Synthesis and Characterization of Amphiphilic Gold Nanoparticles
Summary
Amphiphilic gold nanoparticles can be used in many biological applications. A protocol to synthesize gold nanoparticles coated by a binary mixture of ligands and a detailed characterization of these particles is presented.
Abstract
Gold nanoparticles covered with a mixture of 1-octanethiol (OT) and 11-mercapto-1-undecane sulfonic acid (MUS) have been extensively studied because of their interactions with cell membranes, lipid bilayers, and viruses. The hydrophilic ligands make these particles colloidally stable in aqueous solutions and the combination with hydrophobic ligands creates an amphiphilic particle that can be loaded with hydrophobic drugs, fuse with the lipid membranes, and resist nonspecific protein adsorption. Many of these properties depend on nanoparticle size and the composition of the ligand shell. It is, therefore, crucial to have a reproducible synthetic method and reliable characterization techniques that allow the determination of nanoparticle properties and the ligand shell composition. Here, a one-phase chemical reduction, followed by a thorough purification to synthesize these nanoparticles with diameters below 5 nm, is presented. The ratio between the two ligands on the surface of the nanoparticle can be tuned through their stoichiometric ratio used during synthesis. We demonstrate how various routine techniques, such as transmission electron microscopy (TEM), nuclear magnetic resonance (NMR), thermogravimetric analysis (TGA), and ultraviolet-visible (UV-Vis) spectrometry, are combined to comprehensively characterize the physicochemical parameters of the nanoparticles.
Introduction
The ligand shell of gold nanoparticles can be engineered to exhibit several different properties that can be applied to address challenges in biomedicine1,2,3,4. Such versatility allows for the control of the intermolecular interactions between nanoparticles and biomolecules5,6,7. Hydrophobicity and charge play a decisive role, as well as other surface parameters that affect how nanoparticles interact with biomolecules5,8,9. To tune the nanoparticles' surface properties, the choice of thiolate molecules that make up the ligand shell offers a myriad of possibilities, according to the characteristics sought. For example, a mixture of ligand molecules with hydrophobic and hydrophilic (e.g., charged) end groups are often used to generate amphiphilic nanoparticles10,11.
One prominent example of this type of nanoparticles is protected by a mixture of OT and MUS (hereafter called MUS:OT nanoparticles) that has been shown to possess many relevant properties12,13,14. First, with a ligand shell composition of 66% MUS (hereafter 66:34 MUS:OT), the colloidal stability of the nanoparticles is high, reaching up to 33% in weight in deionized water, as well as in phosphate-buffered saline (1x, 4 mM phosphate, 150 mM NaCl)15. Moreover, these particles do not precipitate at relatively low pH values: for example, at pH 2.3 and with salt concentrations of 1 M NaCl15, these nanoparticles remain colloidally stable for months.The stoichiometric ratio between the two molecules on the ligand shell is important because it dictates the colloidal stability in solutions with a high ionic strength16.
These particles have been shown to traverse the cell membrane without porating it, via an energy-independent pathway1,12. The spontaneous fusion between these particles and lipid bilayers underlies their diffusivity through cell membranes17. The mechanism behind this interaction is the minimization of contact between a hydrophobic solvent-accessible surface area and water molecules upon fusion with lipid bilayers18. Compared to all-MUS nanoparticles (nanoparticles having only the MUS ligand on their shell), the higher hydrophobicity on mixed MUS:OT nanoparticles (for example, at a 66:34 MUS:OT composition) increases the span of the core diameter that can fuse with lipid bilayers18.Different self-assembly organizations of the ligand shell correlate to distinct binding modes of 66:34 MUS:OT nanoparticles with various proteins, such as albumin and ubiquitin, when compared to all-MUS particles19.Recently, it has been reported that 66:34 MUS:OT nanoparticles can be utilized as a broad-spectrum antiviral agent that irreversibly destroys the viruses because of multivalent electrostatic bindings of MUS ligands and nonlocal couplings of OT ligands to capsid proteins14.In all these cases, it has been found that the hydrophobic content, as well as the core size of the nanoparticles, determines how these bio-nano interactions take place. These diverse properties of MUS:OT nanoparticles have prompted many computer simulation studies that aimed to clarify the mechanisms underpinning the interactions between MUS:OT particles and various biological structures such as lipid bilayers20.
The preparation of MUS:OT-protected Au nanoparticles poses a few challenges. First, the charged ligand (MUS) and the hydrophobic ligand (OT) are immiscible. Thus, the solubility of the nanoparticles and of the ligands needs to be taken into account throughout the synthesis, as well as during characterization. Additionally, the purity of the MUS ligand molecules—specifically, the content of inorganic salts in the starting material—influences the quality, reproducibility, as well as the short- and long-term colloidal stability of the nanoparticles.
Here, a detailed synthesis and characterization of this class of amphiphilic gold nanoparticles protected by a mixture of MUS and OT are outlined. A protocol for the synthesis of the negatively charged MUS ligand is reported to ensure the purity and, hence, the reproducibility of different nanoparticle syntheses. Then, the procedure to generate these nanoparticles, based on a common one-phase synthesis, followed by thorough purification, is reported in detail. Various necessary characterization techniques21, such as TEM, UV-Vis, TGA, and NMR, have been combined to obtain all the necessary parameters for any further biological experiments.
Protocol
1. Synthesis of 11-mercapto-1-undecanesulfonate (MUS)
NOTE: This protocol can be used at any scale desired. Here, a 10 g scale-of-product is described.
- Sodium undec-10-enesulfonate
- Add 11-bromo-1-undecene (25 mL, 111.975 mmol), sodium sulfite (28.75 g, 227.92 mmol), and benzyltriethylammonium bromide (10 mg) to a mixture of 200 mL of methanol (MeOH) and 450 mL of deionized (DI) water (4:9 v/v MeOH:H2O ratio) in a 1 L round-bottom flask.
- Reflux the reaction mixture at 102 °C for 48 h. Cap the system with a pressure relief mechanism—for example, a balloon with a needle, or simply a needle. This reaction is not sensitive to atmospheric gases.
