The Unpredictable Chronic Mild Stress Protocol for Inducing Anhedonia in Mice
Summary
Here we present the unpredictable chronic mild stress protocol in mice. This protocol induces a long-term depressive-like phenotype and enables to assess the efficacy of putative antidepressants in reversing the behavioral and neuromolecular depressive-like deficits.
Abstract
Depression is a highly prevalent and debilitating condition, only partially addressed by current pharmacotherapies. The lack of response to treatment by many patients prompts the need to develop new therapeutic alternatives and to better understand the etiology of the disorder. Pre-clinical models with translational merits are rudimentary for this task. Here we present a protocol for the unpredictable chronic mild stress (UCMS) method in mice. In this protocol, adolescent mice are chronically exposed to interchanging unpredictable mild stressors. Resembling the pathogenesis of depression in humans, stress exposure during the sensitive period of mice adolescence instigates a depressive-like phenotype evident in adulthood. UCMS can be used for screenings of antidepressants on the variety of depressive-like behaviors and neuromolecular indices. Among the more prominent tests to assess depressive-like behavior in rodents is the sucrose preference test (SPT), which reflects anhedonia (core symptom of depression). The SPT will also be presented in this protocol. The ability of UCMS to induce anhedonia, instigate long-term behavioral deficits and enable reversal of these deficits via chronic (but not acute) treatment with antidepressants strengthens the protocol's validity compared to other animal protocols for inducing depressive-like behaviors.
Introduction
Major depressive disorder (MDD) is a debilitating condition, that has been indicated as the 11th cause of global burden from disease1, with a lifetime prevalence of 11–16%2,3. MDD has been associated with severe impairments on patients' social and occupational functioning, diminished quality of life, numerous mental and physical disorders and increased risk for mortality4,5,6,7. There are several efficacious pharmacotherapies and psychological interventions for MDD; however, more than the third of the patients do not achieve remission with the existing therapeutic options8,9,10,11. Therefore, better mapping of the pathophysiology of MDD and development of novel drugs are still of utmost importance. In order to address these tasks scientifically validated animal models needs to be utilized.
Unpredictable chronic mild stress (UCMS) is a renowned rodent paradigm used to induce depressive- and anxiety-like behaviors12,13,14,15. The main objective of UCMS is to generate behavioral deficits (such as anhedonia and behavioral despair12,15) in mice and rats, and promote screenings for potential therapeutic pharmacological agents. The procedure was first introduced by Katz16 and subsequently developed by Willner17,18, yielding vast behavioral and neurobiological outcomes reminiscing depressive symptomatology12. It was initially designed for rats and later accommodated to mice13,19. In the procedure, adolescent animals are chronically exposed to different unpredictable mild stressors. Subsequently, pharmacological agents are administered. Behavioral and biological indices are obtained upon treatment termination. One of the more prominent tests conducted following UCMS is the sucrose preference test (SPT). The SPT is based on rodents' innate preference for the sweetened solution rather than water and is widely acknowledged as an essential translational model for assessing anhedonia12,18,20,21 (which is a core symptom in human depression22,23).
While entering the fourth decade since its introduction, UCMS has been applied on mice and rats in myriad studies. The majority of these studies employed UCMS as a method to induce depressive-like behaviors12,13,21,24. Studies have also employed the model to generate anxiogenic effects25,26,27,28,29. Sucrose and saccharin preferences are the main tests used to assess anhedonia following UCMS12,18,30,31,32,33. Other notable outcome measures that are highly incorporated in UCMS literature are: the tail suspension test (TST)28,34,35, the forced swim test (FST)28,34,36,37 (both measuring stress coping/behavioral despair), the open field test (OFT; measuring exploratory behavior, anxiety-like behavior and locomotor activity)25,28,38, the elevated plus maze (EPM; measuring anxiety-like behavior)25,39,40 and additional tests measuring depressive-like behaviors, anxiety-like behaviors, cognitive functioning and social behavior12. Chronic administration of tricyclic antidepressants (TCAs; imipramine35,41,42,43, desipramine18,44,45), tetracyclic antidepressants (TeCAs; maprotiline46,47, mianserin48), selective serotonin reuptake inhibitors (SSRIs; fluoxetine46,47,49, escitalopram30,50, paroxetine51,52), melatonin43,49, agomelatine53, the fatty acid amide hydrolase (FAAH) inhibitor URB59754 and several natural compounds30,37,50,55,56,57,58 have been demonstrated to reverse the UCMS-induced depressive- and anxiety-like symptoms. Overall, these therapeutic effects have not been obtained via acute treatments12 (e.g., paroxetine51,52, imipramine53,54,59,60, fluoxetine53, agomelatine53, URB59754, brofaromine60).
Stress exposure during childhood and adolescence is a major risk factor for the anterior formation of MDD (among several other psychiatric disorders) in adulthood61,62,63. The hypothalamic-pituitary-adrenal (HPA) axis is a major neuroendocrine system regulating the bio-behavioral response to stress64. Long-term stress during the sensitive neurodevelopmental periods of childhood and adolescence impairs the equilibrium of the HPA axis. It might provoke a state of enhanced sympathetic activation, unbalanced reactivity and hypercortisolemia lasting through the resting state; thus, rendering individuals vulnerable to the depression or anxiety-related psychopathologies65,66,67,68. UCMS adequately translates this pathogenesis: stress application during mice' adolescence induces a long-term depressive-like susceptibility. Moreover, the behavioral deficits induced by UCMS, are underlain by significant alterations in HPA axis functioning (e.g., by causing a reduction in hippocampal brain-derived neurotrophic factor [BDNF; a protein highly involved in the equilibrium of the HPA axis69,70]30, or by impairing the regulation of corticosterone secretion to the blood71,72), in similarity to the pathophysiology in humans12,50,73.
UCMS has several bolstering features as a model for depression: e.g. (i) the elicitation of anhedonia (which is regarded an endophenotype of MDD23,74); (ii) UCMS enables to assess wide variety of depressive-like behaviors such as behavioral despair, reduced social behavior, deterioration in fur state and more34; and (iii) chronic (2-4 weeks), but not acute, administration of antidepressants following stress exposure could produce a protracted therapeutic effect parallel to the effect obtained in human patients by the same agents30,75,76,77.
These features strengthen the validity of UCMS compared to other animal models of depression. The FST78 and the TST79 are two models that are used either to induce or to assess depressive-like behavior. As models for inducing depressive-like behaviors they have clear shortfalls compared to UCMS; they do not prompt long-term behavioral changes and might merely reflect an adjustment to acute stress rather than yield a durable depressive-like manifestation76.
