An Automated Method to Determine the Performance of Drosophila in Response to Temperature Changes in Space and Time
Summary
Here we present a protocol to automatically determine the locomotor performance of Drosophila at changing temperatures using a programmable temperature-controlled arena that produces fast and accurate temperature changes in time and space.
Abstract
Temperature is a ubiquitous environmental factor that affects how species distribute and behave. Different species of Drosophila fruit flies have specific responses to changing temperatures according to their physiological tolerance and adaptability. Drosophila flies also possess a temperature sensing system that has become fundamental to understanding the neural basis of temperature processing in ectotherms. We present here a temperature-controlled arena that permits fast and precise temperature changes with temporal and spatial control to explore the response of individual flies to changing temperatures. Individual flies are placed in the arena and exposed to pre-programmed temperature challenges, such as uniform gradual increases in temperature to determine reaction norms or spatially distributed temperatures at the same time to determine preferences. Individuals are automatically tracked, allowing the quantification of speed or location preference. This method can be used to rapidly quantify the response over a large range of temperatures to determine temperature performance curves in Drosophila or other insects of similar size. In addition, it can be used for genetic studies to quantify temperature preferences and reactions of mutants or wild-type flies. This method can help uncover the basis of thermal speciation and adaptation, as well as the neural mechanisms behind temperature processing.
Introduction
Temperature is a constant environmental factor that affects how organisms function and behave1. Differences in latitude and altitude lead to differences in the type of climates organism are exposed to, which results in evolutionary selection for their responses to temperature2,3. Organisms respond to different temperatures through morphological, physiological, and behavioral adaptations that maximize performance under their particular environments4. For instance, in the fruit fly Drosophila melanogaster, populations from different regions have different temperature preferences, body sizes, developmental times, longevity, fecundity, and walking performance at different temperatures2,5,6,7. The diversity observed between flies of different origins is explained in part by genetic variation and plastic gene expression8,9. Similarly, Drosophila species from different areas distribute differently among temperature gradients and show differences in resistance to extreme heat and cold tests10,11,12.
Drosophila has also recently become the model of choice to understand the genetic and neural basis of temperature perception13,14,15,16,17. Broadly, adult flies perceive temperature through cold and hot peripheral temperature sensors in the antennae and through temperature sensors in the brain13,14,15,16,17,18,19,20. The periphery receptors for hot temperatures express Gr28b.d16 or Pyrexia21, while the periphery cold receptors are characterized by Brivido14. In the brain, temperature is processed by neurons expressing TrpA115. Behavioral studies on mutants of these pathways are improving our understanding of how temperature is processed and give insights into mechanisms that vary among populations of Drosophila from different regions.
Here we describe a temperature-controlled arena that produces fast and precise temperature changes. Investigators can pre-program these changes, which allows for standardized and repeatable temperature manipulations without human intervention. Flies are recorded and tracked with specialized software to determine their position and speed at different phases of an experiment. The main measurement presented in this protocol is the walking speed at different temperatures, because it is an ecologically relevant index of physiological performance that can identify individual thermal adaptability5. Together with temperature receptor mutants, this technique can help reveal the mechanisms of thermal adaptation at cellular and biochemical levels.
Protocol
1. Preparation of Fly Food Medium
- Pour 1 L of tap water into a 2 L glass beaker and add a magnetic stir bar. Put the beaker on a magnetic hot plate at 300 °C until boiling temperature is reached.
- Stir at 500 rounds/min and add the following: 10 g of agar, 30 g of glucose, 15 g of sucrose, 15 g of cornmeal, 10 g of wheat germ, 10 g of soy flour, 30 g of molasses, and 35 g of active dry yeast.
- When the mix foams vigorously, turn down the hot plate temperature to 120 °C while continuing stirring.
- Turn the hot plate temperature further down to 30 °C after 10 min and continue stirring until the mix cools to 48 °C. Measure the temperature by inserting a thermometer directly into the food without touching the walls of the beaker.
- Dissolve 2 g of p-hydroxy-benzoic acid methyl ester into 10 mL of 96% ethanol and add it to the mix, together with 5 mL of 1 M propionic acid. Continue stirring for 3 min.
- Turn the hot plate off and pour 45 mL of food into the rearing bottles and 6.5 mL of food into the collection vials.
2. Preparation of Flies
- Place 20 male and 20 female flies in the rearing bottles containing 45 mL of fly food medium. Transfer the flies to new bottles after 3 to 4 days by tapping them down and then tapping them into the fresh bottles. Discard the flies after three changes.
- Place the bottles inside the incubator under 12-h light/12-h dark cycles with a constant temperature of 25 °C.
NOTE: A new generation of flies will eclose after ten days.
