An Induction System for Clustered Stomata by Sugar Solution Immersion Treatment in Arabidopsis thaliana Seedlings
Summary
The goal of this protocol is to demonstrate how to induce clustered stomata in cotyledons of Arabidopsis thaliana seedlings by immersion treatment with a sugar-containing medium solution and how to observe intracellular structures such as chloroplasts and microtubules in the clustered guard cells using confocal laser microscopy.
Abstract
Stomatal movement mediates plant gas exchange, which is essential for photosynthesis and transpiration. Stomatal opening and closing are accomplished by a significant increase and decrease in guard cell volume, respectively. Because shuttle transport of ions and water occurs between guard cells and larger neighboring epidermal cells during stomatal movement, the spaced distribution of plant stomata is considered an optimal distribution for stomatal movement. Experimental systems for perturbing the spaced pattern of stomata are useful to examine the spacing pattern’s significance. Several key genes associated with the spaced stomatal distribution have been identified, and clustered stomata can be experimentally induced by altering these genes. Alternatively, clustered stomata can be also induced by exogenous treatments without genetic modification. In this article, we describe a simple induction system for clustered stomata in Arabidopsis thaliana seedlings by immersion treatment with a sucrose-containing medium solution. Our method is easy and directly applicable to transgenic or mutant lines. Larger chloroplasts are presented as a cell biological hallmark of sucrose-induced clustered guard cells. In addition, a representative confocal microscopic image of cortical microtubules is shown as an example of intracellular observation of clustered guard cells. The radial orientation of cortical microtubules is maintained in clustered guard cells as in spaced guard cells in control conditions.
Introduction
The plant stoma is an essential organ for gas exchange for photosynthesis and transpiration, and stomatal movement is accomplished by significant changes in guard cells through ion-driven uptake and release of water. Under a microscope, we can observe a spaced distribution pattern of stomata on the surfaces of leaves and stems. This spaced distribution of stomata is considered to help stomatal movement, which is regulated by ion and water exchange between guard cells and neighboring epidermal cells1,2. Experimental induction systems for clustered stomata are useful for investigating the importance of the spaced distribution of stomata.
It has been reported that spatial clustering of stomata can be induced by genetic modification of key genes for guard cell differentiation3,4 or treatment with a chemical compound5. We also reported that immersion treatment with a medium solution supplemented with sugars including sucrose, glucose, and fructose caused stomatal clustering in cotyledons of Arabidopsis thaliana seedlings6. Reduced callose in new cell walls separating meristemoids and epidermal cells was observed in the sucrose-treated cotyledon epidermis, suggesting that sucrose solution immersion treatment negatively affects the cell wall, which prevents the leakage and ectopic action of key gene products for guard cell differentiation (e.g. transcription factors) towards adjacent epidermal cells6. A similar mechanism was suggested from studies on gsl8/chor mutants7,8. Our experimental system for reproducible induction of clustered stomata using sucrose-containing medium solution is quite easy and cheap. It can also be used to investigate intracellular structures such as organelles and the cytoskeleton in the clustered guard cells when applied to transgenic lines expressing fluorescent markers that label intracellular structures9,10.
Protocol
1. Preparation of 3% Sucrose-containing 1/2 Murashige-Skoog Medium Solution
- Add 1.1 g of Murashige-Skoog medium salts and 15 g of sucrose to a beaker.
- Add 490 mL of distilled water and mix well using a stir bar.
- Adjust the pH to 5.8 using KOH.
- Dilute to 500 mL with distilled water and transfer the solution into a medium bottle.
- Sterilize the solution by autoclaving (121 °C, 20 min). If not used immediately, this solution may be kept at 4 °C after sterilization.
2. Induction of Clustered Stomata by Sucrose-Containing Medium Solution Immersion Treatment
- Sterilize the seeds.
- Prepare the sterilization solution by adding 500 µL of 5% active chlorine NaClO solution and 1 µL of 10% Triton X-100 to 500 µL sterile water.
- Place ca. 50 transgenic A. thaliana seeds carrying a fluorescent marker such as CT-GFP11 or GFP-TUB612 into a 1.5-mL tube.