NOTE: The solution becomes colorless when the reaction is complete. - Connect the reaction mixture to a rotary evaporator to evaporate MeOH and reduce the volume to approximately 300 mL.
- Transfer the remaining solution to a 1 L addition funnel.
- Extract the remaining aqueous solution 5x with diethyl ether, using the addition funnel. Unreacted 11-bromo-1-undecene stays in the diethyl ether phase and the sulfonated product in H2O.
CAUTION: Release any pressure buildup frequently during the extraction, and consult the correct usage of addition funnels. - Collect the final extracted water solution into a 1 L single-neck round-bottom flask.
- Connect the reaction flask to a rotary evaporator by putting a bit of grease (or Teflon ring strips or any other sealant) between the flask and the trap.
- Decrease the vacuum slowly to evaporate the aqueous phase in a rotary evaporator. Because the product is a surfactant, foaming will occur during the evaporation. To circumvent this problem, follow the instruction in the next step.
- Add ethanol to the mixture to accelerate the evaporation of H2O and prevent foaming. When foaming restarts because of the decrease in ethanol content, stop the evaporation, remove the flask from the rotary evaporator, add more ethanol (about one-third of the total volume), and reconnect the flask to the rotary evaporator. Repeat this process until the solution mixture decreases significantly and does not form bubbles.
- Dry the white powder directly by connecting the flask to a high vacuum. The drier the powder, the less inorganic salts will creep into the subsequent steps.
NOTE: Heat can be used to dry the product—for example, by keeping the flask under vacuum in a 60 °C bath and left overnight. - Suspend the white powder in 400 mL of methanol in a flask. Sonicate to dissolve the maximum amount of product.
NOTE: The goal of this step is to dissolve the product but not the inorganic byproducts, such as excess sodium sulfite and sodium bromide, that have limited solubility in methanol. Use methanol with the lowest water content possible, because water in the methanol will increase the solubility of the inorganic byproducts in the solvent. - To increase the solubility of the product, methanol can be gently heated close to its boiling point (~64 °C).
CAUTION: Make sure to work under a fume hood during the heating of the flask. The fumes of the evaporated methanol are dangerous. - Filter the solution to remove the methanol insoluble inorganic byproducts. Use a filtering flask connected to a vacuum pump and a filtering funnel with quantitative filter paper, or a borosilicate filter. Both the product and the inorganic salts are white powders when dry: the product is soluble in methanol, while the salts are not.
- Transfer the filtered solution from the filtering flask to a 1 L round-bottom flask.
- Connect the flask to a rotary evaporator and evaporate the methanolic solution at 45 °C, redissolve the white powder in methanol, and filter the solution (protocol steps 1.1.7, 1.1.8, and 1.1.9). Repeat this process at least 2x, to decrease the amount of inorganic salt.
- Collect the white, methanol soluble powder (approximately 30 g, at this scale).
- Dissolve approximately 10 mg of product in 500 µL of D2O and transfer the solution to an NMR tube.
- Perform 1H NMR spectrometry on the product in D2O at 400 MHz with 32 scans.
NOTE: The peak assignments for 1H NMR (D2O) are 5.97 (m, 1H), 5.09 (m, 2H), 2.95 (t, 2H), 2.10 (m, 2H), 1.77 (q, 2H), 1.44 (br s, 12H).
- Sodium 11-acetylthio-undecanesulfonate
- Dissolve the approximate 30 g of sodium undec-10-enesulfonate (the reaction product of section 1.1) in 500 mL of methanol inside a 1 L round-bottom flask. Add a 2.6x excess of thioacetic acid to the solution and stir it in front of a UV lamp (250 W) overnight (~12 h). In case a UV lamp is not available, the reaction can be performed by refluxing using a radical initiator, such as azobisisobutyronitrile (AIBN); however, the use of a UV lamp is strongly recommended.
CAUTION: Make sure to work under the fume hood at all times. If the flask needs to be transported to another space where the UV lamp is located, seal the flask to avoid spreading the strong odor of thioacetic acid. Exercise care when operating a UV lamp: completely block the space where the lamp is located and consult the institution’s safety guidelines on how to operate a UV lamp. - Monitor the reaction by taking ~2 mL aliquots from the reaction, evaporate solvent, and add deuterated water to check with 1H NMR. Once the peaks corresponding to the double bond disappear, stop the reaction.
NOTE: Usually, after 12 h in front of the UV lamp, the reaction is complete. If the reaction mixture becomes turbid, add more MeOH and continue the exposure to the UV light for six additional hours. - Evaporate all of MeOH in a rotary evaporator until the solid residue becomes orange-red. If left long enough, the product becomes brown to black.
CAUTION: Work mindfully because of the strong odors from the thioacetic acid. The strong odors of any thiolate spills can be neutralized using an aqueous solution of bleach (sodium hypochlorite). - Using a filtering flask, wash the product with diethyl ether to remove any excess thioacetic acid, until no more colored (orange-yellow) substances appear in the diethyl ether supernatant. Dry the solid under high vacuum and, then, dissolve it in methanol, yielding a yellow to orange solution.
NOTE: Add enough methanol to dissolve the product.
NOTE: The color may vary at this step. - Add 3 g of carbon black to the solution, mix vigorously, and filter the mixture through filtration medium (see Table of Materials) covering two-thirds of a fluted filter paper.
NOTE: The porous structure of carbon black captures the colored side-product material (and some of the product). The filtered solution should be clear. If the filtered solution is still colored (yellow), repeat this process. - Evaporate the solvent completely in a rotary evaporator and collect approximately 35 g of white powder.
- Dissolve ~10 mg of the product in ~500 µL of D2O and transfer the solution to NMR tubes.
- Perform 1H NMR on the product in D2O at 400 MHz with 32 scans.
NOTE: The peak assignments for the H NMR (D2O) are 2.93 (t, 4H), 2.40 (s, 3H), 1.77 (m, 2H), 1.62 (m, 2H), 1.45 (br s, 14H).