- Place the bottles inside the incubator under 12-h light/12-h dark cycles with a constant temperature of 25 °C.
- Anesthetize newly eclosed flies on carbon dioxide pads for a maximum of 4 min and collect them in 2.5 x 9.5 cm fly rearing vials with 6.5 mL of fly food medium using a paintbrush.
- Collect only virgin flies and separate them by sex into groups of 20 flies per rearing vial.
- Place the vials inside incubators for 5-7 days, changing the flies to new vials every 2-3 days and on the days before experiments.
3. Frame of Lights
- Make a wooden frame of 10 cm length, 4 cm width, 4 cm height, and 0.5 cm thick.
- On each of the short edges, create a border of 4 cm length, 4 cm height, and 1.5 cm width towards the inside area of the wooden frame. Leave the internal face of the border open.
- Drill two holes of 0.5 cm diameter at the intersection of one of the long edges of the wooden frame and at each of the borders at the short edges.
- Place 10 cm of a warm white LED strip inside each of the borders on the short edges. Peel the back of the LED strip to immediately glue it in place.
NOTE: For experiments in which illumination needs to be eliminated, the warm white LED strip can be substituted for infrared LED strips. - Connect one end of the LED strip in one of the borders to the switching power supply and its other end to the LED strip on the opposite border.
- Turn the switching power supply on to verify that both LED strips turn on.
- Cover the open side of each border with a white piece of paper.
- Glue another piece of paper to each of the internal phases of the long edges.
4. Temperature-Controlled Arena
- Turn on the temperature-controlled arena (Figure 1A and 1C). Ensure that the fan starts running and the aluminum ring starts warming up.
- Use a USB cable to connect the temperature-controlled arena to the control computer running the TemperaturePhases script with the temperature sequences.
- Open the TemperaturePhases script in the control computer and verify that the temperature sequence is properly set up (Video 1).
- Check that the duration of each experimental phase is set to 60 s by verifying that "par.StimulusDur" is equal to 60 s.
- Check that the 1) number equal to desired number of phases, 2) iterative ON/OFF set-up of the indicative red light emitting diodes (LEDs), 3) 2 °C temperature increase per phase, and 4) 16 °C as the starting temperature are all correct under the "Start the experimental block" section.
NOTE: Allow the flies to acclimate to the Fly Arena for 7 min at 16 °C to avoid an artificial increase of speed during the first experimental phases (Figure 2). - Run the TemperaturePhases script. The software will initialize for 5 seconds as determined in "arena.Wait" and then stop.
- Press the spacebar of the keyboard to begin running the experimental phases once a fly has been blown into the Fly Arena (step 5.3).
NOTE: The TemperaturePhases is the current script controlling the box; however, it is possible to create other custom scripts to use this device that adjust to the requirements of different experiments.
- Connect the camera on top of the arena to the recording computer using the camera's USB cable.
- Open the video recording program (see Table of Materials) in the recording computer by selecting "File | New Movie Recording". A screen showing the image from the camera will open.
- Ensure that the camera image captures all edges of the arena and the indicative red LEDs.
- Start recording by pressing the red button in the middle of the screen's bottom edge showing the camera image once the frame of lights is set around the arena (step 5.4).
NOTE: Small changes in lighting can affect accuracy of the tracking. It is recommended to keep the illumination of the temperature-controlled arena constant by fixing the location of the apparatus.
5. Temperature Behavioral Experiments
- Prepare the Fly Arena (Figure 1C).
- Place a strand of white conductive tape on the top of the copper tiles, ensuring all edges are covered.
- Place the heated aluminum ring around the copper tiles. The edge of the ring fits perfectly around the copper tiles so it is always placed in the same location.
- Clean the glass cover with a clean tissue and place it on the top of the aluminum ring, leaving a gap through which a fly can be blown in.
NOTE: Before the experiments, coat the glass cover with the siliconizing agent to create a slippery surface. Apply the siliconizing agent for 24 h and rinse it with water before use.
- Run the TemperaturePhases script (step 4.3.3) and open the video recording program (step 4.5).
- Blow the fly from a rearing vial (step 2.2.2) into the Fly Arena (e.g., 1 male fly in Figure 3).
- Take a vial of flies from the incubator, tap it twice to force them to go to the bottom, trap one fly with a mouth aspirator, and close the vial and put it back into the incubator.
- Place the fly in the arena through the gap that has been left between the glass cover and aluminum ring (step 5.1.3).
- Close the gap between the glass cover and aluminum ring by pushing the glass cover until it reaches the edge of the aluminum ring as soon as the fly is introduced to the Fly Arena.
- Place the frame of lights around the arena to ensure symmetric illumination.
