI staining, we also showed the distribution of REC-8 within nuclei of the mitotic region.
Three-dimensional rendering of actin staining enabled detailed examination of germline cytoskeletal structures. The germline appears to have two separate actin structures, though they maintain physical contact. While the reconstruction of the cytoskeleton for the entire germline is possible as a single entity, the best results can be obtained if the regions of interest are preselected into mitotic, transition, and meiotic regions. The distal germline cytoskeleton has an undefined shape and evolves into a filamentous structure at the proximal end. Our protocol therefore enables three-dimensional reconstruction of the germline actin cytoskeleton by defining specific parameters to accommodate differences in actin distribution. The most critical aspect of the analysis is the definition of the parameters such as nuclei size and surface detail requirement. Lowering the nuclei size below the actual size will risk the software to pick bright spots within the nuclei as separate objects. Similarly, high surface details values will lead to the loss of accurate three-dimensional structures. The proposed method relies on the precision of dissection and the quality of the germline staining. A damaged germline may lead to the release of nuclei from the germline, causing inaccurate numbering. High background from the staining will also cause the inaccurate nuclei count and improper three-dimensional rendering.
Our method extends previously known automated methods of germline analysis where we are able to analyze the nuclei number and distribution/expression of germline proteins in space with a single protocol20. Secondly, our method enables scoring of smaller structures such as chromosomes within the germline. Chromosome segregation is an important aspect of oocyte development21. Using our method, it is possible to detect the number and position of chromosomes where the distance can be defined by calibrating against wild type oocytes. Any mutation affecting the chromosome separation can be studied using this tool. Also, the method can be extended to study chromosomes in embryos at the 2-cell stage. The computational analysis also provides the number of sperm in the spermatheca, number of chromosomes in an oocyte and germline cytoskeletal structures. Taken together, the method provides comprehensive analysis of the C. elegans germline using a single protocol while reducing analysis time considerably. The method eliminates the requirement of multiple tools for each analysis (nuclei number, protein distribution, and cytoskeletal structure) and removes the coding time required in any specific software. Finally, this method can be effectively extended to other worm studies including the three-dimensional analysis of sedated live worms.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We thank Monash Microimaging for their technical support. Some strains were provided by the Caenorhabditis Genetics Center, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). This work was supported by a Monash University Biomedicine Discovery Fellowship, NHMRC Project Grant (GNT1105374), NHMRC Senior Research Fellowship (GNT1137645) and veski innovation fellowship: VIF 23 to Roger Pocock.
Materials
| C. elegans strains: wild type (N2, Bristol), rnp-8(tm2435) I/hT2[bli-4(e937) let-?(q782) qIs48] (I;III), cpb-3(bt17) I, glp-1 (e2141) III | Caenorhabditis Genetics Center (CGC) | ||
| OP50 Escherichia coli bacteria | Homemade | ||
| Nematode Growth Media (NGM) plates | Homemade | ||
| polyclonal rabbit anti-REC-8 | SDIX | 29470002 | |
| Alexa 488 conjugated antibody raised in goat | Thermofisher Scientific | A-21236 | |
| Cytoskeletal dye phalloidin | Thermofisher Scientific | A-12380 | |
| DAPI | Thermofisher Scientific | 62248 | |
| Poly-L-lysine | Sigma Aldrich | P5899 | |
| Tetramisol | Sigma Aldrich | P5899 | |
| MgSO4 | Sigma Aldrich | M7506 | |
| 1M HEPES buffer, pH 7.4 | Sigma Aldrich | G0887 | |
| 10X PBS pH 7.4 | Thermofisher Scientific | AM9625 | |
| Tween-20 | Sigma Aldrich | P1389 | |
| EGTA | Sigma Aldrich | E3889 | |
| 37% Paraformaldehyde solution | Merck Millipore | 1040031000 | |
| Normal goat serum | Sigma Aldrich | G9023 | |
| Fluoroshield fixing reagent | Sigma Aldrich | F6182 | |
| Ethanol | Millipore | 1009832511 | |
| Methanol | Sigma Aldrich | 34860 | |
| 20°C & 25°CIncubator | Any brand | ||
| Light microscope | Any brand | ||
| Confocal microscope | Any brand (Leica, Zeiss) | ||
| Computer equipped with Imaris suit 8.4.1 or later version, full licence to use the software and Matlab software. | Bitplane | ||
| Phospho buffered saline, pH 7.4 | Homemade | ||
| Teflon microscope slides | Tekdon | 941-322-8288 |
References
- Hubbard, E. J. Caenorhabditis elegans germ line: a model for stem cell biology. Dev Dyn. 236 (12), 3343-3357 (2007).