- Dissolve the approximate 30 g of sodium undec-10-enesulfonate (the reaction product of section 1.1) in 500 mL of methanol inside a 1 L round-bottom flask. Add a 2.6x excess of thioacetic acid to the solution and stir it in front of a UV lamp (250 W) overnight (~12 h). In case a UV lamp is not available, the reaction can be performed by refluxing using a radical initiator, such as azobisisobutyronitrile (AIBN); however, the use of a UV lamp is strongly recommended.
- 11-mercapto-1-undecanesulfonate (MUS)
- Reflux sodium 11-acetylthio-undecanesulfonate at 102 °C in 400 mL of 1 M HCl for 12 h to cleave the thioacetate group and obtain a thiol.
- Transfer the product to a 1.5 L or 2 L round-bottom flask. Add 200 mL of 1 M NaOH to the final solution and top it with 400 mL of DI water to have a final volume of 1 L. This will keep the solution acidic and prevent the crystallization of inorganic salts as byproduct.
NOTE: A complete neutralization of the solution to pH 7 will result in the crystallization of a product insoluble in methanol. - Keep the clear solution at 4 °C and it will crystallize overnight. The product crystallizes as fine crystals that are viscous when wet.
NOTE: To accelerate the crystallization, add presynthesized MUS to the solution, if available. - Decant the clear supernatant and centrifuge down the viscous white product in 50 mL centrifuge tubes for 5 min at 4,000 x g.
- Decant the supernatant into another flask and dry the white pellets under high vacuum—depending on the centrifuge available, this can be 2 – 16 tubes or more.
NOTE: Filtering is not advised because of the surfactant nature of the product; excessive foaming will occur and most of the product will be lost. - Collect approximately 12 g (about 30% yield) of methanol-soluble MUS from this purification step.
NOTE: Be mindful that the powder is fine and electrostatic—it tends to stick to spatulas and the surfaces of containers. Also, more material can be extracted from the supernatant of the centrifugation step by reducing the volume (to about a third of its original value) and keeping it at 4 °C. Decrease the volume even more (by 75%) to increase the yield at this step. - Dissolve ~10 mg of the product in ~500 µL of D2O and transfer the solution to NMR tubes.
- Perform 1H NMR on the product in D2O at 400 MHz with 32 scans.
NOTE: The peak assignments of H NMR (D2O) are 2.93 (t, 4H), 2.59 (t, 3H), 1.78 (m, 2H), 1.65 (m, 2H), 1.44 (br s, 14H). The calculated molar mass (including the sodium counterion) of the product is 290.42 g/mol.
2. Nanoparticle Synthesis: Preparation of the Reagents
- Clean all glassware (one 250 mL and one 500 mL single-neck round-bottom flask, a 100 mL addition funnel, and a small funnel) with fresh aqua regia (three parts hydrochloric acid to one part nitric acid). Rinse the glassware with an excess amount of water inside a fume hood and remove all the fumes. Then, rinse the glassware with ethanol and dry it in a laboratory glassware oven (40 – 60 °C is recommended).
- Weigh 177.2 mg (0.45 mmol) of gold (III) chloride trihydrate (HAuCl4∙3H2O) in a small glass vial (10 or 20 mL clean glass vials, or on weighing paper).
- Weigh 87 mg (0.3 mmol) MUS in a glass vial of 20 mL.
- Add 10 mL of methanol to dissolve the MUS. Sonicate it in an ultrasonic bath until no solid material is visible, to ensure complete dissolution.
NOTE: Alternatively, using a heat gun or a warm bath (~60 °C), heat the solution gently. When heated, run cold water through the outside of the flask to bring it back to room temperature. - Add 26 µL (0.15 mmol) of OT to the methanol solution and agitate it to mix the ligands.
- Weigh 500 mg (13 mmol) of sodium borohydride (NaBH4) and add it to 100 mL of ethanol in the 250 mL round-bottom flask. Stir vigorously using magnetic stirring (600 – 800 rpm). (The NaBH4 takes 10 to 20 min, depending on the grade, to form a clear solution in ethanol.)
3. Synthesis of Gold Nanoparticles
- Dissolve gold salt in 100 mL of ethanol in the 500 mL round-bottom flask and start stirring at 800 rpm with a magnetic bar on a stirring plate. Make sure the gold salt dissolves completely.
- Place a 100 mL addition funnel above the round-bottom flask. Put a funnel on the top of the addition funnel with a quantitative paper filter inside. When the NaBH4 is dissolved in ethanol, start filtering the solution into the addition funnel through the filter paper in the funnel.
- Add the ligand solution to the reaction mixture. Wait 15 min for the formation of gold-thiolate complex. The color change of the reaction mixture from translucent yellow to turbid yellow indicates the formation of gold-thiolate complex.
- Start adding the filtered NaBH4 solution from the addition funnel dropwise. Adjust the interval time of the drops so that the addition of NaBH4 takes about 1 h.
- After the complete addition of NaBH4, remove the funnel. Keep stirring the reaction for another hour. At the end of the reaction, remove the magnetic stirring bar using a magnet placed on the outside of the flask.
- Use a septum to close the flask and pierce a needle into the septum to release the H2 gas that will evolve after the reaction.
- Keep the reaction mixture inside a laboratory refrigerator (4 °C) to precipitate the nanoparticles overnight.
4. Workup of the Synthesis
- Decant the supernatant ethanol to reduce the volume.
- Transfer the remaining precipitant to 50 mL centrifuge tubes and centrifuge for 3 min at 4,000 x g.
- Decant the supernatant, disperse the nanoparticles again with ethanol by vortexing, and centrifuge them again. Repeat this washing process 4x.
- Dry the nanoparticles under vacuum to remove the residual ethanol.
- To clean the nanoparticles from free hydrophilic ligands/molecules, dissolve the precipitates in 15 mL of DI water and transfer them to the centrifuge tubes with a filtration membrane of 30 kDa cutoff molecular weight. Dialysis is also amenable for this procedure.
- Centrifuge these tubes for 5 min at 4,000 x g to concentrate the nanoparticle solution.
- Add 15 mL of DI water to this solution and centrifuge to concentrate again. Repeat this cleaning process at least 10x.