- Mark the location (e.g., using a permanent marker) of the frame of lights around the Fly Arena (Figure 1C) to ensure that the frame is always placed in the same location.
- Start recording with the video recording program (step 4.5.2) and press the spacebar on the keyboard of the control computer to begin running the experimental phases (step 4.3.4).
- After all experimental phases are done, save the video in .mp4 or .avi format and remove the fly from the Fly Arena with the mouth aspirator.
NOTE: The end of the experimental phases can be determined by both indicative red LEDs being turned off or by the TemperaturePhases script stopping.- Stop the video recording by pressing the stop button in the middle of the screen's bottom edge in the recording program. Press "File | Save as" to save the video.
6. Video Tracking and Data Analysis
- Use the FlySteps tracking software (Video 2) to track the videos.
- Open the "configuration_file.ini" inside the "FlyTracker" folder.
- Set the location of the videos in "video_folder" and the names of the videos in "video_files".
- Specify the borders of the Fly Arena in "arena_settings" based on (x, y) pixel coordinates of multiple points at the edge of the arena.
- Specify the location of the indicative red LEDs in "led_settings" based on (x, y) pixel coordinates of the location of the center of the LEDs.
- Check the location of the borders of the Fly Arena by setting "debug" to "true" in "arena_settings", clicking "Save", and running the script in the terminal.A screen capture of the video will appear with a blue square formed by the coordinates inputted in "arena_settings".
NOTE: This square surrounds the area to be tracked. - Change "debug" in "arena_settings" to "false", click "Save", and run the screen in the terminal once more.
NOTE: This will start the tracking process.
NOTE: Flies can walk out of the tracking area onto the heated aluminum ring. This happens during the first seconds of an experiment, after which flies stop touching the heated ring and remain inside the tracking area.
NOTE: Videos can be tracked with other tracking software according to the experimenter's preferences.
- Use the (x,y) location of each fly provided by the tracking software to calculate the measure of interest for the temperature performance. Custom scripts (e.g., FlyStepsAnalysis de Supplementary) can be used.
- Compare the temperature performance curves of different fly groups using repeated measurements (RM) analysis of variance (ANOVA) and post-hoc multiple comparisons using statistical software (see Table of Materials).
Representative Results
The temperature-controlled arena (Figure 1A) consists of three copper tiles whose temperature can be individually controlled through a programmable circuit. Each copper tile possesses a temperature sensor that gives feedback to the programmable circuit. The circuit activates a power supply to increase the temperature of each tile. Passive thermoelectric elements act as constant heating elements to maintain the desired temperature, while a heat sink cooled by a fan provides constant cooling. The magnitude of temperature change determines the speed of the process in a non-linear manner. An increase of 2 °C requires only 0.1 s, and an increase of 18 °C requires 4 s. A screen connected to the programmable circuit (Figure 1C) informs the user of the temperature measured by the temperature sensors in each of the tiles. The copper tiles are surrounded by an aluminum ring constantly heated to 50 °C (Figure 1B and 1C) by semiconductors around the periphery. This ring forms the edges of the Fly Arena (Figure 1C), the area in which flies are to be placed. The Fly Arena is covered by a siliconized glass cover (Figure 1A and 1C), which provides a 3 mm high space which ensures that flies can walk but not fly. Next to the Fly Arena are two red LEDs (Figure 1C) that can be programmed to mark different experimental phases. For example, for the results shown in Figure 2A, each LED is associated with a different temperature, while in Figure 2B, each LED indicates 60 s. The FlySteps software can register when each of the indicative LEDs is on, and the researcher can then use this information to automatically determine the experimental phases based on temperature or time.
The temperature-controlled arena can be used to compare the behavioral response of flies from different genetic backgrounds to dynamic temperature changes. For example, flies from different species can be exposed to gradually increasing temperatures (Figure 3) to compare differences in thermal performance. The speed of all species increases as temperature increases until reaching a point of maximum performance, after which it decayed and perished. However, each species has a particular response curve with specific maximum response speeds and thermal tolerances. Previous reports have shown that Drosophila from different species differ among developmental timing, longevity, fecundity, body dimensions, sexual communication, and temperature tolerance3,6,7,8,22. Thus, our description of species-specific locomotion in a temperature gradient adds to this body of work.
The temperature-controlled arena can also be used to explore the response to conditioning experiments based on temperature. The simplest form of this approach is an operant conditioning paradigm in which flies are trained to prefer one side of the arena over the other, by warming up the side that will be avoided23,24,25. We exposed individual flies to 40 °C in the middle and one of the side tiles, while leaving the other side tile at a comfortable 22 °C (Figure 4). Wild-type flies quickly stopped moving along the arena and remained in the comfortable location. In contrast, the classic memory mutant Dunce kept exploring the arena and spent less time than controls in the comfortable location. The differences between performance of the wild-type flies and Dunce mutants became larger when all tiles were set to 22 °C and comparisons were made between the treatment groups. Dunce mutants also showed greater differences between training and test phases in comparison to the wild-type flies (Figure 4). These results suggest an effect of memory on remaining in the comfortable location.
