[(DPEPhos)(bcp)Cu]PF6: A General and Broadly Applicable Copper-Based Photoredox Catalyst
Summary
Detailed and general protocols are presented for the synthesis of [(DPEPhos)(bcp)Cu]PF6, a general copper-based photoredox catalyst, and for its use in synthetic chemistry for the direct arylation of C-H bonds in (hetero)arenes and radical cyclization of organic halides.
Abstract
Our group recently reported the use of [(DPEPhos)(bcp)Cu]PF6 as a general copper-based photoredox catalyst which proved efficient to promote the activation of a broad variety of organic halides, including unactivated ones. These can then participate in various radical transformations such as reduction and cyclization reactions, as well as in the direct arylation of several (hetero)arenes. These transformations provide a straightforward access to a range of small molecules of interest in synthetic chemistry, as well as to biologically active natural products. Altogether, [(DPEPhos)(bcp)Cu]PF6 acts as a convenient photoredox catalyst which appears to be an attractive, cheap and complementary alternative to the state-of-the-art iridium- and ruthenium-based photoredox catalysts. Here, we report a detailed protocol for the synthesis of [(DPEPhos)(bcp)Cu]PF6, as well as NMR and spectroscopic characterizations, and we illustrate its use in synthetic chemistry for the direct arylation of (hetero)arenes and radical cyclization of organic halides. In particular, the direct arylation of N-methylpyrrole with 4-iodobenzonitrile to afford 4-(1-methyl-1H-pyrrol-2-yl)benzonitrile and the radical cyclization of N-benzoyl-N-[(2-iodoquinolin-3-yl)methyl]cyanamide to afford natural product luotonin A are detailed. The scope and limitations of this copper-based photoredox catalyst are also briefly discussed.
Introduction
Radical transformations have been known for decades to provide remarkably efficient pathways in synthetic chemistry which are often complementary to transformations based on cationic, anionic or pericyclic processes1. While particularly promising for various types of transformations, radical-based chemistry has however long been underexploited, mainly because of the need for highly toxic reagents which considerably limits its attractiveness. Moreover, radical processes have long been considered as transformations associated with poor levels of control in terms of regio- and/or stereoselectivity, or leading to extensive dimerization and/or polymerization issues.
Alternative strategies have recently been developed in order to facilitate the generation and better control the reactivity of radical species. Among them, photoredox catalysis has become one of the most powerful methods as it allows the convenient generation of radical species using a light-responsive compound, namely the photoredox catalyst, and visible light irradiation2,3. Visible light itself is indeed able to promote population of the excited state of the photoredox catalyst which becomes, consequently, both a stronger reductant and oxidant than in its corresponding ground state. These enhanced redox properties make single-electron transfer processes, not feasible in the ground state, possible under mild conditions from the excited state. Over the past decade, visible light photoredox catalysis has become an attractive and powerful technique in organic synthesis and has allowed the development of numerous remarkably efficient and selective transformations based on radical intermediates generated under sustainable, mild and user-friendly conditions.
While most photoredox processes reported to date are dominated by the use of iridium- and ruthenium-based photoredox catalysts, as well as by some organic dyes such as pyrylium and acridinium derivatives4, cheaper alternatives are still highly demanded for the development of complementary processes of interest for industrial applications. In this regard, the use of copper-based photoredox catalysts appears particularly appealing as they are not only cheaper but also provide opportunities to activate a broader and/or different range of substrates, which therefore opens new perspectives in photoredox catalysis5,6,7,8. Despite some promising early works reported by the Kutal9, Mitani10 and Sauvage11 groups, photoactivatable copper complexes have, however, only been scarcely used in photoredox catalysis, most probably because of their short-lived excited states compared to their ruthenium- and iridium-based congeners. More recently, recent remarkable contributions by Peters and Fu12,13,14,15, Reiser16,17,18,19,20 and other groups21,22,23,24,25 have clearly brought attention back to copper-based photoredox catalysts and demonstrated their unique potential.