NOTE: One indication that the water-soluble impurities have been removed is the absence of foaming when agitating the aqueous waste; after all, most of the impurities are disulfides of MUS with itself or with OT (this can be determined by collecting the material and performing 1H NMR). - After the centrifugation, transfer the concentrated nanoparticles to a 15 mL centrifuge tube. To turn the nanoparticles into a manageable powder, either precipitate them in a solvent such as acetone or freeze-dry the remaining aqueous solution. When freeze-dried, the nanoparticles tend to form a loose powder that sticks to surfaces and may be difficult to manipulate.
5. Characterization of the Nanoparticles
- Purity
- To check whether the nanoparticles are free from unbound ligands, dissolve 5 mg of dry nanoparticles in 600 µL of D2O and perform a 1H NMR measurement of the particles. If there are no sharp peaks of the ligands, it means the nanoparticles are free from small organic molecules.
- Ligand ratio
- Prepare a 20 mg/mL methanol-d4 solution of iodine. Add 600 µL of this solution to the ~5 mg of nanoparticles in a glass vial, to etch the nanoparticles.
- Wrap the cap of the vial with paraffin film and sonicate it in an ultrasonic bath for 20 min. Transfer the solution to an NMR tube and acquire a 1H NMR (400 MHz) spectrum with 32 scans.
- Ligand density
- Transfer 2 to 8 mg of nanoparticles to a TGA crucible. Choose a temperature range from 30 °C to 900 °C and a speed of 5 °C per minute under N2 gas.
- Size distribution
- TEM
- Prepare 0.1 mg/mL nanoparticle solution in DI water. Drop 5 µL of the prepared solution onto the 400-mesh carbon-supported copper grid. Wait until it dries.
- Transfer the grid in a TEM holder and insert it into the microscope. Acquire 5 – 10 images with a magnification of at least 64,000X, operated at 200 kV.
NOTE: To increase the contrast, an objective aperture of 20 nm can be inserted.
- UV-Vis spectra
- Prepare a 0.2 mg/mL nanoparticle solution in DI water.
- Put the required amount of this solution in the quartz cuvette and scan from 200 nm to 700 nm.
- TEM
Representative Results
The reaction steps to synthesize MUS are shown in Figure 1. The 1H NMR spectra of the product of each step are represented in Figure 2. The synthesis workflow of the binary MUS:OT amphiphilic gold nanoparticles is described in Figure 3. Following the synthesis, the workup of the nanoparticles consisted of washing the particles several times with ethanol and DI water. Prior to any characterization of nanoparticles, the cleanliness of the nanoparticles from unbound free ligands was monitored by 1H NMR in D2O, as shown in Figure 4. The size distribution of the nanoparticles was characterized by TEM (Figure 5a,b). Localized surface plasmon resonance absorption was measured by acquiring UV-Vis spectra (Figure 5c).
The ratio of the two ligands was determined by etching the gold core using iodine, acquiring the 1H NMR, and calculating the relative amounts of each ligand using the integrated values. Figure 6 shows the representative spectra, as well as the procedure of NMR peak assignments. To find the ligand ratio between MUS and OT, we calculated the integrals of the peaks between 0.8 – 1 (I1), 1.12 – 1.55 (I2), 1.6 – 1.9 (I3), and 2.6 – 3 (I4) ppm. The I1 peak contains signal from three OT hydrogens, the I2 peak from a combination of 14 MUS hydrogens and 10 OT hydrogens, and the I3 and I4 peaks from four MUS hydrogens and two OT hydrogens (for each peak). Therefore, to find the OT percentage, it is necessary to normalize I1 to 3 and apply the following expressions.
For I2,
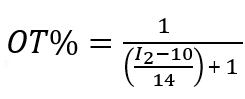
For I3 and I4,
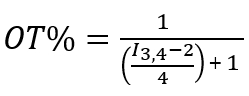
These calculations indicate the ratio of OT to MUS, assuming there is one arbitrary unit of OT in the system. For Figure 6B, the three integrals gave similar values for OT percentage (i.e., 15.3, 15.9, and 15.9 from I2, I3, and I4, respectively).
The surface coverage of the nanoparticles is examined by TGA as shown in Figure 7. TGA, NMR, and TEM data (Figure 3) are combined to calculate the ligand density, which is the number of ligands on a unit of surface area, approximating the particles to a sphere. (This calculation assumes that Na boils as NaHSO3.) TEM data shows that the average diameter of the nanoparticles is 2.4 nm, pointing to approximately 18.08 nm2 (Apar = 4pr2) of surface area (Apar) and 7.23 nm3 (Vpar = 4pr3/3) of volume per particle (Vpar). The density of gold is 19.9 g/cm3 and the mass of one particle is 1.3969 x 10-16 mg (Massparticle = Vpar x the density of gold = 7.23 nm3 x 19.9 g/cm3 x 10-18 mm3/nm3). The remaining mass around 800 °C corresponds to the gold core, and there are approximately 3.7 x 1016 particles (Npar) that are estimated using Npar = (Massgold/Massparticle) = 5.17 mg / 1.3969 x 10-16 mg. The total surface area (Atot) of the particles is 6.69 x 1017 nm2 (Atot = Npar x Apar = 3.69 x 1016 x 18.08 nm2). The NMR of iodine-etched nanoparticles showed that the MUS:OT ratio is 85:15 and the amount of organic content in TGA is 0.00146 g. Therefore, there are 3.26 x 1018 ligands (Nligand) following the formula of Nligand = [Massorganic / ((ROT x MwOT) + (RMUS x MwMUS)) / (RMUS + ROT)] x NAvogadro = [0.00146 g / ((15 x 146 g/mol) + (85 x 267.42 g/mol)) / (85 + 15] x (6.02 x 1023) = 3.26 x 1018. Finally, the ligand density is 4.8 ligands/nm2, calculated by dividing the Nligand by Atot (4.8 = 3.26 x 1018/6.69 x 1017 nm2). The stoichiometric ratios vs. the NMR ratios of the OT, resulting from various syntheses, are compared in Figure 8.