Combinations of temperature and location are also useful to understand the function of different temperature receptors during dynamic temperature changes. We exposed individual D. melanogaster Gr28b.d and TrpA1GAL4 mutants to increasing temperatures (2 °C increase every 60 s) while providing a comfortable location at 22 °C (Figure 5). The comfortable location shifted from left to right, and vice versa, per iteration. Results show that the periphery temperature receptor Gr28b.d mutants behave as the control, as they spend more time in the comfortable location as temperature increases. However, brain temperature receptor TrpA1GAL4 mutants are not affected by increasing temperatures and do not change their locations in the arena. The increases and decrease in the curve of TrpA1GAL4 mutants show the effect in flies that were already sitting in the comfortable location before it became comfortable and remained there during that phase. The consistency of peaks and valleys of the curve of TrpA1GAL4 suggest that these flies remained still for most of the experiment; hence, they were constantly counted when their location was the one considered comfortable. This conclusion was confirmed by visual inspection of the recorded videos. These results support previous physiological reports suggesting that periphery perception of fast and large changes does not depend on Gr28b.d17 and that flies possess a main central mechanism to sense temperature based on TrpA114,21.
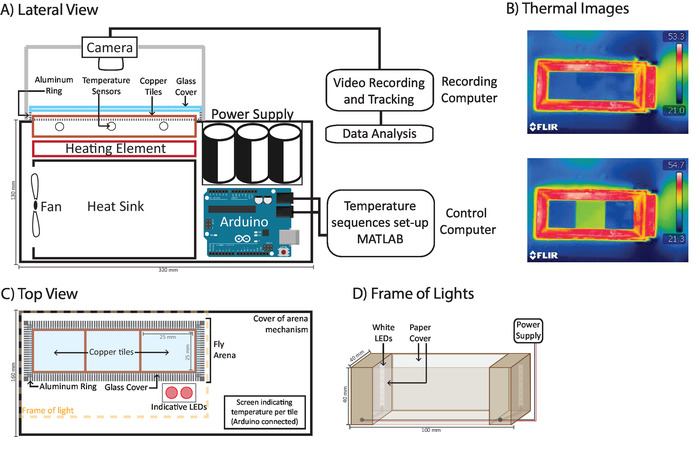
Figure 1: Diagram of temperature controlled-arena. (A) A lateral view of the temperature-controlled arena. A programmable circuit connects a power supply and temperature sensors to heating elements under copper tiles to control their temperature. Tiles are constantly cooled down through a heat sink connected to a fan. A heated aluminum ring over which a glass cover rests surrounds the tiles. (B) Thermal imaging showing the tiles set at 24 °C (top) and side tiles at 24 °C with a middle tile at 30 °C (bottom). (C) A top view of the arena. A camera records the copper tiles, aluminum ring, and red LEDs, then automatically determines experimental phases. A screen in the corner of the box, not recorded by the camera, displays the current tile temperature. (D) Ring of light: two warm white LED strips inside a wooden box covered in white paper ensure constant and symmetric illumination of the whole arena. Please click here to view a larger version of this figure.
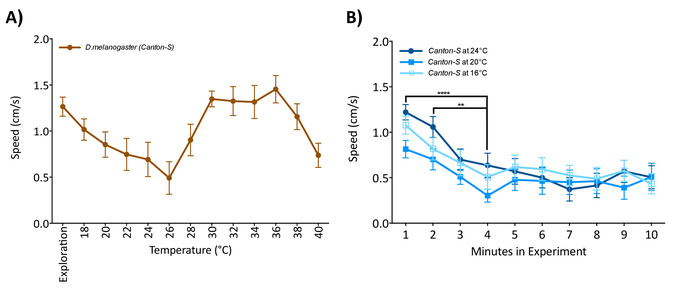
Figure 2: Flies must acclimate to the arena before starting the temperature protocol. (A) Single male flies were introduced to the arena and allowed to explore at a constant 16 °C for 1 min, after which the temperature started increasing. (B) Single flies exposed to 16 °C, 20 °C, or 24 °C (no group differences; two-way ANOVA F (2,570) = 4.156, p = 0.162) have a higher locomotion at the beginning of the experiment than after 5 min (two-way RM ANOVA F (9,570) = 7.803, p < 0.0001). Data are mean and standard error of the mean (± SEM) of 20 virgin female flies 5 to 7 days old tested over multiple days. Asterisk indicates significant difference among groups (****p < 0.0001; Tukey's multiple comparison test, p = 0.05). Please click here to view a larger version of this figure.