As part of our recent interest in copper-catalyzed radical processes26,27, we recently reported a general and broadly applicable copper-based photoredox catalyst, [(DPEPhos)(bcp)Cu]PF6 (DPEPhos: bis[(2-diphenylphosphino)phenyl] ether; bcp: bathocuproine), which turned out to be particularly efficient for the activation of organic halides under visible light irradiation (Figure 1A)28,29,30. Upon irradiation with visible light and in the presence of an amine as sacrificial reductant, a wide range of unactivated aryl and alkyl halides was shown to be easily activated by catalytic amounts of [(DPEPhos)(bcp)Cu]PF6 and therefore to participate in various radical transformations including reductions, cyclizations and direct arylation of several electron-rich (hetero)arenes. Furthermore, [(DPEPhos)(bcp)Cu]PF6 has also proven successful at promoting photoinduced radical domino cyclizations of ynamides and cyanamides, providing an efficient and straightforward access to complex tri-, tetra- and pentacyclic nitrogen heterocycles at the core structures of various natural products. This strategy permitted the efficient synthesis of rosettacin, luotonin A, and deoxyvasicinone, natural products that exhibit anticancer, antimicrobial, anti-inflammatory and antidepressant activities. These transformations are illustrated in Figure 1C. From a mechanistic standpoint, the photoinduced activation of organic halides with [(DPEPhos)(bcp)Cu]PF6 proceeds through a rare Cu(I)/Cu(I)*/Cu(0) catalytic cycle, which has been confirmed by extensive mechanistic and photophysical studies. In particular, excitation of the ground state [(DPEPhos)(bcp)Cu]PF6 [Cu(I)] upon irradiation by visible light leads to the formation of the corresponding excited complex [(DPEPhos)(bcp)Cu]PF6* [Cu(I)*] which is then reduced by the sacrificial amine to generate the corresponding [(DPEPhos)(bcp)Cu]PF6 [Cu(0)] species. This Cu(0) intermediate is reductive enough to reduce the carbon–halogen bond of various organic halides to generate the corresponding radicals, which can then participate in the aforementioned transformations, together with regeneration of the starting catalyst (Figure 1B).
In the following section, we first describe the protocol to synthesize the photoactivatable [(DPEPhos)(bcp)Cu]PF6 (whose NMR and spectroscopic characterizations are presented in the representative results section). The synthesis is straightforward and particularly convenient, and simply requires addition of 1 equivalent of DPEPhos and 1 equivalent of bcp to a solution of tetrakisacetonitrile copper(I) hexafluorophosphate in dichloromethane. The desired [(DPEPhos)(bcp)Cu]PF6 is then isolated by precipitation from diethyl ether and can be easily obtained on a multigram scale (Figure 2A). Importantly, the isolated copper complex is not particularly sensitive to oxygen and moisture and can therefore be conveniently handled with no specific precautions other than being stored away from light.
Secondly, we describe the protocols to activate organic halides using [(DPEPhos)(bcp)Cu]PF6 under visible light irradiation by focusing on two different transformations. The first reaction is the direct arylation of N-methylpyrrole with 4-iodobenzonitrile using catalytic amounts of [(DPEPhos)(bcp)Cu]PF6 as photoredox catalyst, dicyclohexylisobutylamine as the sacrificial reductant and potassium carbonate as the base under irradiation at 420 nm (Figure 2B). The second reaction is the radical cyclization of N-benzoyl-N-[(2-iodoquinolin-3-yl)methyl]cyanamide, using the same catalyst and sacrificial reductant, whose cyclization directly leads to luotonin A, a natural product displaying interesting anticancer activities (Figure 2C). Detailed protocols are provided for both transformations.
Protocol
1. Synthesis of [(DPEPhos)(bcp)Cu]PF6
- Add 3.73 g (10.00 mmol) of tetrakisacetonitrile copper(I) hexafluorophosphate and 5.39 g (10.00 mmol) of DPEPhos to a 2 L round bottom flask equipped with a magnetic stir bar.
- Fit the round bottom flask with a three neck vacuum adapter connected to a vacuum line and an argon line.
- Evacuate the flask under vacuum and backfill with argon three times. Replace the three neck vacuum adapter by a rubber septum.
NOTE: The reaction can be performed under air with slightly reduced efficiency (see discussion section for details). - Add 800 mL of dry dichloromethane.
NOTE: Dichloromethane is freshly distilled from CaH2. The reaction can also be performed in regular dichloromethane (99.8%) with similar efficiency (see discussion section for details). - Stir the reaction mixture for 2 h in the dark (reaction flask covered with aluminum foil) at room temperature (23-25 ºC) under an argon atmosphere.
- Add 3.60 g (10.00 mmol) of bcp to a 500 mL round bottom flask equipped with a magnetic stir bar.
- Fit the round bottom flask with a three neck vacuum adapter connected to a vacuum line and an argon line.
- Evacuate the flask under vacuum and backfill with argon three times. Replace the three neck vacuum adapter by a rubber septum.