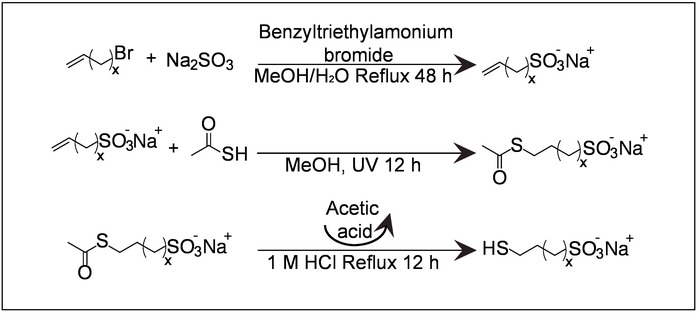
Figure 1: Schematic of MUS synthesis. MUS synthesis is the key point for the reproducibility of amphiphilic nanoparticle synthesis. If MUS has a high salt content, the stoichiometric ratio of the ligands may deviate. X = 9. Please click here to view a larger version of this figure.
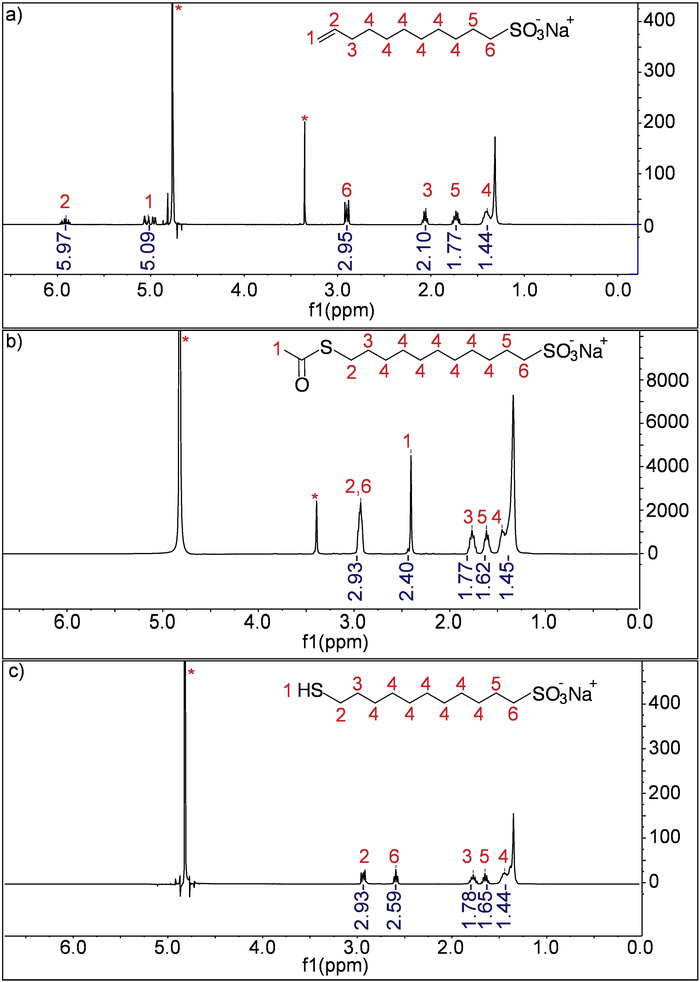
Figure 2: NMR spectra of the molecules after each step in the MUS synthesis (400 MHz). (A) This panel shows the 1H NMR spectrum of sodium undec-10-enesulfonate in D2O. (B) This panel shows the 1H NMR spectrum of sodium 11-acetylthio-undecanesulfonate in D2O. (C) This panel shows the 1H NMR spectrum of 11-mercapto-1-undecanesulfonate in D2O. In all spectra, * indicates the solvent peaks. Please click here to view a larger version of this figure.
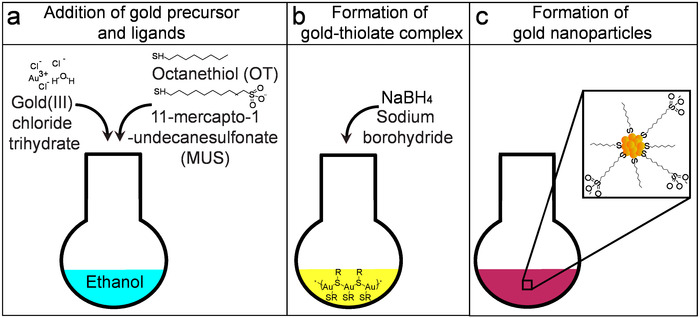
Figure 3: Schematic of the amphiphilic nanoparticle synthesis. (A) This panel shows the preparation of the one-phase chemical reduction reaction using ethanol as solvent. (B) Gold-thiolate complex is allowed to form before the addition of a reducing agent. At this stage, the solution of gold salt became turbid. (C) During the dropwise addition of the reducing agent, gold nanoparticles are formed. Please click here to view a larger version of this figure.
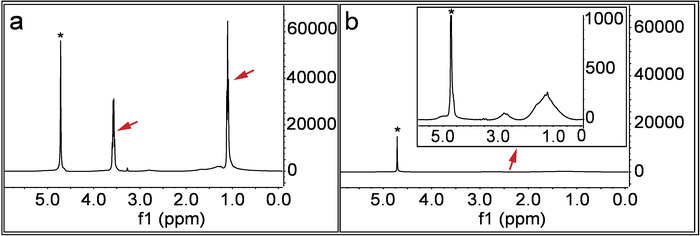
Figure 4: Cleanliness of the nanoparticles from unreacted free ligands. (A) This panel shows the 1H NMR spectrum of the nanoparticles right after the synthesis and vacuum-drying. D2O is used as the solvent for the 1H NMR analysis. Sharp peaks shown by red arrows indicate the existence of free unbound ligands. (B) This panel shows the 1H NMR spectrum of the nanoparticles after a thorough purification (i.e., washes and centrifugation with ethanol and DI water). The red arrow points to the magnified part of the spectrum, in which the peaks are broad, not sharp as before indicating the absence of free ligands. In both spectra, * indicates the solvent peaks. Please click here to view a larger version of this figure.