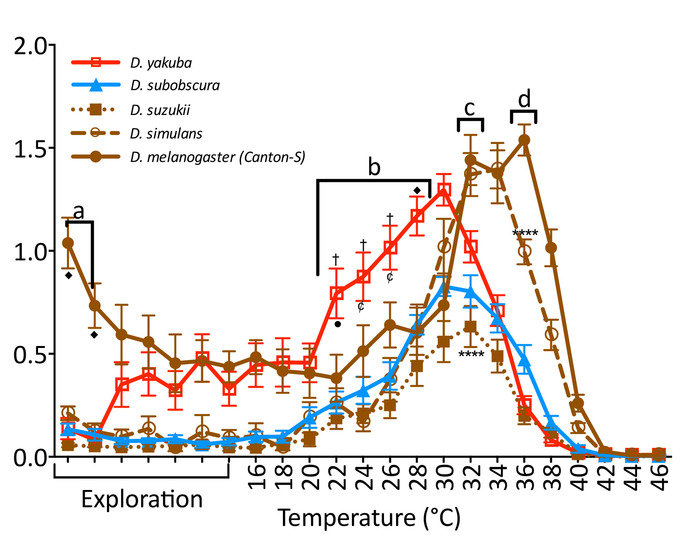
Figure 3: Locomotion of 5 Drosophila species exposed to gradually increasing temperatures. Individual male flies from temperate (blue), tropical (red), and cosmopolitan (brown) Drosophila species were exposed to an increasing temperature gradient (2 °C every 60 s) between 16 and 46 °C. The first 7 min were constantly at 22 °C to allow flies to explore the arena. Species were significantly different (two-way RM ANOVA F(4,70) = 28.46, p < 0.001). (a) D. melanogaster (brown; filled circles) was faster when introduced to the arena. (b) D. yakuba (red; empty squares) was faster as temperature increased. (c) D. suzukii (brown; filled square) was slower than the other cosmopolitan flies at its maximum performance point. (d) D. simulans (brown; empty circles) was in decay at the maximum point of D. melanogaster. Each point represents the mean (± SEM) of 15 male flies 5 to 7 days old tested over several days. Significance indicated by symbols (♦ = difference from all, p < 0.0001; †= difference from all except D. melanogaster, p < 0.0001; • = difference from D. melanogaster, p < 0.01; ¢ = difference from D. melanogaster, p < 0.001; **** = difference between named groups, p < 0.0001; Tukey's multiple comparison test, p = 0.05). Please click here to view a larger version of this figure.
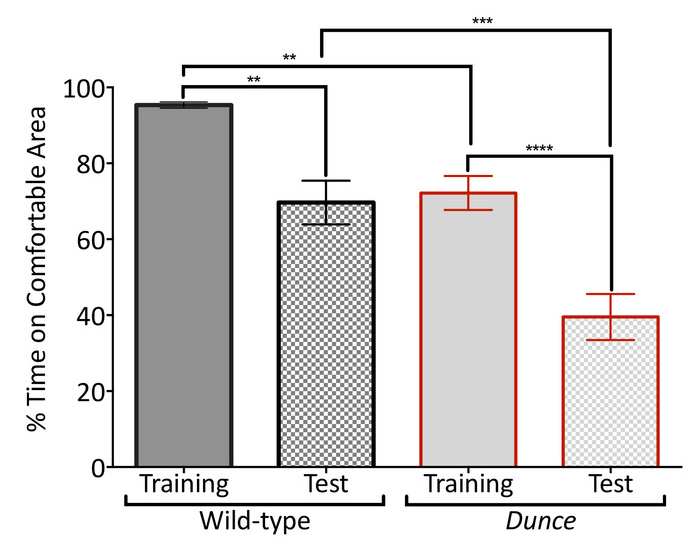
Figure 4: The temperature-controlled arena can be used for operant conditioning. D. melanogaster Canton-S strain (wild-type; black border) and dnc1 (Dunce; red border) mutants were trained to prefer a lateral tile at 22 °C after warming the middle and opposite lateral tiles to 40 °C for 4 min (training, no pattern). Memory of the heated areas is then tested by setting all tiles to 22 °C (test; grid pattern). Flies were conditioned to prefer tiles on the left in half of the experiments, then tiles on the right in the other half. The percentage of total time inside the tile at 22 °C during training and testing was measured to compare performances. Groups were significantly different (one-way ANOVA F(3,76) = 23.23, p < 0.0001), with Dunce performing worse than wild-type overall. Data are mean (± SEM) of 20 virgin female flies 5 to 7 days old tested over several days. Asterisks indicate significance difference among groups (****p > 0.0001; ***p > 0.001; **p > 0.01; Tukey's multiple comparison test, p = 0.05) Please click here to view a larger version of this figure.