An alternative animal model of depression is the social defeat model. Unlike the FST and the TST this model (like UCMS) require the application of chronic stress (id est [i.e.], the recurrent subjection of the animal to aversive social encounters with dominant counterparts)76,77,80,81,82. The main advantage of the social defeat model is that it employs social stimuli as stressors, thus reflecting the role of psychosocial stress in the pathogenesis of human depression. Similar to UCMS, the social defeat model elicits long-term depressive-like behaviors and neuroendocrine alterations. Yet again parallel to UCMS, the social defeat-induced deficits could be reversed via chronic, but not acute, administration of antidepressants. Overall, there is large support for the utilization of both UCMS and social defeat as pre-clinical apparatuses for investigating the pathophysiology of depression76,77,81,82. However, a major shortfall of the social defeat model is that it could only be applied on male rodents, as females do not exhibit sufficient aggressive behavior toward each other83. Contrastingly, UCMS has been shown to produce several depressive-like effects on both male and female mice34.
Predictable chronic mild stress (PCMS) is another rodent model that enforces a regimen of daily recurring exposure to restraint stress28,84,85,86,87. Several studies have shown that PCMS increased anxiety-like behaviors28,87; albeit, there are contradictory reports vis-à-vis PCMS ability to induce long-term depressive-like behaviors. Unlike UCMS, PCMS has produced less satisfactory results referring to its ability to induce an anhedonic-like state28,84,86. This is consistent with the human phenomenology, in which unpredictable stressors are more harmful than predictable ones88.
Protocol
All methods described here have been approved by the Institutional Animal Care and Use Committee of the Academic College Tel-Aviv-Yaffo.
1. Animals
- Use pre-adolescent (i.e., 3 weeks old) Institute of Cancer Research (ICR) outbred male mice.
- Randomize mice to two equally sized stress group (UCMS vs. naïve). Use 15 mice per treatment group (e.g.: if there are 3 pharmacological treatment groups use 90 mice overall; 2 [UCMS vs. naïve] × 3 [treatments] × 15 [mice] = 90)
- House mice according to the stress group; namely, house naïve mice with naïve mice only, and house UCMS group mice with mice from the UCMS group only.
- House animals in standard home cages (30 × 15 × 14 cm; 5 mice per cage; each cage containing mice from all treatment groups [i.e., pharmacological treatment groups]; maintain mice in the same cage throughout the experiment, except when indicated otherwise).
- Fill home cages with fresh sawdust (replaced twice a week) and add a piece of cotton wool for enrichment.
- House animals in the home cage for an acclimation period of one week. Allow ad libitum access to rodent chow and water (except during UCMS stressor applications).
- Keep a consistent 12 h light/dark cycle (except when indicated otherwise). During UCMS procedures maintain naïve mice in their home cage.
2. UCMS
- Designate a separate room in the lab, for the sole use of the UCMS protocol.
- Design a 4-week stressor regimen in which each of the seven stressors (i.e., wet cage, dampened sawdust, tilted cage, empty cage, social stress, mice restraint and disruption of light/dark cycle) is utilized once a week, on a different day each week (for a possible design see Supplemental Table 1).
- Following 1 week of acclimation (see 1.6) initiate stressors application (ensure that mice are approximately 4 weeks old).
- Each day, before the stressor application, transfer the cages of the UCMS group from the housing room to the UCMS room.
- During stressor applications, block access to the rodent chow and water for the UCMS group (except during reversal of light/dark cycle).
NOTE: This could be obtained by the replacement of the original cage lid (which contains food and water), to an empty cage lid. - Apply the following stressors according to the regimen designed earlier (see 2.2):
- Wet cage
- Place mice together with their home cage counterparts in an empty cage (i.e., cage without sawdust).
- Fill the empty cage with water kept at 24 ± 1 °C to a depth of 1 cm (pour with caution to avoid direct water spillage on mice). Keep mice in the wet cage for 4 h.
- Transfer each mouse to a separate individual transient drying cage with a heat lamp above it, a heating pad under it and paper towel bedding. Place a thermometer in the transient cage to verify the temperature does not exceed 37 °C.
- Keep each mouse in the transient cage until it is dry and looks invigorated (approximately 10–15 min). Return mice to home cage with same counterparts.
- Dampened sawdust
- Pour water kept at 24 ± 1 °C to the home cage until the sawdust is moderately dampened (pour with caution to avoid direct water spillage on mice).
NOTE: It is not necessary to use fresh sawdust before pouring the water. - After 4 h, dry mice in transient cages as described in 2.6.1.3. Place mice with home cage counterparts in a sterile cage with fresh sawdust.
- Pour water kept at 24 ± 1 °C to the home cage until the sawdust is moderately dampened (pour with caution to avoid direct water spillage on mice).
- Tilted cage
- Tilt cages at 45° against the wall for 4 h.
NOTE: During this period, stressor mice remain in their home cage with their counterparts.
- Tilt cages at 45° against the wall for 4 h.
- Empty cage
- Transfer mice, along with their specific home cage counterparts, from the home cage to an empty cage for 4 h.
- Social stress
- Transfer mice, along with their specific home cage counterparts, from the home cage to a cage which was housed by a different group of mice for a period of at least 3 d prior to stressor application. Keep mice in the unfamiliar cage for 4 h.
NOTE: To avoid uncertainty place a sticker on each cage to indicate mice origin cage.
- Transfer mice, along with their specific home cage counterparts, from the home cage to a cage which was housed by a different group of mice for a period of at least 3 d prior to stressor application. Keep mice in the unfamiliar cage for 4 h.
- Mice Restraint
- Place each mouse separately in a clean mouse restrainer for 4 h. Return mice to home cage with same counterparts.
- Disruption of light/dark cycle
- Transfer mice, in their home cage with their specific counterparts, to the UCMS room. Keep the light on, for 24 consecutive h.
NOTE: Only during this stressor mice will be allowed ad libitum access to rodent chow and water.
- Transfer mice, in their home cage with their specific counterparts, to the UCMS room. Keep the light on, for 24 consecutive h.
- Wet cage
- Following stressor application, return cages of the UCMS group from the UCMS room to the housing room.
- During the 4 weeks of stress exposure, keep the naïve group in their home cages located in the housing room.
NOTE: Naïve mice are not transferred to the UCMS rooms because exposure to other mice undergoing stress procedure could induce a stressogenic effect, even without direct exposure to the stressor89,90. - Monitoring of Animals during UCMS
- During stressor applications (except during disruption of light/dark cycle) monitor mice every 30 min by an experienced experimenter. If an atypical distress (e.g., trembling, lethargy, lack of movement) is observed (special caution should be placed on potential hypothermia during 'wet cage' and 'dampened sawdust') relieve the mouse from the stressor immediately.