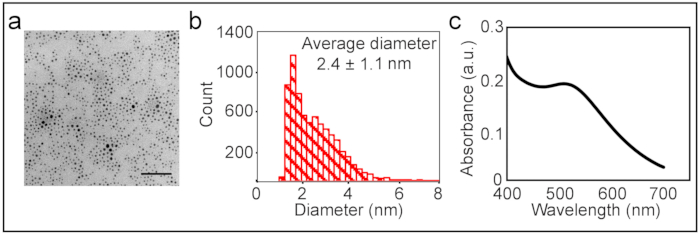
Figure 5: Size distribution of nanoparticles. (A) This panel shows a representative TEM image of MUS:OT nanoparticles. The scale bar is 20 nm. (B) This panel shows a histogram of the core size of nanoparticles based on several TEM images. (C) UV-Vis spectra of the nanoparticles showed the characteristic surface plasmon resonance peak of the nanoparticles at around 520 nm. Please click here to view a larger version of this figure.
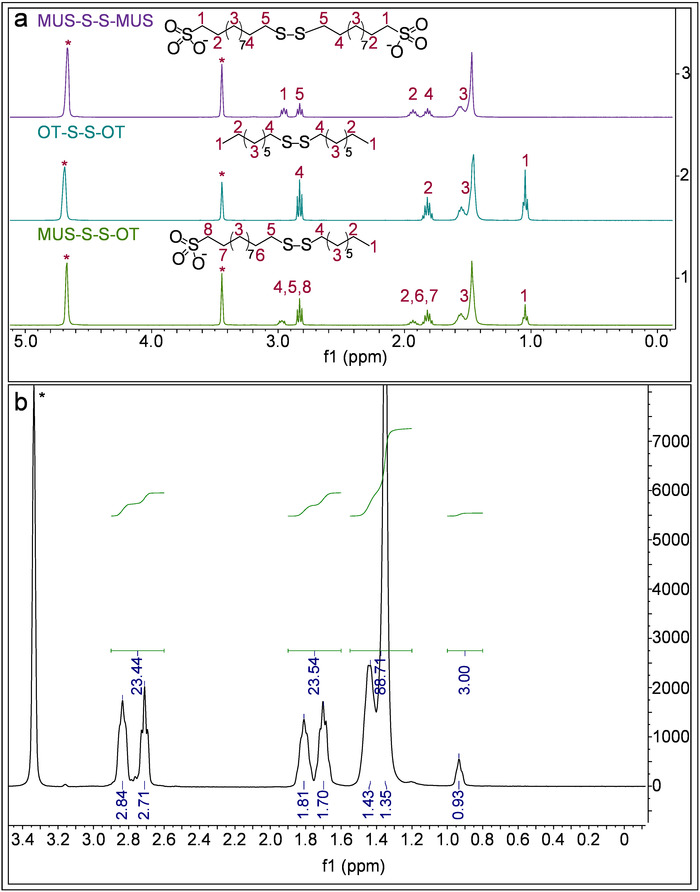
Figure 6: Ligand ratio calculation. (A) This panel shows representative NMR spectra of combinations of disulfides (as references for ligands after core etching) and peak assignments for different protons in MeOD-d4. (B) This panel shows 1H NMR spectra of etched nanoparticles in MeOD-d4. In all spectra, * indicates the solvent peaks. Please click here to view a larger version of this figure.
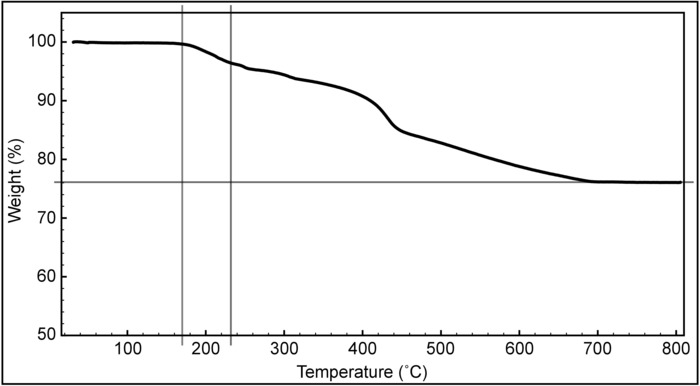
Figure 7: Ligand density analysis. A TGA measurement of the nanoparticles was done to determine the ratio and density of organic material (ligands). The graph of the measurements is plotted as the weight percentage vs. the temperature. OT desorbs first, between 176 °C to 233 °C (vertical lines). MUS degrades to smaller molecules and is totally burned at around 800 °C. The remaining weight percentage corresponds to the gold core of the nanoparticles. Please click here to view a larger version of this figure.
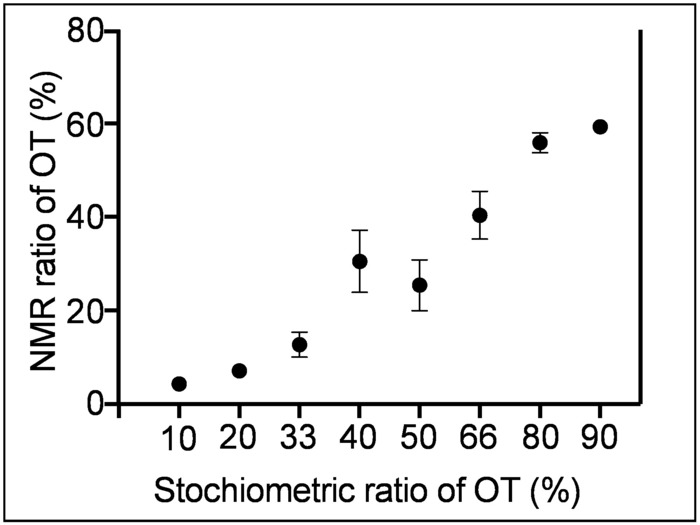
Figure 8: Comparison of stoichiometric and NMR ratios of OT on the particles. It is possible to tune the amphiphilicity of the nanoparticles by changing the starting stoichiometric ratio between MUS and OT in the reaction. The error bars show the upper and lower limit of OT content acquired using the indicated stoichiometric ratios. Stoichiometric ratios of 10%, 20%, etc., up to 90% OT, were synthesized to observe the limits of OT content on the nanoparticle surfaces. Please click here to view a larger version of this figure.