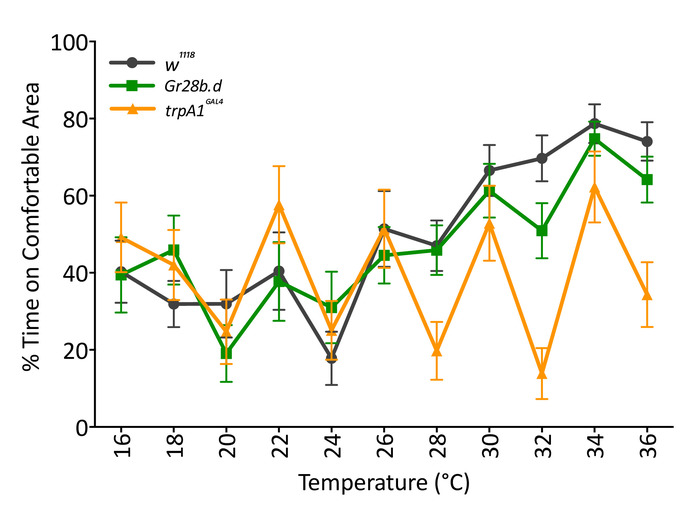
Figure 5: Response of temperature mutants to increasing temperature when a comfortable location is provided. Temperature mutants Gr28b.d (green; squares) respond as controls (w1118, black; circles) by increasing the percentage of time in the comfortable area as temperature increases (two-way RM ANOVA F (1,38) = 0.5107, p = 0.479). TrpA1GAL4 mutants (yellow; triangles) are different from controls (w1118, black), as they do not increase the time in the comfortable area as temperature increases (two-way RM ANOVA F (1,38) = 1.670, p = 0.019). Data are mean (± SEM) of 20 male flies 5 to 7 days old tested over several days. TrpA1GAL4 is significantly different from Gr28b.d and the control (p < 0.05; Tukey's multiple comparison test, p = 0.05). Please click here to view a larger version of this figure.
Discussion
Here we have presented an automated temperature-controlled arena (Figure 1) that produces precise temperature changes in time and space. This method allows exposure of individual Drosophila not only to pre-programmed gradual increases of temperature (Figure 2 and Figure 3), but also to dynamic temperature challenges in which each tile of the fly arena was heated independently to a different temperature (Figure 4 and Figure 5).
The temperature-controlled arena uses an innovative approach to the heating process. Instead of producing temperature changes in the tiles through thermoelectric Peltier heating elements used in traditional methods, the temperature-controlled arena uses current to warm up a copper mass with the copper tiles, and flies are placed at the top. The copper mass is constantly cooled down by a heat sink block connected to a fan. Peltier-like elements are used to maintain the desired temperature of the copper mass once it has been warmed up. Because these elements are not the main temperature generators, they suffer less stress, which extends their life span and permits faster temperature changes. A programmable circuit that receives feedback from temperature sensors under each of the copper tile, which can also activate the low voltage power supply, coordinates the heating mechanism. Researchers can specify when and where temperature changes occur and determine the intensity and direction of such changes. Furthermore, coupling the method with specialized tracking software, such as FlySteps, permits analysis of all aspects relating to Drosophila's movement, such as the overall speed at certain temperatures or time spent in certain locations (Figure 2, Figure 3, Figure 4, Figure 5). Nevertheless, all results must consider characteristics inherent to fly behavior that might affect their locomotion. For example, if flies are not allowed to explore the arena and settle before changing the temperature, speed measurements might be artificially high (Figure 2). Flies can also leave odorants that affect subsequent flies; hence, the glass cover must be cleaned, and tape covering the tiles must be changed between subjects. Given that locomotion declines as flies age26, it is important that flies are standardized for age to avoid variation in results. In our arena, flies have also shown centrophobism, preferring edges over the middle area. Experimenters must control for this by changing the location of comfortable areas to prevent overestimating site preference.
The current characteristics of the arena and requirements of the tracking process could limit some experimental procedures. For example, the close environment of the arena does not include access points through which odours could be introduced, which prevents studies in which this stimulus is important. Similarly, the FlyStepts tracker necessitates videos with uniform backgrounds, which limits the possibility of adding food or other items to the fly's environment. The arena could be adapted to include a connection to a gas valve, and software developments exist that may allow for more objects to be present. Future projects may take advantage of these possibilities to adapt the temperature-controlled arena to specific experimental needs.