- Add 1 mL of 70% ethanol solution and mix well by inverting five times. Leave for 1 min.
- The seeds will sink to the bottom of the tube. On a clean bench, gently remove the 70% ethanol using a micropipette, and add 1 mL of sterilization solution. Mix well by inverting five times and leave for 5 min.
- Wash the seeds. Still working under aseptic conditions on a clean bench, gently remove the solution using a micropipette, and add 1 mL of sterile water. Repeat this step five times.
- Add 1.5 mL of sterilized 3% sucrose-containing 1/2 Murashige-Skoog medium solution to each well of a 24-well plate on a clean bench.
- Add two sterilized seeds into each well. Tape the lid onto the 24-well plate using two layers of parafilm.
- Transfer the 24-well plate to a growth chamber set at 23.5 °C with a 12-h/12-h light-dark cycle using 100 µmol m−2 s−1 white light and incubate for 14 days.
3. Microscopic Observation of Clustered Stomata
- Place 30 µL of 3% sucrose-containing 1/2 Murashige-Skoog medium solution from a well of the 24-well plate onto the center of a glass slide (size: 76 × 26 mm, thickness: 1.0-1.2 mm).
- Remove a cotyledon from a 14-day-old seedling using dissecting scissors. Float the cotyledon with the observation side facing up on the solution drop.
- Prepare the cotyledon specimen according to our previous method13. Essentially, place 30 µL of the solution on the center of a cover glass (size: 18 × 18 mm, thickness: 0.12-0.17 mm). Turn the cover glass upside down and place it on the cotyledon gently. Wipe off excess buffer using a lint-free tissue.
- Set a specimen on the stage of a confocal laser microscope and select clustered guard cells for observation using bright field illumination.
- Acquire confocal images of fluorescently labelled intracellular structures according to the microscope manufacturer's instructions.
Representative Results
Here, the protocol for a simple method of inducing stomatal clustering with sucrose-containing medium solution in A. thaliana seedlings has been presented. The clustered guard cells grown in sucrose-containing medium solution (Figure 1B) have larger chloroplasts than guard cells grown in sucrose-free control conditions (Figure 1A). The enlargement of chloroplasts was confirmed with CT-GFP11, a chloroplast stroma marker, and chlorophyll autofluorescence (Figure 1C-F), suggesting that sucrose treatment resulted in starch grain accumulation in the chloroplasts via sucrose solution uptake. In addition, confocal observation of GFP-TUB612 revealed that cortical microtubules were radially oriented even in sucrose-treated clustered guard cells, like those in spaced guard cells in sucrose-free control conditions (Figure 2). These observations suggest that the sucrose-induced clustered guard cells have a normal orientation for cortical microtubules and cellulose microfibrils to enable stomatal opening in response to environmental cues9.
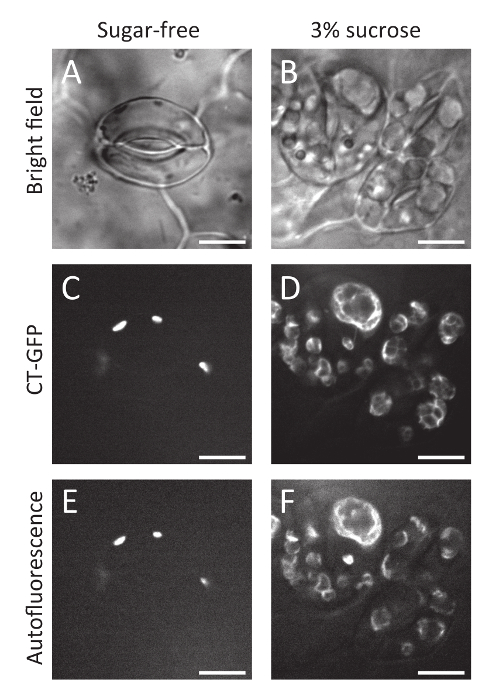
Figure 1: Chloroplasts in clustered guard cells treated with sucrose-containing medium solution. Bright field (A, B), chloroplast stroma marker CT-GFP (C, D), and chlorophyll autofluorescence (E, F) images of guard cells grown in sugar-free control conditions (A, C, E) and 3% sucrose conditions (B, D, F). Scale bars = 10 µm. Please click here to view a larger version of this figure.