- Inspect each mouse daily for wounds or other physical or behavioral abnormalities. If such are inspected consult with the laboratory's veterinarian to decide whether the mouse should be excluded from the experiment.
- Weigh each mouse every 3 days. Robust reduction in body weight (i.e., >10% reduction from baseline weight or >15% reduction from last measured weight) must be reported to the laboratory's veterinarian and the mouse must be excluded from the experiment.
3. Screening for Antidepressants
- On the day following cessation of the UCMS protocol, start administration of putative therapeutic pharmacological agents (i.e., escitalopram [15 mg/kg; i.p.; 3 weeks; on administration per day]30,50, or novel herbal treatment [NHT; 30 mg/kg; i.p.; 3 weeks; one administration per day]30,50).
NOTE: For doses of other drugs not included in this protocol see: flouxetine46, 47, 49, 89, 90, paroxetine51, imipramine35,41,42,43, desipramine18,44,45, maprotiline46,47, mianserin48, melatonin43,49, URB59754 and other natural compounds37,55,56,57,58.
NOTE: NHT is an herbal formula that was developed in our lab. It is composed of 4 constituents:
Crataegus Pinnatifida, Triticum Aestivum, Lilium Brownie, and Fructus Zizyphi Jujube. The formula is prepared by dissolving the constituents (acquired as freeze-dried granules) together in saline containing 1% DMSO to give a final concentration of 0.47 mg/ml (each)27,50. - Include a control treatment group and administer saline (i.p.; 3 weeks; one injection per day) to this group.
- Calculate drug dose according to mice' weight, as obtained in the preceding weighing.
- Administer drugs chronically (usually one drug administration per day via intraperitoneal injection [i.p.] for 3 weeks).
NOTE: There is no need to anesthetize the mice before the i.p. injection. - Continue to weigh mice every 3 d; the last weighing should be 3 d before the last drug administration.
4. Assessment of Hedonic Tone in the SPT
- Following the treatment phase, remove each mouse from the home cage and place it individually in a cage filled with fresh sawdust and a piece of cotton wool for enrichment.
- Prepare two bottles, one with distilled water and another with 2% sucrose solution (other substance could be used: e.g., saccharin91, ethanol30).
NOTE: Each bottle should contain the same volume of fluid. Neither prior acclimation nor habituation phases are needed before the introduction of fluids. - Weigh the two bottles and set them at the cage lid to allow mice ad libitum access to both solutions for a period of (one of the following): 24/48/72/144 h.
- Place the two bottles at both ends of the cage lid. Place rodent chow between the two bottles to allow ad libitum access to food.
- Replace bottles every 24 h, using sterile bottles with fresh fluid.
NOTE: As mice are housed individually there is no need to change the sawdust even after 144 h92. - Switch nozzles' positions every 12 h (when test duration is 24 h) or once a day (when test duration exceeds 24 h) to counterbalance the possibility that the results were confounded by position preference.
- Weigh bottles each day to estimate consumption from each bottle.
- Calculate sucrose preference as theratio of sucrose intake from total fluid intake (i.e., sucrose/sucrose + water).
Representative Results
In order to corroborate the efficacy of the UCMS procedure for inducing depressive-like deficits, a manipulation check was conducted. Male ICR outbred mice were randomly assigned to either UCMS or naïve conditions (4 weeks, as described in protocol 2.2). Subsequently, the SPT (6 days, as described in protocol 4) was administered to assess whether mice after undergoing UCMS demonstrated hedonic deficits. Shortly after, mice were sacrificed and the hippocampus was dissected out entirely for BDNF (a protein highly implicated in the pathophysiology of depression70,93) assessment via enzyme-linked immunosorbent assay (ELISA). See Figure 1 for the study design.
Independent samples t-test revealed a significant difference between the groups in their sucrose preference (t(23) = 2.32, p < 0.05). The UCMS group demonstrated diminished sucrose preference compared to the naïve group (see Figure 2A). This suggests that the UCMS protocol was effective in inducing anhedonia. Independent samples t-test on hippocampal BDNF levels revealed a significant difference between the groups (t(23) = 2.43, p < 0.05). The UCMS group demonstrated diminished hippocampal BDNF levels compared to the naïve group (see Figure 2B). This suggests that the UCMS protocol led to the diminution in hippocampal BDNF levels, as evident in human depression94.
In another study from our lab, we examined the potential antidepressant-like effects of two drugs following UCMS protocol.30 Following the UCMS procedure (as described in 2.) male ICR outbred mice received chronic (3 weeks) treatment with the SSRI escitalopram (15 mg/kg; i.p.), NHT (30 mg/kg; i.p.; for more information regarding NHT see references25,30,50,75) or saline. Following treatment phase, the SPT was conducted and hippocampal BDNF levels were assessed. See Figure 3 for the study design.
Two-way analysis of variance (ANOVA) on sucrose preference revealed significant treatment (F(2,92) = 4.01, p < 0.05) and UCMS × treatment interaction (F(2,92) = 4.92, p < 0.01) effects (see Figure 4A). Sidak post-hoc analysis revealed that the UCMS-saline group demonstrated a significant decrease in sucrose preference compared to the naïve-saline group (p < 0.001); no decreases were observed in the UCMS-escitalopram and in the UCMS-NHT groups compared to the naïve groups (not significant [N.S.]). Additionally, the UCMS-saline group demonstrated decreased sucrose preference compared to both the UCMS-escitalopram (p < 0.05) and the UCMS-NHT (p < 0.001) groups. These suggest that both escitalopram and NHT normalized the UCMS-induced anhedonia.
Two-way ANOVA on hippocampal BDNF levels revealed significant UCMS (F(1,22) = 8.92, p < 0.01), treatment (F(2,22) = 18.36, p < 0.001) and UCMS × treatment interaction (F(2,22) = 5.19, p < 0.05) effects (see Figure 4B). Sidak post-hoc analysis revealed that the UCMS-saline group demonstrated a significant decrease in hippocampal BDNF levels compared to the naïve-saline group (p < 0.001); no similar decreases were observed in the UCMS-escitalopram and in the UCMS-NHT groups compared to the naïve groups (N.S.). Additionally, the UCMS-saline group demonstrated decreased hippocampal BDNF levels compared to both the UCMS-escitalopram and the UCMS-NHT groups (p < 0.001 in both contrasts). These suggest that both escitalopram and NHT normalized the UCMS-induced reduction in BDNF levels in the hippocampus.