Finally, we have shown in the results that different species of Drosophila perform differently as temperature increases (Figure 3) and that temperature mutants do not respond in the same way as controls (Figure 5). This shows that this new method may be used to explore Drosophila's thermal behavior and how it is affected by natural selection and functional characteristics. Finally, it illustrates that our method may help further understanding of thermal adaptation and speciation as well as the interactions of temperature receptors with other stimuli in future studies.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported in part by a scholarship from the Behavioural and Cognitive Neuroscience Program of the University of Groningen and a graduate scholarship from the Consejo Nacional de Ciencia y Tecnología (CONACyT) from Mexico, granted to Andrea Soto-Padilla, and a grant from the John Templeton Foundation for the study of time awarded to Hedderik van Rijn and Jean-Christophe Billeter. We are also thankful to Peter Gerrit Bosma for his participation in developing the FlySteps tracker.
Scripts TemperaturePhases,FlySteps, and FlyStepAnalysis can be found as supplementary information and in the following temporary and publicly available link:
https://dataverse.nl/privateurl.xhtml?token=c70159ad-4d92-443d-8946-974140d2cb78
Materials
| Arduino Due | Arduino | A000062 | Software RUG |
| Electronics Board | Ruijsink Dynamic Engineering | FF-Main-02-2014 | |
| Power supply Boost | XP-Power 48. V 65 W | ECS65US48 | Set to 53 Volt |
| Power supply Tile Heating | XP-Power 15. V 80 W | VFT80US15 | |
| Power supply Cooling | XP-Power 15. V 130 W | ECS130U515 | |
| Peltier elements | Marlow Industries | RC12-4 | 2 Elements, controlled DC feed |
| Heat sink | Fisher Technik | LA 9/150-230V | Decoupled for vibration |
| Temperature sensors | Measurement Specialties | MCD_10K3MCD1 | Micro Thermistor Probe |
| Copper block/tiles | Ruijsink Dynamic Engineering | FF-CB-01-2014 | |
| Auminum ring | Ruijsink Dynamic Engineering | FF-RoF-02-2015 | |
| Tesa 4104 white tape 25 x 66 mm | RS Components | 111-2300 | White conductive tape |
| Red LEDs | Lucky Ligt | ll-583vc2c-v1-4da | Wavelength between 625 nm, 20 mAmp and 6 V |
| Warm white LED strip | Ledstripkoning | HQ-3528-SMD | 60 LEDs per meter |
| Switch Power Supply | Generic | T-36-12 | |
| Logitech c920 | Logitech Europe S.A | PN960-001055 | |
| QuickTime Player | Apple Computer | Recording program | |
| Tracking analysis software | R | Packages: pacman | |
| Tracking analysis software | MATLAB | ||
| Thermal Imaging | FLIR T400sc | ||
| Graphs and Statisticts Software | Graph Pad Prism | ||
| Sigmacote | Sigma-Aldrich | SL2-100ML | Siliconising agent |
| Fly rearing bottles | Flystuff | 32-130 | 6oz Drosophila stock bottle |
| Flypad | Flystuff | 59-114 | |
| Fly rearing vials | Dominique Dutscher | 789008 | Drosophila tubes narrow 25×95 mm |
| Incubator | Sanyo | MIR-154 | |
| Magnetic hot plate | Heidolph | 505-20000-00 | MR Hei-Standard |
| Agar | Caldic Ingredients B.V. | 010001.26.0 | |
| Glucose | Gezond&wel | 1019155 | Dextrose/Druivensuiker |
| Sucrose | Van Gilse | Granulated sugar | |
| Cornmeal | Flystuff | 62-100 | |
| Wheat germ | Gezond&wel | 1017683 | |
| Soy flour | Flystuff | 62-115 | |
| Molasses | Flystuff | 62-117 | |
| Active dry yeast | Red Star | ||
| Tegosept | Flystuff | 20-258 | 100% |
References
- Abram, P. K., Boivin, G., Moiroux, J., Brodeur, J. Behavioural effects of temperature on ectothermic animals unifying thermal physiology and behavioural plasticity. bioRxiv. , (2016).
- Rajpurohit, S., Schmidt, P. S. Measuring thermal behavior in smaller insects: A case study in Drosophila melanogaster demonstrates effects of sex, geographic origin, and rearing temperature on adult behavior. Fly. 10 (4), 149-161 (2016).
- Jezovit, J. A., Levine, J. D., Schneider, J. Phylogeny environment and sexual communication across the Drosophila genus. The Journal of Experimental Biology. 220 (1), 42-52 (2017).
- Sinclair, B. J., Williams, C. M., Terblanche, J. S. Variation in Thermal Performance among Insect Populations. Physiological and Biochemical Zoology. 85 (6), 594-606 (2012).