- Add 200 mL of dry dichloromethane and gently stir the suspension until complete dissolution of the bcp.
- Add the solution of bcp in dichloromethane to the reaction mixture using a cannula.
- Stir for an additional hour in the dark (reaction flask covered with aluminum foil) at room temperature (23-25 ºC) under an argon atmosphere.
- Filter the mixture through a pad of Celite, wash with ca. 100 mL of dichloromethane and concentrate the filtrate to ca. 50-100 mL under reduced pressure.
- Add the concentrate dropwise to 1 L of diethyl ether using an addition funnel and with vigorous stirring to induce precipitation of the desired complex.
- Collect the precipitate by filtration through a fritted glass (pore size 3) and wash the precipitate with ca. 100 mL of diethyl ether.
- Dry the bright yellow precipitate under vacuum at room temperature (23-25 ºC) for 5 h to recover 10.1 g (91% yield) of the copper complex.
NOTE: A 75% yield was obtained when the reaction was performed under air using distilled dichloromethane; a 89% yield was obtained when the reaction was performed under argon using regular dichloromethane; when performing the reaction under air with regular dichloromethane, the reaction was found to be less efficient and led to much lower purity (ca 70%, as estimated by 1H NMR with an internal standard). - Characterize [(DPEPhos)(bcp)Cu]PF6 as previously reported31.
2. Direct arylation of N-methylpyrrole with 4-iodobenzonitrile
- Add 55 mg (0.05 mmol) of [(DPEPhos)(bcp)Cu]PF6, 59 mg (0.25 mmol) of dicyclohexylisobutylamine (Cy2NiBu), 138 mg (1.0 mmol) of K2CO3 and 114 mg (0.50 mmol) of 4-iodobenzonitrile to an oven-dried 10 mL vial.
- Add a magnetic stir bar, seal the vial with a rubber septum, evacuate the vial under vacuum and backfill with argon three times.
- Add 5 mL of acetonitrile and 890 μL (10.00 mmol) of N-methylpyrrole. Replace the rubber septum by a screw cap.
NOTE: Acetonitrile is freshly distilled from CaH2 and degassed using freeze-pump-thaw cycles prior to use to ensure high yields and reproducibility. - Stir the reaction mixture for 3 days at room temperature (23-25 ºC) in a photoreactor under 420 nm wavelength irradiation.
NOTE: As an alternative to the use of a photoreactor, the reaction can also be conveniently performed using blue LEDs strips or a photochemistry device with a blue LED lamp (440 nm, 34 W). These experimental setups are shown in Figure 3 (see Representative results) and a discussion on their respective results is provided in the "Discussion" section. - Filter the reaction mixture through a pad of Celite, wash with ca. 5 mL of diethyl ether and concentrate the filtrate under reduced pressure.
- Purify the crude residue by flash column chromatography over silica gel (eluent system petroleum ether/EtOAc: 90/10).
- Dry the pure compound under vacuum at room temperature (23-25 ºC) for 3 h to recover 65 mg (72% yield) of the desired C2-arylated pyrrole.
NOTE: The use of blue LEDs strips afforded a 76% yield (69 mg) while the use of a photochemistry device with a blue LED lamp (440 nm, 34 W) afforded an 86% yield (78 mg). - Characterize the pure compound as previously reported32.
3. Cyclization of N-benzoyl-N-[(2-iodoquinolin-3-yl)methyl]cyanamide to luotonin A
- Add 37 mg (0.09 mmol) of N-benzoyl-N-[(2-iodoquinolin-3-yl)methyl]cyanamide, 9 mg (9.0 μmol) of [(DPEPhos)(bcp)Cu]PF6, 11 mg (0.04 mmol) of Cy2NiBu and 25 mg (0.18 mmol) of K2CO3 to an oven-dried 7 mL vial equipped with a magnetic stir bar.
- Seal the vial with a rubber septum, evacuate the vial under vacuum and backfill with argon three times.
- Add 2 mL of acetonitrile. Replace the rubber septum by a screw cap.
NOTE: Acetonitrile is distilled from CaH2 and degassed using freeze-pump-thaw cycles prior to use to ensure high yields and reproducibility. - Stir the reaction mixture for 5 days at room temperature (23-25 ºC) in a photoreactor under 420 nm wavelength irradiation.
- Filter the reaction mixture through a pad of Celite, wash with ca. 2 mL of dichloromethane and concentrate the filtrate under reduced pressure.
- Purify the crude residue by flash column chromatography over silica gel (eluent system: petroleum ether/EtOAc 60:40).