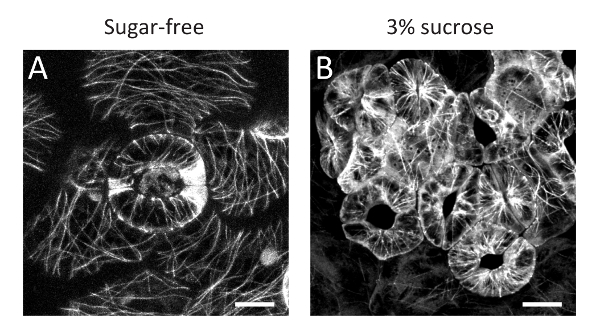
Figure 2: Cortical microtubules in clustered guard cells treated with sucrose solution. Cortical microtubules labelled with GFP-TUB6 of guard cells in the sugar-free control (A) and clustered guard cells in 3% sucrose conditions (B). Scale bars = 10 µm. Please click here to view a larger version of this figure.
Discussion
We have presented protocols for induction of clustered stomata in A. thaliana seedlings by immersion treatment with a sucrose-containing medium solution. As shown here, this method is very simple and requires no specialized skill but can efficiently induce clustered stomata. More than 45% of guard cells are clustered with 3% sucrose-containing medium solution (mean values of more than 20 independent observations)6. Moreover, this experimental system can be directly applied to transgenic or mutant lines as shown for transgenic lines expressing CT-GFP (Figure 1) or GFP-TUB6 (Figure 2). Although only snapshot images are shown here, it would also be possible to perform time-sequential observations during stomatal development.
Note that this method is based on an artificial exogeneous treatment, so we cannot exclude the possibility that phenomena that are not directly related to the stomatal distribution are caused by sucrose solution immersion treatment. In fact, guard cell chloroplasts are enlarged by the treatment (Figure 1). This might be due to starch grain accumulation in the chloroplasts via sucrose solution uptake. In addition, a smaller stomatal aperture was observed in the sucrose-induced clustered guard cells9, suggesting that sucrose-mediated hyperosmotic stress suppressed stomatal opening. Nevertheless, the radial orientation of cortical microtubules was maintained (Figure 2). In addition, the stomatal aperture of the clustered guard cells significantly increases in response to fusicoccin treatment, as in the case of spaced stomata9. Thus, although it will be necessary to carefully judge whether this experimental model system is useful depending on your research purposes, our system would provide insightful information concerning relationship between stomatal distribution and response.
As mentioned in the Introduction, sugar solution treatment might decrease the cell wall integrity, resulting in leakage of key gene products for stomatal differentiation (e.g. transcription factors) to adjacent epidermal cells. We assume that the sucrose-induced ectopic localization of these gene products causes clustered stomata. However, this working hypothesis is not sufficiently supported by molecular biological evidence. Screening for sugar-insensitive mutants would be a promising way to clarify the molecular mechanisms underlying sugar solution-induced stomatal clustering.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We are grateful to Prof. Seiichiro Hasezawa for his kind support of our work. This work was supported by grants from the Japan Society for the Promotion of Science (JSPS) KAKENHgrant numbers 17K19380 and 18H05492, from The Sumitomo Foundation for a Grant for Basic Science Research Projects grant number 160146, and The Canon Foundation to T.H. This experimental system was developed under a financial support from the JSPS KAKENHgrant number 26891006 to K. A. We thank Robbie Lewis, MSc, from Edanz Group (www.edanzediting.com/ac) for editing a draft of the manuscript.