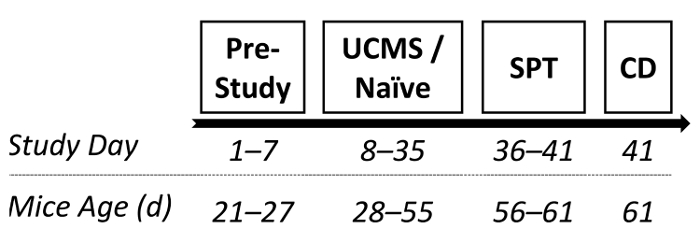
Figure 1: A diagram depicting a possible experimental design. Following 1 week of acclimation, mice were randomly assigned to either UCMS or naïve conditions (persisting 4 weeks). Subsequently, sucrose preference was examined and mice were prepared for BDNF assessment. SPT: sucrose preference test; CD: cervical dislocation. Please click here to view a larger version of this figure.
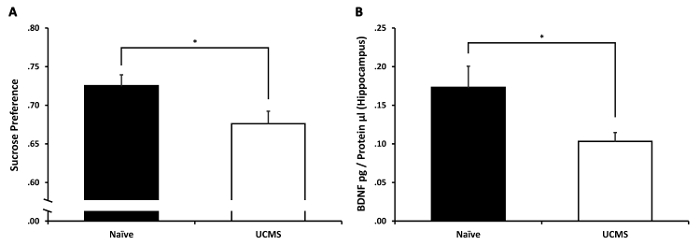
Figure 2: The effects of UCMS on sucrose preference and hippocampal BDNF levels. (A) Mice subjected to 4 weeks of UCMS demonstrated a significant reduction in sucrose preference compared to naïve mice. (B) Mice subjected to 4 weeks of UCMS demonstrated a significant reduction in hippocampal BDNF levels compared to naïve mice. n = 12–13 mice per group. Results are expressed as mean ± SEM. *p < 0.05 Please click here to view a larger version of this figure.
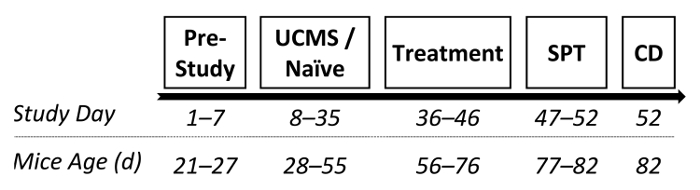
Figure 3: A diagram depicting a possible experimental design. Following 1 week of acclimation, mice were randomly assigned to either UCMS or naïve conditions (persisting 4 weeks). Subsequently, mice received chronic treatment with saline, escitalopram (15 mg/kg; i.p.) or NHT (30 mg/kg; i.p.), lasting 3 weeks (one administration per day). Following treatment, sucrose preference was examined and mice were prepared for BDNF assessment. SPT: sucrose preference test; CD: cervical dislocation. Please click here to view a larger version of this figure.
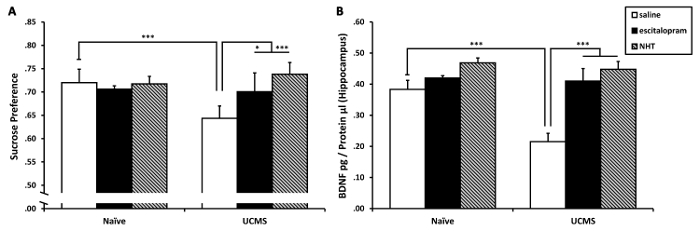
Figure 4: The effects of chronic treatment with escitalopram and NHT on UCMS-induced reductions in sucrose preference and hippocampal BDNF levels. (A) Both escitalopram and NHT prevented the UCMS-induced reduction in sucrose preference; n = 15–17 mice per group. (B) Both escitalopram and NHT prevented the UCMS-induced reduction in hippocampal BDNF levels; n = 4–6 mice per group. Results are expressed as mean ± SEM. *p < 0.05 ***p < 0.001. This figure has been modified from a previously published study from our lab and is reprinted under PLoS ONE open access license ("CC-BY") which allows reprint30. Please click here to view a larger version of this figure.
Supplemental Table 1: Schedule of unpredictable chronic mild stress (UCMS). Please click here to download this file.
Discussion
Insofar as MDD is a widespread highly debilitating disorder, only partially addressed by current therapeutic options, the scientific quest for better treatments is still a pressing issue. Along with innovations in psychological techniques, additional pharmacotherapies are required for the large portion of patients who do not respond to the existing drugs. Meticulous animal models for depression are the key element in this task. Such models facilitate screenings for innovative antidepressants and expand the understanding of the etiology of the disorder. UCMS is one of the more prominent rodent models of depression. Its' stature is exhibited by vast publications and notable insights12,18,82,95,96,97.
Anhedonia is one of the core symptoms of MDD22,23,74. A more severe anhedonic tone has been associated with poorer prognosis for MDD patients74,98. A major strength of UCMS as a model of depression is its ability to generate anhedonia31 as exemplified in the SPT. Sucrose is an innate reinforcer for various rodent species51,52,59,99; this explains the overall support of SPT as a realistic model of hedonic tone in rodents14,31,100. Due to the focal role of anhedonia in any animal model of depression, it has been suggested that when considering implementing UCMS into a lab, the first step should be a verification of the procedure ability to induce an anhedonic state12. This will facilitate a better standardization across labs and could be the foundation for future studies shedding more light on the disorder.
Another feature that supports the validity of UCMS as a model of depression is that the behavioral and molecular alterations induced by UCMS are reversed by chronic, but not acute, treatment with agents that have been previously verified as effective antidepressants12. The protracted therapeutic effect is similar to the effects of antidepressants in humans, which usually start manifesting only after 2-3 weeks of treatment101,102. In this regard, UCMS possess a superior face validity compared to the FST78 and the TST103, in which the effects are obtained also following acute treatment. Unlike the TST and the FST, this shortfall is not evident in the social defeat model of depression, which (along with UCMS) stand out as an excellent animal model of depression. However, compared to the FST and the TST, UCMS and other chronic stress models are much more lengthy and expensive.
Notable mice strains have been employed in UCMS studies. Among the more frequent strains are the C57BL/6 and the BALB/cJ21,34. We have utilized male ICR outbred mice as numerous studies have demonstrated the efficiency of UCMS in this strain. Moreover, the utilization of ICR outbred mice bolsters the ecological validity of the protocol, due to the high between animals genetic variability of this strain (compared to transgenic mice strains)30,75,104,105,106.
For suggested doses of other drugs not included in this protocol, but were used in other UCMS protocols see: flouxetine46,47,49,104,105, paroxetine51, imipramine35,41,42,43, desipramine18,44,45, maprotiline46,47, mianserin48, melatonin43,49, URB59754 and other natural compounds37,55,56,57,58.