- Dry the pure compound under vacuum at room temperature (23-25 ºC) for 3 h to recover 20 mg (79% yield) of the desired luotonin A.
- Characterize the desired luotonin A as previously reported29.
Representative Results
Synthesis of [(DPEPhos)(bcp)Cu]PF6
As shown by the protocol described in the above section, the synthesis of [(DPEPhos)(bcp)Cu]PF6 is particularly convenient and can be easily performed on a multigram scale. The 1H and 13C NMR spectra indicate formation of the pure complex (Figure 4A,B). The spectroscopic data correspond to those previously reported31.
1H NMR (400 MHz, CDCl3) δ (ppm): 7.83 (s, 2H), 7.60-7.47 (m, 12H), 7.40-7.32 (m, 2H), 7.29-7.17 (m, 8H), 7.11-6.94 (m, 18H), 2.52 (s, 6H). 13C NMR (100 MHz, CDCl3) δ (ppm): 158.5, 150.3, 144.0, 136.5, 133.9, 133.1, 133.0, 132.9, 132.5, 132.2, 132.0, 131.7, 130.1, 129.6, 129.5, 129.2, 128.7, 128.7, 128.6, 125.8, 125.8, 125.5, 125.4, 120.4, 27.5.
The UV/Vis absorption and emission spectra have also been recorded and are shown in Figure 4C,D. The UV/Vis absorption spectrum (acetonitrile, 10-4 M) displays two main absorption bands with two maxima at 385 nm and 485 nm. The emission spectrum (acetonitrile, 10-4 M), obtained by excitation at 445 nm, displays a maximum at 535 nm.
Copper-catalyzed photoredox direct arylation of (hetero)arenes with aryl halides
The direct arylation of N-methylpyrrole with 4-iodobenzonitrile is representative of the use of [(DPEPhos)(bcp)Cu]PF6 to promote the direct arylation of various electron-rich (hetero)arenes under visible light irradiation. The scope of the transformation is depicted in Figure 5. These results are briefly discussed in the next section.
As for the characterization of 4-(1-methyl-1H-pyrrol-2-yl)benzonitrile, the 1H and 13C NMR spectra indicate formation of the pure compound (Figure 6A,B). The spectroscopic data correspond to those previously reported32.
1H NMR (400 MHz, CDCl3) δ (ppm): 7.66 (d, J = 8.6 Hz, 2H), 7.50 (d, J = 8.6 Hz, 2H), 6.78 (app. t, J = 2.1 Hz, 1H), 6.35 (dd, J = 3.7 and 1.8 Hz, 1H), 6.23 (dd, J = 3.7 and 2.7 Hz, 1H), 3.71 (s, 3H). 13C NMR (100 MHz, CDCl3) δ (ppm): 137.8, 132.7, 132.3, 128.4, 125.9, 119.1, 110.8, 109.8, 108.7, 35.6.
Figure 3 illustrates the three experimental setups that have been successfully used to perform the direct arylation of (hetero)arenes with aryl halides under photoredox conditions. The reaction can be performed in a photoreactor with irradiation at 420 nm wavelength (Figure 3A), using commercially available blue LEDs strips (Figure 3B), or using a photochemistry device with a blue LED lamp (440 nm, 34 W) (Figure 3C). A brief discussion on the difference in efficiency between the three experimental setups is given in the next section.
Photoinduced radical domino cyclization of ynamides and cyanamides
The cyclization of N-benzoyl-N-[(2-iodoquinolin-3-yl)methyl]cyanamide to luotonin A is representative of the use of [(DPEPhos)(bcp)Cu]PF6 to promote the radical domino cyclization of ynamides and cyanamides containing a suitably-placed iodoaryl subunit. These cyclizations, which provide an efficient and straightforward access to tri-, tetra- and pentacyclic nitrogen heterocycles at the core structures of various natural products, are shown in Figure 7. These results are briefly discussed in the next section.
The 1H and 13C NMR spectra indicate formation of the pure natural product luotonin A (Figure 8A,B). The spectroscopic data correspond to those previously reported33.
1H NMR (300 MHz, CDCl3) δ (ppm): 8.49-8.41 (m, 3 H), 8.12 (d, J = 7.8 Hz, 1H), 7.95 (d, J = 7.5 Hz, 1 H), 7.89-7.82 (m, 2 H), 7.71-7.66 (m, 1 H), 7.58 (t, J = 8.1 Hz, 1 H), 5.34 (s, 2 H). 13C NMR (75 MHz, CDCl3) δ (ppm): 160.8, 152.7, 151.3, 149.5 (2 C), 134.7, 131.6, 130.8 (2 C), 129.5, 128.9 (2 C), 128.6, 128.0, 127.5, 126.5, 121.4, 47.4.