- Gibert, P., Huey, R., Gilchrist, G. Locomotor performance of Drosophila melanogaster: Interactions among developmental and adult temperautures, age, and geography. Evolution. 55 (1), 205-209 (2001).
- Trotta, V., et al. Thermal plasticity in Drosophila melanogaster: A comparison of geographic populations. BMC Evolutionary Biology. 6, 1-13 (2006).
- Klepsatel, P., Gálikova, M., De Maio, N., Huber, C. D., Christian, S., Flatt, T. Variation in thermal performance and reaction norms among populations of Drosophila melanogaster. Evolution. 67 (12), 3573-3587 (2013).
- Latimer, C. A. L., Wilson, R. S., Chenoweth, S. F. Quantitative genetic variation for thermal performance curves within and among natural populations of Drosophila serrata. Journal of Evolutionary Biology. 24, 965-975 (2011).
- Chen, J., Nolte, V., Schlotterer, C. Temperature-related reaction norms of gene expression: Regulatory architecture and functional implications. Molecular Biology and Evolution. , (2015).
- Kellermann, V., Overgaard, J., Hoffmann, A. A., Flojgaard, C., Svenning, J. -. C., Loeschcke, V. Upper thermal limits of Drosophila are linked to species distributions and strongly constrained phylogenetically. Proceedings of the National Academy of Sciences. 109 (40), 16228-16233 (2012).
- Andersen, J. L., Manenti, T., Sørensen, J. G., Macmillan, H. A., Loeschcke, V., Overgaard, J. How to assess Drosophila cold tolerance: Chill coma temperature and lower lethal temperature are the best predictors of cold distribution limits. Functional Ecology. 29 (1), 55-65 (2015).
- Krstevska, B., Hoffmann, A. A. The effects of acclimation and rearing conditions on the response of tropical and temperate populations of Drosophila melanogaster and D. simulans to a temperature gradient (Diptera: Drosophilidae). Journal of Insect Behavior. 7 (3), 279-288 (1994).
- Frank, D. D., Jouandet, G. C., Kearney, P. J., Macpherson, L. J., Gallio, M. Temperature representation in the Drosophila brain. Nature. 519 (7543), 358-361 (2015).
- Gallio, M., Ofstad, T. A., Macpherson, L. J., Wang, J. W., Zuker, C. S. The coding of temperature in the Drosophila brain. Cell. 144 (4), 614-624 (2011).
- Hamada, F. N., et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 454 (7201), 217-220 (2008).
- Ni, L., et al. A gustatory receptor paralogue controls rapid warmth avoidance in Drosophila. Nature. 500 (7464), 580-584 (2013).
- Liu, W. W., Mazor, O., Wilson, R. I. Thermosensory processing in the Drosophila brain. Nature. 519 (7543), 353-357 (2015).
- Neely, G. G., et al. TrpA1 Regulates Thermal Nociception in Drosophila. Public Library of Science ONE. 6 (8), e24343 (2011).
- Zhong, L., et al. Thermosensory and non-thermosensory isoforms of Drosophila melanogaster TRPA1 reveal heat sensor domains of a thermoTRP channel. Cell Reports. 1 (1), 43-55 (2012).
- Barbagallo, B., Garrity, P. A. Temperature sensation in Drosophila. Current Opinion in Neurobiology. 34, 8-13 (2015).
- Tang, X., Platt, M. D., Lagnese, C. M., Leslie, J. R., Hamada, F. N. Temperature integration at the AC thermosensory neurons in Drosophila. Journal of Neuroscience. 33 (3), 894-901 (2013).
- Petavy, G., David, J. R., Gibert, P., Moreteau, B. Viability and rate of development at different temperatures in Drosophila: A comparison of constant and alternating thermal regimes. Journal of Thermal Biology. 26 (1), 29-39 (2001).
- Diegelmann, S., Zars, M., Zars, T. Genetic dissociation of acquisition and memory strength in the heat-box spatial learning paradigm in Drosophila. Learning & Memory. 13 (1), 72-83 (2006).
- Zars, M., Zars, T. High and low temperatures have unequal reinforcing properties in Drosophila spatial learning. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 192 (7), 727-735 (2006).
- Zars, T., Wolf, R., Davis, R., Heisenberg, M. Tissue-specific expression of a type I adenylyl cyclase rescues the rutabaga mutant memory defect: in search of the engram. Learning & Memory. 7 (1), 18-31 (2000).
- Jones, M. A., Grotewiel, M. Drosophila as a model for age-related impairment in locomotor and other behaviors. Experimental Brain Research. 46 (5), 320-325 (2011).