There are several additional outcome measures frequently applied in UCMS protocols, among them: (i) FST: a measure for behavioral despair (see reference78 for protocol delineation); (ii) TST: another measure for behavioral despair (see reference103 for protocol delineation); (iii) splash test and evaluation of coat state: two indicators of grooming behavior and putative measures of apathy (see reference107 for protocol delineation); (iv) sociability/preference for social novelty: measures for social behavior108 (see reference109 for protocol delineation); and (v) sexual behavior: another measure for hedonic tone (see reference75 for protocol delineation). Furthermore, UCMS is used to assess neuromolecular, endocrine and other biological measures pertinent to depression50,72,110,111,112,113,114,115,116 (specifically, see references117,118 for BDNF assessment via ELISA protocol delineation).
There are several critical steps within the UCMS protocol: (i) it is vital that mice from all treatment groups will be housed together and not in separate cages. For example, if there are 3 treatment groups (e.g., escitalopram, NHT, and saline) there will be 2 mice from 2 of the groups and 1 mouse from the remaining group in each cage. The hybrid group housing will thwart the possibility that the results were underlain by the housing conditions and not the treatment per se. (ii) naïve mice must be housed separately from stressed mouse, since housing with stressed mice is stressful90,119,120 and, therefore, could impede or attenuate the stress manipulation. (iii) previous UCMS protocols have instructed single housing rather than group housing32,121; we have suggested the latter as single housing might cause further susceptibility to stress in mice and rats122,123,124,125. (iv) the UCMS schedule must be designed diligently to ensure unpredictability (i.e., random exposure to each stressor once a week); however, schedules could be modified throughout the experiment as long as the unpredictability is kept.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Gali Breuer for her assistance in the video production. This research was supported by the Israel Ministry of Science, Technology & Space (grant no. 313552), by the National Institute for Psychobiology in Israel (NIPI-208-16-17b) and by the Open University Foundation.
Materials
| Heating lamp | Ikea | AA-19025-3 | |
| Heating pillow | Sachs | EF-188B | |
| Mice restrainer | |||
| Portable electronic balance (*.** g) | |||
| Standard rubber stopper, size 5 | Ancare | #5.5R | To avoid spillage during SPT |
| Straight open drinking tube (2.5") | Ancare | OT-100 | To avoid spillage during SPT (insert drinking tube into rubber stopper) |
| 2% sucrose solution | |||
| 50ml conical centrifuge tube | For the SPT | ||
| Pre-adolescent (approximately 20-days old) ICR outbred mice | Envigo | Hsd:ICR (CD-1) |
References
- Murray, C. J., et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study. Lancet. 380 (9859), 2197-2223 (2010).
- Bromet, E., et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Medicine. 9, (2011).
- Kessler, R. C., et al. The Epidemiology of Major Depressive Disorder. JAMA: The Journal of the American Medical Association. 289 (23), 3095 (2003).
- Doom, J. R., Haeffel, G. J. Teasing apart the effects of cognition, stress, and depression on health. American Journal of Health Behavior. 37 (5), 610-619 (2013).
- Mykletun, A., Bjerkeset, O., Øverland, S., Prince, M., Dewey, M., Stewart, R. Levels of anxiety and depression as predictors of mortality: The HUNT study. British Journal of Psychiatry. 195 (2), 118-125 (2009).
- Moussavi, S., Chatterji, S., Verdes, E., Tandon, A., Patel, V., Ustun, B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 370 (9590), 851-858 (2007).
- Otte, C., et al. Major depressive disorder. Nature Reviews Disease Primers. 2, (2016).
- Rush, A. J., et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Amerian Journal of Psychiatry. 163 (11), 1905-1917 (2006).
- Cuijpers, P., Karyotaki, E., Weitz, E., Andersson, G., Hollon, S. D., Van Straten, A. The effects of psychotherapies for major depression in adults on remission, recovery and improvement: A meta-analysis. Journal of Affective Disorder. 159, 118-126 (2014).
- Lam, R. W., et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder. Canadian Journal of Psychiatry. 61 (9), 510-523 (2016).
- Kupfer, D. J., Frank, E., Phillips, M. L. Major depressive disorder: New clinical, neurobiological, and treatment perspectives. Lancet. 379 (9820), 1045-1055 (2012).
- Willner, P. Chronic mild stress (CMS) revisited: Consistency and behavioural- neurobiological concordance in the effects of CMS. Neuropsychobiology. 52 (2), 90-110 (2005).
- Surget, A., Belzung, C. Unpredictable chronic mild stress in mice. Experimental Animal Model in Neurobehavior Research. , 79-112 (2009).
- Hoffman, K. L. 2 -What can animal models tell us about depressive disorders?. Modelling Neuropsychiatric Disorder in Laboratory Animals. , (2016).
- Cryan, J. F., Holmes, A. The ascent of mouse: advances in modelling human depression and anxiety. Nature Review Drug Discovery. 4 (9), 775-790 (2005).
- Katz, R. J., Roth, K. A., Carroll, B. J. Acute and chronic stress effects on open field activity in the rat: Implications for a model of depression. Neuroscience and Biobehavior Reviews. 5 (2), 247-251 (1981).
- Willner, P. The validity of animal models of depression. Psychopharmacology (Berlin). 83 (1), 1-16 (1984).
- Willner, P., Towell, A., Sampson, D., Sophokleous, S., Muscat, R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berlin). 93 (3), 358-364 (1987).
- Ducottet, C., Belzung, C. Behaviour in the elevated plus-maze predicts coping after subchronic mild stress in mice. Physiology and Behavior. 81 (3), 417-426 (2004).
- Treadway, M. T., Zald, D. H. Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neuroscience and Biobehavioral Reviews. 35 (3), 537-555 (2011).
- Pothion, S., Bizot, J. C., Trovero, F., Belzung, C. Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behavioural Brain Research. 155 (1), 135-146 (2004).
- American Psychiatric Association. . Diagnostic and Statistical Manual of Mental Disorders. 5th Edition (DSM-5). , (2013).
- Pizzagalli, D. A. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annual Review Clinical Psychology. 10, 393-423 (2014).
- Nollet, M., Le Guisquet, A. -. M., Belzung, C. Models of depression: unpredictable chronic mild stress in mice. Current Protocols in Pharmacology. , (2013).
- Doron, R., Lotan, D., Rak-Rabl, A., Raskin-Ramot, A., Lavi, K., Rehavi, M. Anxiolytic effects of a novel herbal treatment in mice models of anxiety. Life Science. 90 (25-26), 995-1000 (2012).
- Rössler, A. S., Joubert, C., Chapouthier, G. Chronic mild stress alleviates anxious behaviour in female mice in two situations. Behavioural Processes. 49 (3), 163-165 (2000).
- Maslova, L. N., Bulygina, V. V., Markel, A. L. Chronic stress during prepubertal development: Immediate and long-lasting effects on arterial blood pressure and anxiety-related behavior. Psychoneuroendocrinology. 27 (5), 549-561 (2002).