Discussion
This protocol describes first the synthesis of MUS ligand and, then, the synthesis and characterization of amphiphilic MUS:OT gold nanoparticles. Synthesizing MUS with minimal salt content enables a better reliability of the stoichiometric ratio between the ligands during the nanoparticle synthesis, which is a key factor for the reproducible synthesis of MUS:OT nanoparticles with a target hydrophobic content (Figure 8). The use of methanol as a common solvent for MUS and OT, along with the synthesis of the particles in ethanol, allows for a reliable synthesis of MUS:OT gold nanoparticles. The methods of characterization presented here make up a minimal list of experiments necessary to acquire sufficient information on the nanoparticles to verify the outcome of their synthesis.
There are four critical steps in this protocol: (i) the synthesis of MUS with a low salt content along with the removal of colored impurities in the second step and a crystallization of pure MUS in the end; (ii) tuning and determining the stoichiometric ratio between MUS and OT; (iii) the workup of the nanoparticles; and (iv) the characterization of the nanoparticles.
During the formation of nanoparticles, MUS preferentially binds to the nanoparticle surfaces, which might be related to the solubility of the resulting nanoparticles. For example, a 2:1 stoichiometric feed ratio between MUS and OT results in 15% of OT on the surface when calculated using the data from the 1H NMR of etched gold nanoparticles. Therefore, a higher content of OT must be used during the synthesis of the particles (Figure 8) to obtain a nanoparticle with a lower MUS to OT ratio; in other words, a more hydrophobic particle. In order to assess the stoichiometric ratio between the ligands on the surface of the nanoparticles, it is necessary to ensure there are no unbound ligands in the solution. The presence of unbound ligands affects the determination of the ligand ratio on the nanoparticles and the density, along with subsequent tests and experiments that may lead to incorrect interpretations. Repetitive cleaning cycles with different solvents (such as ethanol and DI water) are required to eliminate all unbound ligands and other impurities (byproducts of sodium borohydride, gold ions, etc.). 1H NMR is important to confirm the purity of the nanoparticles. The line-broadening effect of ligands owing to the complex chemical environment on the nanoparticles broaden the peaks corresponding to the ligands, while any sharp signal comes from unbound molecules22. Furthermore, due to restricted mobility, the NMR peaks corresponding to the methylene adjacent to the thiol groups cannot be detected, which is another signature of the nanoparticle when inspected using 1H NMR. Once the nanoparticles are clean, then the metal core is etched with iodine. Iodine etching is a well-established method to quantify the ligand ratio on nanoparticles. For instance, two decades ago, Murray et al. reported the determination of monolayer composition on gold nanoparticles after iodine etching, in which the iodine decomposes the gold core and releases the thiolate ligands as disulfides23. The reliability of the iodine etching method has been established using other methods; for example, Harkness et al. reported that the ligand ratio obtained from NMR is within 1% deviation from mass spectroscopy measurements24.
TGA is a straightforward method to calculate the organic content on the nanoparticles. The estimation of the surface ligand density assumes that all thiolate ligands bind to surface gold atoms and all free ligands have been removed during purification. To determine the ligand density, several assumptions are made, mainly that the particles are spherical, which is used to calculate the surface area, as well as the packing density, of the gold core. TEM provides a size distribution of the nanoparticle gold cores that can be used to calculate the approximate surface area of a nanoparticle. The nanoparticle synthesis described here produces a polydisperse population of particles with an average diameter of 2 – 3 nm and a size deviation of up to 30%. Also, the average radius, used to calculate the average volume of one particle (approximating the particles to spheres), combined with the density of gold, enables the calculation of the mass of one nanoparticle. Then, the mass measured by TGA over 800 °C enables the calculation of the number of particles initially present. By using this value and the average core size, the total surface area of the gold nanoparticles can be estimated. The ligand ratio calculated from the data acquired with 1H NMR spectroscopy allows for the calculation of the number of moles of the ligands on the surface of the nanoparticles. The molar ratio between the ligands over the surface area of gold nanoparticles provides the ligand density (Figure 7). Clean nanoparticles have approximately 4 ligands per nm2. TGA data can also be used to estimate the ligand ratio, if the temperature interval in which they desorb from the gold surface is known for each ligand, and desorption occurs at separate temperature ranges.
In summary, this protocol provides a straightforward way to synthesize the MUS ligand with a low salt content and MUS:OT amphiphilic gold nanoparticles. One of the key factors of the reproducibility of these nanoparticles is the low inorganic salt content in the MUS used. These nanoparticles are stable both as powder and in solution (e.g.,H2O and physiologically-relevant ones), which should be emphasized as a prerequisite for many applications. Thorough characterization of the size and the surface properties of amphiphilic nanoparticles is essential for future applications in which the degree of amphiphilicity may play a key role.
Divulgations
The authors have nothing to disclose.
Acknowledgements
Z.P.G. and F.S. thank the Swiss National Science Foundation and, specifically, NCCR 'Molecular Systems Engineering'. Z.L. and F.S. thank the support of the Swiss National Science Foundation Division II grant. All authors thank Quy Ong for fruitful discussions and for proofreading the manuscript.