- Joshi, P. M., Riddle, M. R., Djabrayan, N. J., Rothman, J. H. Caenorhabditis elegans as a model for stem cell biology. Dev Dyn. 239 (5), 1539-1554 (2010).
- Kershner, A., et al. Germline stem cells and their regulation in the nematode Caenorhabditis elegans. Adv Exp Med Biol. 786, 29-46 (2013).
- Byrd, D. T., Knobel, K., Affeldt, K., Crittenden, S. L., Kimble, J. A DTC niche plexus surrounds the germline stem cell pool in Caenorhabditis elegans. PLoS One. 9 (2), 88372 (2014).
- Kimble, J., Crittenden, S. L. Controls of germline stem cells, entry into meiosis, and the sperm/oocyte decision in Caenorhabditis elegans. Annu Rev Cell Dev Biol. 23, 405-433 (2007).
- Hansen, D., Hubbard, E. J., Schedl, T. Multi-pathway control of the proliferation versus meiotic development decision in the Caenorhabditis elegans germline. Dev Biol. 268 (2), 342-357 (2004).
- Crittenden, S. L., et al. A conserved RNA-binding protein controls germline stem cells in Caenorhabditis elegans. Nature. 417 (6889), 660-663 (2002).
- Eckmann, C. R., Crittenden, S. L., Suh, N., Kimble, J. GLD-3 and control of the mitosis/meiosis decision in the germline of Caenorhabditis elegans. Génétique. 168 (1), 147-160 (2004).
- Schumacher, B., et al. Translational repression of C. elegans p53 by GLD-1 regulates DNA damage-induced apoptosis. Cell. 120 (3), 357-368 (2005).
- McMullen, P. D., et al. Macro-level modeling of the response of C. elegans reproduction to chronic heat stress. PLoS Comput Biol. 8 (1), 1002338 (2012).
- Hubbard, E. J., Korta, D. Z., Dalfo, D. Physiological control of germline development. Adv Exp Med Biol. 757, 101-131 (2013).
- Wolke, U., Jezuit, E. A., Priess, J. R. Actin-dependent cytoplasmic streaming in C. elegans oogenesis. Development. 134 (12), 2227-2236 (2007).
- Jones, A. R., Francis, R., Schedl, T. GLD-1, a cytoplasmic protein essential for oocyte differentiation, shows stage- and sex-specific expression during Caenorhabditis elegans germline development. Dev Biol. 180 (1), 165-183 (1996).
- Hasegawa, E., Karashima, T., Sumiyoshi, E., Yamamoto, M. C. elegans CPB-3 interacts with DAZ-1 and functions in multiple steps of germline development. Dev Biol. 295 (2), 689-699 (2006).
- Austin, J., Kimble, J. glp-1 is required in the germ line for regulation of the decision between mitosis and meiosis in C. elegans. Cell. 51 (4), 589-599 (1987).
- Berry, L. W., Westlund, B., Schedl, T. Germ-line tumor formation caused by activation of glp-1, a Caenorhabditis elegans member of the Notch family of receptors. Development. 124 (4), 925-936 (1997).
- Fox, P. M., Schedl, T. Analysis of Germline Stem Cell Differentiation Following Loss of GLP-1 Notch Activity in Caenorhabditis elegans. Génétique. 201 (1), 167-184 (2015).
- Pasierbek, P., et al. A Caenorhabditis elegans cohesion protein with functions in meiotic chromosome pairing and disjunction. Genes and Development. 15 (11), 1349-1360 (2001).
- Klass, M., Wolf, N., Hirsh, D. Development of the male reproductive system and sexual transformation in the nematode Caenorhabditis elegans. Dev Biol. 52 (1), 1-18 (1976).
- Korta, D. Z., Tuck, S., Hubbard, E. J. S6K links cell fate, cell cycle and nutrient response in C. elegans germline stem/progenitor cells. Development. 139 (5), 859-870 (2012).
- Laband, K., et al. Chromosome segregation occurs by microtubule pushing in oocytes. Nat Commun. 8 (1), 1499 (2017).