- Zhu, S., Shi, R., Wang, J., Wang, J. -. F., Li, X. -. M. Unpredictable chronic mild stress not chronic restraint stress induces depressive behaviours in mice. Neuroreport. 25 (14), 1151-1155 (2014).
- Bondi, C. O., Rodriguez, G., Gould, G. G., Frazer, A., Morilak, D. A. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 33 (2), 320-331 (2008).
- Burstein, O., et al. Escitalopram and NHT normalized stress-induced anhedonia and molecular neuroadaptations in a mouse model of depression. PLoS One. 12 (11), (2017).
- Willner, P., Muscat, R., Papp, M. Chronic mild stress-induced anhedonia: A realistic animal model of depression. Neuroscience and Biobehavioral Reviews. 16 (4), 525-534 (1992).
- Papp, M., Willner, P., Muscat, R. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berlin). 104 (2), 255-259 (1991).
- Kumar, B., Kuhad, A., Chopra, K. Neuropsychopharmacological effect of sesamol in unpredictable chronic mild stress model of depression: Behavioral and biochemical evidences. Psychopharmacology (Berlin). 214 (4), 819-828 (2011).
- Mineur, Y. S., Belzung, C., Crusio, W. E. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behavioral Brain Research. 175 (1), 43-50 (2006).
- Ibarguen-Vargas, Y., et al. Deficit in BDNF does not increase vulnerability to stress but dampens antidepressant-like effects in the unpredictable chronic mild stress. Behavioral Brain Research. 202 (2), 245-251 (2009).
- Luo, D. D., An, S. C., Zhang, X. Involvement of hippocampal serotonin and neuropeptide Y in depression induced by chronic unpredicted mild stress. Brain Research Bulletin. 77 (1), 8-12 (2008).
- Bhutani, M. K., Bishnoi, M., Kulkarni, S. K. Anti-depressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neurochemical changes. Pharmacolology and Biochemistry Behavior. 92 (1), 39-43 (2009).
- Lin, Y. H., Liu, A. H., Xu, Y., Tie, L., Yu, H. M., Li, X. J. Effect of chronic unpredictable mild stress on brain-pancreas relative protein in rat brain and pancreas. Behavior Brain Research. 165 (1), 63-71 (2005).
- Cox, B. M., Alsawah, F., McNeill, P. C., Galloway, M. P., Perrine, S. A. Neurochemical, hormonal, and behavioral effects of chronic unpredictable stress in the rat. Behavior Brain Research. 220 (1), 106-111 (2011).
- Lagunas, N., Calmarza-Font, I., Diz-Chaves, Y., Garcia-Segura, L. M. Long-term ovariectomy enhances anxiety and depressive-like behaviors in mice submitted to chronic unpredictable stress. Hormones and Behavior. 58 (5), 786-791 (2010).
- Papp, M., Klimek, V., Willner, P. Parallel changes in dopamine D2 receptor binding in limbic forebrain associated with chronic mild stress-induced anhedonia and its reversal by imipramine. Psychopharmacology (Berlin). 115 (4), 441-446 (1994).
- Harkin, A., Houlihan, D. D., Kelly, J. P. Reduction in preference for saccharin by repeated unpredictable stress in mice and its prevention by imipramine. Journal of Psychopharmacology. 16 (2), 115-123 (2002).
- Detanico, B. C., et al. Antidepressant-like effects of melatonin in the mouse chronic mild stress model. European Journal of Pharmacology. 607 (1-3), 121-125 (2009).
- Kubera, M., et al. Prolonged desipramine treatment increases the production of interleukin-10, an anti-inflammatory cytokine, in C57BL/6 mice subjected to the chronic mild stress model of depression. Journal of Affective Disorder. 63 (1-3), 171-178 (2001).
- Moreau, J. L., Jenck, F., Martin, J. R., Mortas, P., Haefely, W. E. Antidepressant treatment prevents chronic unpredictable mild stress-induced anhedonia as assessed by ventral tegmentum self-stimulation behavior in rats. European Neuropsychopharmacoly. 2 (1), 43-49 (1992).
- Muscat, R., Papp, M., Willner, P. Reversal of stress-induced anhedonia by the atypical antidepressants, fluoxetine and maprotiline. Psychopharmacology (Berlin). 109 (4), 433-438 (1992).
- Yalcin, I., Belzung, C., Surget, A. Mouse strain differences in the unpredictable chronic mild stress: a four-antidepressant survey. Behavioural Brain Research. 193 (1), 140-143 (2008).
- Moreau, J. L., Bourson, A., Jenck, F., Martin, J. R., Mortas, P. Curative effects of the atypical antidepressant mianserin in the chronic mild stress-induced anhedonia model of depression. Journal of Psychiatry Neuroscience. 19 (1), 51-56 (1994).
- Kopp, C., Vogel, E., Rettori, M. C., Delagrange, P., Misslin, R. The effects of melatonin on the behavioural disturbances induced by chronic mild stress in C3H/He mice. Behavioural Pharmacology. 10 (1), 73-83 (1999).
- Doron, R., et al. Escitalopram or novel herbal mixture treatments during or following exposure to stress reduce anxiety-like behavior through corticosterone and BDNF modifications. PLoS One. 9 (4), (2014).
- Elizalde, N., et al. Long-lasting behavioral effects and recognition memory deficit induced by chronic mild stress in mice: Effect of antidepressant treatment. Psychopharmacology (Berlin). 199 (1), 1-14 (2008).
- Casarotto, P. C., Andreatini, R. Repeated paroxetine treatment reverses anhedonia induced in rats by chronic mild stress or dexamethasone. European Neuropsychopharmacology. 17 (11), 735-742 (2007).
- Papp, M., Gruca, P., Boyer, P. -. A., Mocaër, E. Effect of agomelatine in the chronic mild stress model of depression in the rat. Neuropsychopharmacology. 28 (4), 694-703 (2003).
- Bortolato, M., et al. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biological Psychiatry. 62 (10), (2007).
- Liu, Y., et al. Antidepressant-like effects of tea polyphenols on mouse model of chronic unpredictable mild stress. Pharmacology Biochemistry Behavior. 104 (1), 27-32 (2013).
- Dai, Y., et al. Metabolomics study on the anti-depression effect of xiaoyaosan on rat model of chronic unpredictable mild stress. Journal of Ethnopharmacology. 128 (2), 482-489 (2010).
- Zhang, D., Wen, X. S., Wang, X. Y., Shi, M., Zhao, Y. Antidepressant effect of Shudihuang on mice exposed to unpredictable chronic mild stress. Jouranl of Ethnopharmacology. 123 (1), 55-60 (2009).