Materials
| 11-bromo-1-undecene | Sigma Aldrich | 467642-25 ml | |
| Sodium Sulfite | Sigma Aldrich | S0505-250 g | |
| Benzyltriethyl-ammonium bromide | Sigma Aldrich | 147125-25 g | |
| Methanol | VWR | BDH1135-2.5 LP | |
| DI water | Millipore | ZRXQ003WW | Deionized water |
| 1 L round bottom flask | DURAN | 24 170 56 | |
| Diethyl ether | Sigma Aldrich | 1.00930 EMD Millipore | |
| Stirring bar | Sigma Aldrich | Z329207, | |
| Dow Corning High Vacuum Grease | Sigma Aldrich | Z273554 ALDRICH | |
| Filtering flask | DURAN | 20 201 63 | |
| Filtering Buchner Funnel | FisherSci | 11707335 | |
| Ethanol >99.8%, ACS, Reagent | VWR | 2081.321DP | |
| Deuterium dioxide | Sigma Aldrich | 151882 ALDRICH | |
| Thioacetic acid 96% | Sigma Aldrich | T30805 ALDRICH | |
| Carbon black | Sigma Aldrich | 05105-1KG | |
| Celite | Sigma Aldrich | D3877 SIGMA-ALDRICH | Filtration medium |
| Condenser | Sigma Aldrich | Z531154 | |
| Hydrochloric acid, ACS reagent 37% | Sigma Aldrich | 320331 SIGMA-ALDRICH | |
| Sodium Hydroxide, BioXtra, pellets (anhydrous) | Sigma Aldrich | S8045 SIGMA-ALDRICH | |
| Centrifuge tubes | VWR | 525-0155P | |
| 250 mL round bottom flask | DURAN | 24 170 37 | |
| 500 mL round bottom flask | DURAN | 24 170 46 | |
| Nitric acid, fACS reagent 70% | Sigma Aldrich | 438073 SIGMA-ALDRICH | |
| Gold(III) chloride trihydrate >99.9% trace metal basis | Sigma Aldrich | 520918 ALDRICH | |
| 1-octanethiol >98.5% | Sigma Aldrich | 471836 ALDRICH | |
| Sodium Borohydride purum p.a.>96% | Sigma Aldrich | 71320 ALDRICH | |
| Separatory funnel | SIgma Aldrich | Z330655 SIGMA | |
| Funnel | DURAN | 21 351 46 | |
| 2V folded filtering papers | Whatman | 1202-150 | |
| Amicon filters | Merck | UFC903024 | |
| Iodine, ACS reagent, >99.8%, solid | Sigma Aldrich | 207772 SIGMA-ALDRICH | |
| 5 mm NMR-Tubes, Type 5HP (high precision) | Armar | 32210.503 | Length 178 mm |
| Methanol-d4 99.8 atom%D | Armar | 16400.2035 | |
| TGA crucible | Thepro | 9095-9270.45 | |
| 400 mesh carbon supported copper grid | Electron Microscopy Science | CF400-Cu | |
| quartz cuvette | Hellma Analytics | 100-1-40 |
References
- Verma, A., et al. Effect of surface properties on nanoparticle-cell interactions. Small. 6 (1), 12-21 (2010).
- Yeh, Y. -. C., et al. Gold nanoparticles: preparation, properties, and applications in bionanotechnology. Nanoscale. 4 (6), 1871-1880 (2012).
- Mirza, A. Z. A novel drug delivery system of gold nanorods with doxorubicin and study of drug release by single molecule spectroscopy. Journal of Drug Targeting. 23 (1), 52-58 (2015).
- Mirza, A. Z., et al. Fabrication and characterization of doxorubicin functionalized PSS coated gold nanorod. Arabian Journal of Chemistry. , (2014).
- Nel, A. E., et al. Understanding biophysicochemical interactions at the nano-bio interface. Nature Materials. 8 (7), 543-557 (2009).
- Yeo, E. L. L., et al. Protein Corona around Gold Nanorods as a Drug Carrier for Multimodal Cancer Therapy. ACS Biomaterials Science and Engineering. 3 (6), 1039-1050 (2017).
- Lin, J., et al. Cell membranes open "doors" for cationic nanoparticles/ biomolecules: Insights into uptake kinetics. ACS Nano. 7 (12), 10799-10808 (2013).
- Saha, K., et al. Regulation of Macrophage Recognition through the Interplay of Nanoparticle Surface Functionality and Protein Corona. ACS Nano. 10 (4), 4421-4430 (2016).
- Moyano, D. F., et al. Fabrication of corona-free nanoparticles with tunable hydrophobicity. ACS Nano. 8 (7), 6748-6755 (2014).
- Pengo, P., et al. Gold nanoparticles with patterned surface monolayers for nanomedicine: current perspectives. European Biophysics Journal. 46 (8), 749-771 (2017).
- Kuna, J. J., et al. The effect of nanometre-scale structure on interfacial energy. Nature Materials. 8 (10), 837-842 (2009).
- Verma, A., et al. Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nature Materials. 7 (7), 588-595 (2008).
- Van Lehn, R. C., et al. Lipid tail protrusions mediate the insertion of nanoparticles into model cell membranes. Nature Communications. 5, 4482-4493 (2014).
- Cagno, V., et al. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nature Materials. 17 (2), 195-203 (2018).
- Uzun, O., et al. Water-soluble amphiphilic gold nanoparticles with structured ligand shells. Chemical Communications. 2 (2), 196-198 (2008).
- Huang, R., et al. Colloidal stability of self-assembled monolayer-coated gold nanoparticles: The effects of surface compositional and structural heterogeneity. Langmuir. 29 (37), 11560-11566 (2013).
- Carney, R. P., et al. Electrical method to quantify nanoparticle interaction with lipid bilayers. ACS Nano. 7 (2), 932-942 (2013).
- Van Lehn, R. C., et al. Effect of particle diameter and surface composition on the spontaneous fusion of monolayer-protected gold nanoparticles with lipid bilayers. Nano Letters. 13 (9), 4060-4067 (2013).
- Huang, R., et al. Effects of surface compositional and structural heterogeneity on nanoparticle-protein interactions: Different protein configurations. ACS Nano. 8 (6), 5402-5412 (2014).
- Van Lehn, R. C., et al. Free energy change for insertion of charged, monolayer-protected nanoparticles into lipid bilayers. Soft Matter. 10 (4), 648-658 (2014).
- Ong, Q., et al. Characterization of Ligand Shell for Mixed-Ligand Coated Gold Nanoparticles. Accounts of Chemical Research. 50 (8), 1911-1919 (2017).
- Marbella, L. E., et al. NMR techniques for noble metal nanoparticles. Chemistry of Materials. 27 (8), 2721-2739 (2015).
- Templeton, A. C., et al. Reactivity of Monolayer-Protected Gold Cluster Molecules Steric Effects. Journal of American Chemical Society. 120 (8), 1906-1911 (1998).
- Harkness, K. M., et al. A structural mass spectrometry strategy for the relative quantitation of ligands on mixed monolayer-protected gold nanoparticles. Analytical Chemistry. 82 (22), 9268-9274 (2010).