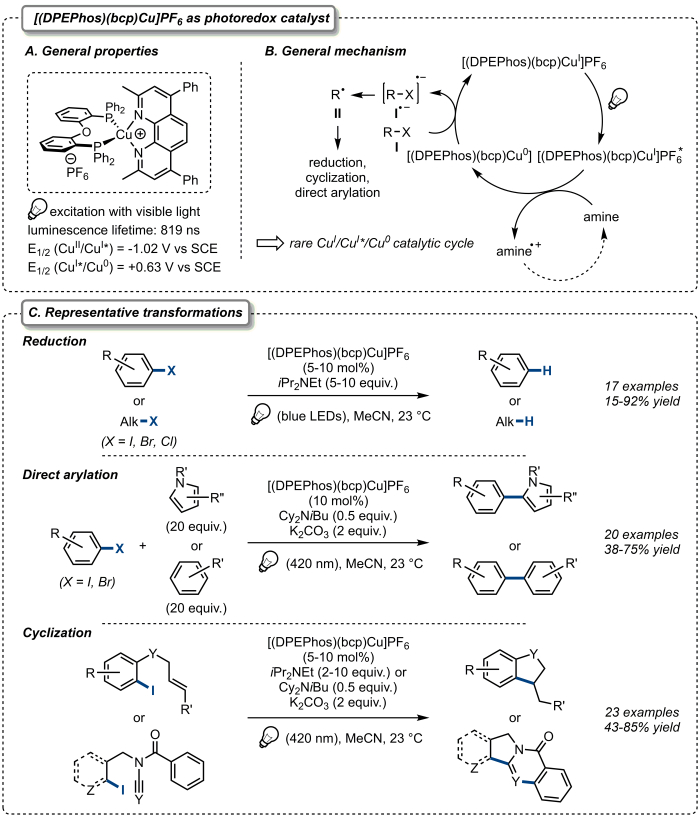
Figure 1. [(DPEPhos)(bcp)Cu]PF6 as a general copper-based photoredox catalyst. (A). General properties of [(DPEPhos)(bcp)Cu]PF6. (B). General mechanism for the activation of organic halides. (C). Representative transformations including reduction, direct arylation and cyclization reactions. Please click here to view a larger version of this figure.
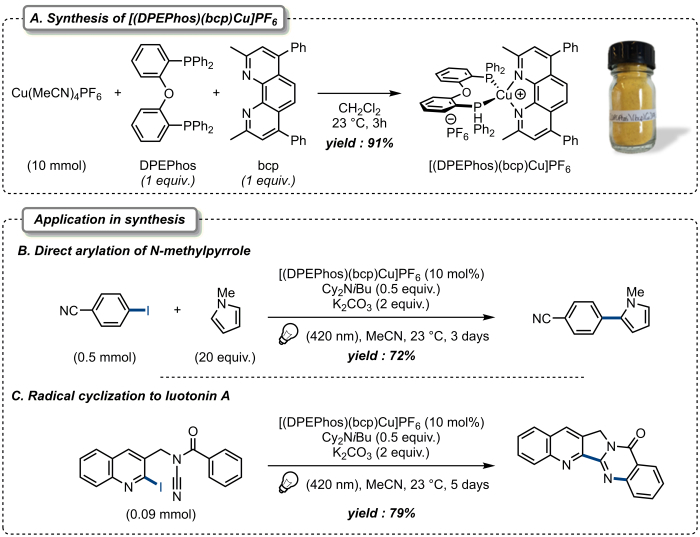
Figure 2. Synthesis of [(DPEPhos)(bcp)Cu]PF6 and application in organic/natural product synthesis. (A). Synthesis of [(DPEPhos)(bcp)Cu]PF6. (B). Direct arylation of N-methylpyrrole. (C). Radical cyclization to luotonin A. Please click here to view a larger version of this figure.

Figure 3. Experimental setups used for the direct arylation of (hetero)arenes under photoredox conditions. (A). Photoreactor. (B). Blue LEDs strips. (C). Photochemistry device and blue LED lamp. Please click here to view a larger version of this figure.