- Li, Y. C., et al. Antidepressant-like effects of curcumin on serotonergic receptor-coupled AC-cAMP pathway in chronic unpredictable mild stress of rats. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 33 (3), 435-449 (2009).
- Monleon, S., Parra, A., Simon, V. M., Brain, P. F., D’Aquila, P., Willner, P. Attenuation of sucrose consumption in mice by chronic mild stress and its restoration by imipramine. Psychopharmacology (Berlin). 117 (4), 453-457 (1995).
- Papp, M., Moryl, E., Willner, P. Pharmacological validation of the chronic mild stress model of depression. European Journal of Pharmacology. 296 (2), 129-136 (1996).
- Jansen, K., et al. Childhood trauma, family history, and their association with mood disorders in early adulthood. Acta Psychiatrica Scandinavica. (4), (2016).
- Kessler, R. C. THE EFFECTS OF STRESSFUL LIFE EVENTS ON DEPRESSION. Annual Review of Psychology. 48 (1), 191-214 (1997).
- Brady, K. T., Back, S. E. Childhood trauma, posttraumatic stress disorder, and alcohol dependence. Alcohol Research. 34 (4), 408-413 (2012).
- Pariante, C. M., Lightman, S. L. The HPA axis in major depression: classical theories and new developments. Trends in Neurosciences. 31 (9), 464-468 (2008).
- De Bellis, M. D., et al. Developmental traumatology part I: biological stress systems. Biological Psychiatry. 45 (10), 1259-1270 (1999).
- de Kloet, E. R., Joëls, M., Holsboer, F. Stress and the brain: from adaptation to disease. Nature Reviews Neurosciences. 6 (6), 463-475 (2005).
- Heim, C., Newport, D. J., Mletzko, T., Miller, A. H., Nemeroff, C. B. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 33 (6), 693-710 (2008).
- Trickett, P. K., Noll, J. G., Susman, E. J., Shenk, C. E., Putnam, F. W. Attentuation of cortisol across development for victims of sexual abuse. Developmental Psychopathology. 22 (1), 165-175 (2010).
- Bremne, J. D., Vermetten, E. Stress and development: behavioral and biological consequences. Developmental Psychopathology. 13 (3), 473-489 (2001).
- Nestler, E. J., Barrot, M., DiLeone, R. J., Eisch, A. J., Gold, S. J., Monteggia, L. M. Neurobiology of depression. Neuron. 34 (1), 13-25 (2002).
- Liu, D., et al. Resveratrol reverses the effects of chronic unpredictable mild stress on behavior, serum corticosterone levels and BDNF expression in rats. Behavioural and Brain Research. 264, 9-16 (2014).
- Silberman, D. M., Wald, M., Genaro, A. M. Effects of chronic mild stress on lymphocyte proliferative response. Participation of serum thyroid hormones and corticosterone. Int Immunopharmacol. 2 (4), 487-497 (2002).
- Bielajew, C., Konkle, A. T., Merali, Z. The effects of chronic mild stress on male Sprague-Dawley and Long Evans rats: I. Biochemical and physiological analyses. Behavioural and Brain Research. 136 (2), 583-592 (2002).
- Vrieze, E., et al. Dimensions in major depressive disorder and their relevance for treatment outcome. Journal of Affective Disorder. 155 (1), 35-41 (2014).
- Doron, R., et al. A novel herbal treatment reduces depressive-like behaviors and increases BDNF levels in the brain of stressed mice. Life Sciences. 94 (2), 151-157 (2014).
- Nestler, E. J., Hyman, S. E. Animal models of neuropsychiatric disorders. Nature Neurosciences. 13 (10), 1161-1169 (2010).
- Yan, H. -. C., Cao, X., Das, M., Zhu, X. -. H., Gao, T. -. M. Behavioral animal models of depression. Neuroscience Bulletin. 26 (4), 327-337 (2010).
- Yankelevitch-Yahav, R., Franko, M., Huly, A., Doron, R. The Forced Swim Test as a Model of Depressive-like Behavior. Journal of Visualized Experiment. (97), (2015).
- Cryan, J. F., Mombereau, C., Vassout, A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosciences and Biobehavioral Reviews. 29 (4-5), 571-625 (2005).
- Berton, O., et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 80 (5762), 864-868 (2006).
- Krishnan, V., Nestler, E. J. Animal models of depression: Molecular perspectives. Current Topics in Behavioral Neurosciences. 7 (1), 121-147 (2011).
- Belzung, C., Lemoine, M. Criteria of validity for animal models of psychiatric disorders: focus on anxiety disorders and depression. Biology of Mood and Anxiety Disorder. 1 (1), 9 (2011).
- Björkqvist, K. Social defeat as a stressor in humans. Physiology and Behavior. 73 (3), 435-442 (2001).
- Parihar, V. K., Hattiangady, B., Kuruba, R., Shuai, B., Shetty, A. K. Predictable chronic mild stress improves mood, hippocampal neurogenesis and memory. Molecular Psychiatry. 16 (2), 171-183 (2011).
- Haile, C. N., GrandPre, T., Kosten, T. A. Chronic unpredictable stress, but not chronic predictable stress, enhances the sensitivity to the behavioral effects of cocaine in rats. Psychopharmacology (Berlin). 154 (2), 213-220 (2001).
- Suo, L., et al. Predictable chronic mild stress in adolescence increases resilience in adulthood. Neuropsychopharmacology. 38 (8), 1387-1400 (2013).
- Gameiro, G. H., et al. Nociception- and anxiety-like behavior in rats submitted to different periods of restraint stress. Physiology and Behavior. 87 (4), 643-649 (2006).
- Anisman, H., Matheson, K. Stress, depression, and anhedonia: Caveats concerning animal models. Neuroscience and Biobehavioural Reviews. 29 (4-5), 525-546 (2005).
- Carr, W. J., Martorano, R. D., Krames, L. Responses of mice to odors associated with stress. J Comp Physiol Psychol. 71, 223-228 (1970).
- Zalaquett, C., Thiessen, D. The effects of odors from stressed mice on conspecific behavior. Physiology and Behavior. 50 (1), 221-227 (1991).
- Burstein, O., Shoshan, N., Doron, R., Akirav, I. Cannabinoids prevent depressive-like symptoms and alterations in BDNF expression in a rat model of PTSD. Progess in Neuro-Psychopharmacology Biological psychiatry. 84 (Part A), 129-139 (2018).
- Hedrich, H. J., Nicklas, W. Housing and Maintenance. Lab Mouse. , 521-545 (2012).
- Molendijk, M. L., Spinhoven, P., Polak, M., Bus, B. A. A., Penninx, B. W. J. H., Elzinga, B. M. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484). Molecular Psychiatry. 19 (7), 791-800 (2014).