Materials
| 24-well plate | Sumitomo Bakelite | MS-0824R | |
| 488 nm laser | Furukawa Denko | HPU-50101-PFS2 | |
| 488 nm laser | Olympus | Sapphire488-20/O | |
| 510 nm long-pass filter | Olympus | BA510IF | |
| 524 – 546 nm band-pass filter | Semrock | FF01-535/22-25 | |
| 530 nm short-pass filter | Olympus | BA530RIF | |
| 561 nm laser | CVI Melles Griot | 85-YCA-025-040 | |
| 604 – 644 nm band-pass filter | Semrock | FF01-624/40-25 | |
| Confocal laser scanning head | Yokogawa | CSU10 | |
| Confocal laser scanning head | Olympus | FV300 | |
| Cooled CCD camera | Photometrics | CoolSNAP HQ2 | |
| Image acquisition software | Molecular Devices | MetaMorph version 7.8.2.0 | |
| Image acquisition software | Olympus | FLUOVIEW v5.0 | |
| Immersion oil | Olympus | Immersion Oil Type-F | ne = 1.518 (23 degrees) |
| Inverted microscope | Olympus | IX-70 | |
| Inverted microscope | Olympus | IX-71 | |
| Murashige and Skoog Plant Salt Mixture | FUJIFILM Wako Pure Chemical Corporation | 392-00591 | Murashige T and Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum 15(3), 473-497. |
| Objective lens | Olympus | UPlanApo 100x / 1.35 NA Oil Iris 1.35 | NA = 1.35 |
| Objective lens | Olympus | UPlanAPO 40x / 0.85 NA | NA = 0.85 |
| Sucrose | FUJIFILM Wako Pure Chemical Corporation | 196-00015 |
References
- Raschke, K., Fellows, M. P. Stomatal movement in Zea mays: shuttle of potassium and chloride between guard cells and subsidiary cells. Planta. 101 (4), 296-316 (1971).
- Higaki, T., Hashimoto-Sugimoto, M., Akita, K., Iba, K., Hasezawa, S. Dynamics and environmental responses of PATROL1 in Arabidopsis subsidiary cells. Plant and Cell Physiology. 55 (4), 773-780 (2013).
- Bergmann, D. C., Sack, F. D. Stomatal development. Annual Review of Plant Biology. 58, 163-181 (2007).
- Pillitteri, L. J., Torii, K. U. Mechanisms of stomatal development. Annual Review of Plant Biology. 63, 591-614 (2012).
- Sakai, Y., et al. The chemical compound bubblin induces stomatal mispatterning in Arabidopsis by disrupting the intrinsic polarity of stomatal lineage cells. Development. 144 (3), 499-506 (2017).
- Akita, K., Hasezawa, S., Higaki, T. Breaking of plant stomatal one-cell-spacing rule by sugar solution immersion. PLOS One. 8 (9), 72456 (2013).
- Chen, X. Y., et al. The Arabidopsis callose synthase gene GSL8 is required for cytokinesis and cell patterning. Plant Physiology. 150 (1), 105-113 (2009).
- Guseman, J. M., et al. Dysregulation of cell-to-cell connectivity and stomatal patterning by loss-of-function mutation in Arabidopsis chorus (glucan synthase-like 8). Development. 137 (10), 1731-1741 (2010).
- Akita, K., Hasezawa, S., Higaki, T. Cortical microtubules and fusicoccin response in clustered stomatal guard cells induced by sucrose solution immersion. Plant Signaling and Behavior. 13 (4), 1454815 (2018).
- Akita, K., Hasezawa, S. Sugar solution induces clustered lips. Cytologia. 79 (2), 125-126 (2014).
- Holzinger, A., Buchner, O., Lütz, C., Hanson, M. R. Temperature-sensitive formation of chloroplast protrusions and stromules in mesophyll cells of Arabidopsis thaliana. Protoplasma. 230 (1-2), 23-30 (2007).
- Abe, T., Hashimoto, T. Altered microtubule dynamics by expression of modified α-tubulin protein causes right-handed helical growth in transgenic Arabidopsis plants. The Plant Journal. 43 (2), 191-204 (2005).
- Higaki, T. Real-time imaging of plant cell surface dynamics with variable-angle epifluorescence microscopy. Journal of Visualized Experiments. (106), 53437 (2015).