- Chen, B., Dowlatshahi, D., MacQueen, G. M., Wang, J. F., Young, L. T. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biological Psychiatry. 50 (4), 260-265 (2001).
- Tye, K. M., et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 493 (7433), 537-541 (2013).
- Hamani, C., et al. Deep brain stimulation reverses anhedonic-like behavior in a chronic model of depression: Role of serotonin and brain derived neurotrophic factor. Biological Psychiatry. 71 (1), 30-35 (2012).
- Hill, M. N., Hellemans, K. G. C., Verma, P., Gorzalka, B. B., Weinberg, J. Neurobiology of chronic mild stress: Parallels to major depression. Neuroscience and Biobehavior Reviews. 36 (9), 2085-2117 (2012).
- Kasch, K. L., Rottenberg, J., Ba Arnow, ., Gotlib, I. H. Behavioral activation and inhibition systems and the severity and course of depression. Journal of Abnormal Psychology. 111 (4), 589-597 (2002).
- Faull, J. R., Halpern, B. P. Reduction of sucrose preference in the hamster by gymnemic acid. Physiology and Behavior. 7 (6), 903-907 (1971).
- Moreau, J. -. L., Scherschlicht, R., Jenck, F., Martin, J. R. Chronic mild stress-induced anhedonia model of depression; sleep abnormalities and curative effects of electroshock treatment. Behavioural Pharmacology. 6 (7), 682-687 (1995).
- Blier, P. Optimal use of antidepressants: when to act?. J Psychiatry Neurosci. 34 (1), 80 (2009).
- Frazer, A., Benmansour, S. Delayed pharmacological effects of antidepressants. Mol Psychiatry. 7, S23-S28 (2002).
- Can, A., Dao, D. T., Terrillion, C. E., Piantadosi, S. C., Bhat, S., Gould, T. D. The Tail Suspension Test. Journal of Visualized Experiments. (58), (2011).
- Song, L., Che, W., Min-wei, W., Murakami, Y., Matsumoto, K. Impairment of the spatial learning and memory induced by learned helplessness and chronic mild stress. Pharmacology Biochemistry and Behavior. 83 (2), 186-193 (2006).
- Mao, Q. Q., Ip, S. P., Ko, K. M., Tsai, S. H., Che, C. T. Peony glycosides produce antidepressant-like action in mice exposed to chronic unpredictable mild stress: Effects on hypothalamic-pituitary-adrenal function and brain-derived neurotrophic factor. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 33 (7), 1211-1216 (2009).
- Lutz, C. M., Linder, C. C., Davisson, M. T. Strains, Stocks and Mutant Mice. Lab Mouse. , 37-56 (2012).
- Yalcin, I., Aksu, F., Belzung, C. Effects of desipramine and tramadol in a chronic mild stress model in mice are altered by yohimbine but not by pindolol. European Journal of Pharmacology. 514 (2-3), 165-174 (2005).
- Van Boxelaere, M., Clements, J., Callaerts, P., D’Hooge, R., Callaerts-Vegh, Z. Unpredictable chronic mild stress differentially impairs social and contextual discrimination learning in two inbred mouse strains. PLoS One. 12 (11), (2017).
- Nadler, J. J., et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes, Brain Behavior. 3 (5), 303-314 (2004).
- Girard, I., Garland, T. Plasma corticosterone response to acute and chronic voluntary exercise in female house mice. Journal of Applied Physiology. 92 (4), 1553-1561 (2002).
- Gumuslu, E., et al. The antidepressant agomelatine improves memory deterioration and upregulates CREB and BDNF gene expression levels in unpredictable chronic mild stress (UCMS)-exposed mice. Drug Target Insights. 2014 (8), 11-21 (2014).
- Willner, P., Golembiowska, K., Klimek, V., Muscat, R. Changes in mesolimbic dopamine may explain stress-induced anhedonia. Psychobiology. 19 (1), 79-84 (1991).
- Peng, Y. L., Liu, Y. N., Liu, L., Wang, X., Jiang, C. L., Wang, Y. X. Inducible nitric oxide synthase is involved in the modulation of depressive behaviors induced by unpredictable chronic mild stress. Journal of Neuroinflammation. 9, (2012).
- Liu, B., et al. Icariin exerts an antidepressant effect in an unpredictable chronic mild stress model of depression in rats and is associated with the regulation of hippocampal neuroinflammation. Neurosciences. 294, 193-205 (2015).
- Yalcin, I., Aksu, F., Bodard, S., Chalon, S., Belzung, C. Antidepressant-like effect of tramadol in the unpredictable chronic mild stress procedure: Possible involvement of the noradrenergic system. Behavioural Pharmacology. 18 (7), 623-631 (2007).
- Mineur, Y. S., Belzung, C., Crusio, W. E. Functional implications of decreases in neurogenesis following chronic mild stress in mice. Neurosciences. 150 (2), 251-259 (2007).
- Simchon-Tenenbaum, Y., Weizman, A., Rehavi, M. Alterations in brain neurotrophic and glial factors following early age chronic methylphenidate and cocaine administration. Behav Brain Research. 282, 125-132 (2015).
- Hnasko, R. . ELISA: Methods and Protocols. , (2015).
- Watanabe, S. Social factors modulate restraint stress induced hyperthermia in mice. Brain Research. 1624, 134-139 (2015).
- Mineur, Y. S., Prasol, D. J., Belzung, C., Crusio, W. E. Agonistic behavior and unpredictable chronic mild stress in mice. Behaviour Genetics. 33 (5), 513-519 (2003).
- Frisbee, J. C., Brooks, S. D., Stanley, S. C., d’Audiffret, A. C. An Unpredictable Chronic Mild Stress Protocol for Instigating Depressive Symptoms, Behavioral Changes and Negative Health Outcomes in Rodents. Journal of Visualized Experiments. (106), (2015).
- Westenbroek, C., Ter Horst, G. J., Roos, M. H., Kuipers, S. D., Trentani, A., Den Boer, J. A. Gender-specific effects of social housing in rats after chronic mild stress exposure. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 27 (1), 21-30 (2003).
- Bartolomucci, A., et al. Individual housing induces altered immuno-endocrine responses to psychological stress in male mice. Psychoneuroendocrinology. 28 (4), 540-558 (2003).
- Võikar, V., Polus, A., Vasar, E., Rauvala, H. Long-term individual housing in C57BL/6J and DBA/2 mice: Assessment of behavioral consequences. Genes, Brain and Behavior. 4 (4), (2005).
- Krohn, T. C., Sørensen, D. B., Ottesen, J. L., Hansen, A. K. The effects of individual housing on mice and rats: a review. Animal Welfare. 15 (4), 343-352 (2006).