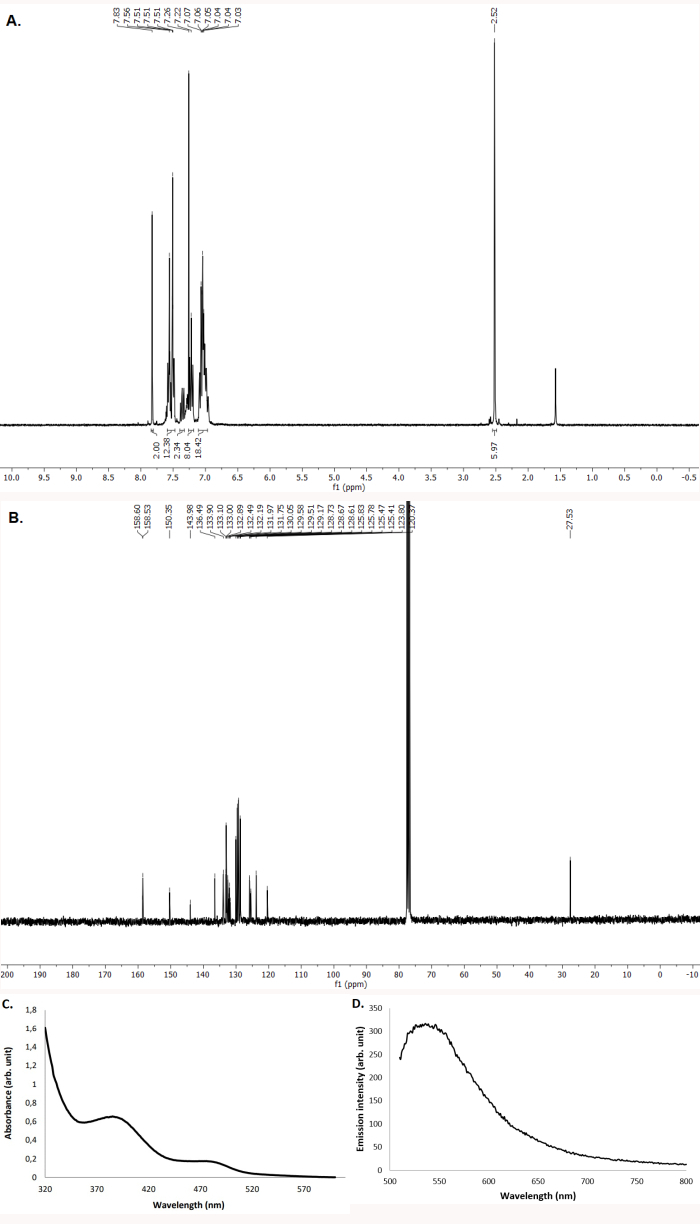
Figure 4. Characterization of [(DPEPhos)(bcp)Cu]PF6. (A). 1H NMR spectrum (CDCl3, 400 MHz, 23 °C). (B). 13C NMR spectrum (CDCl3, 100 MHz, 23 °C). (C). UV/Vis absorption spectrum (acetonitrile, argon, 23 °C). (D). Emission spectrum (acetonitrile, argon, 23 °C). Please click here to view a larger version of this figure.
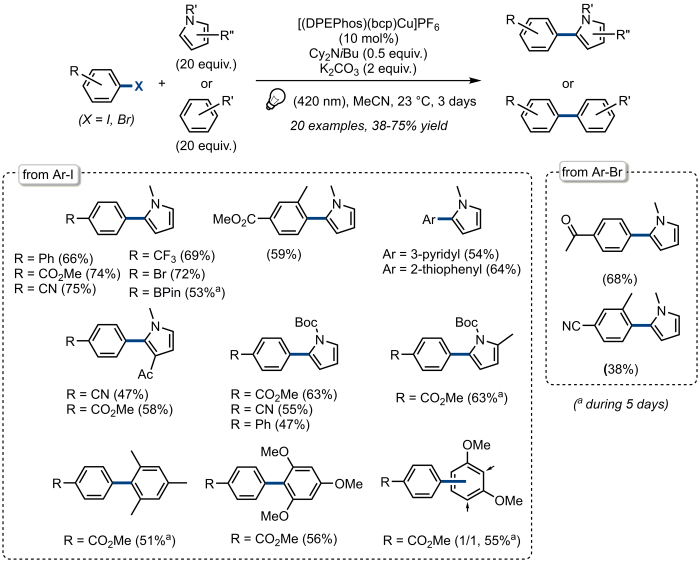
Figure 5. Copper-catalyzed photoredox direct arylation of (hetero)arenes with aryl halides. Substrate scope. Please click here to view a larger version of this figure.
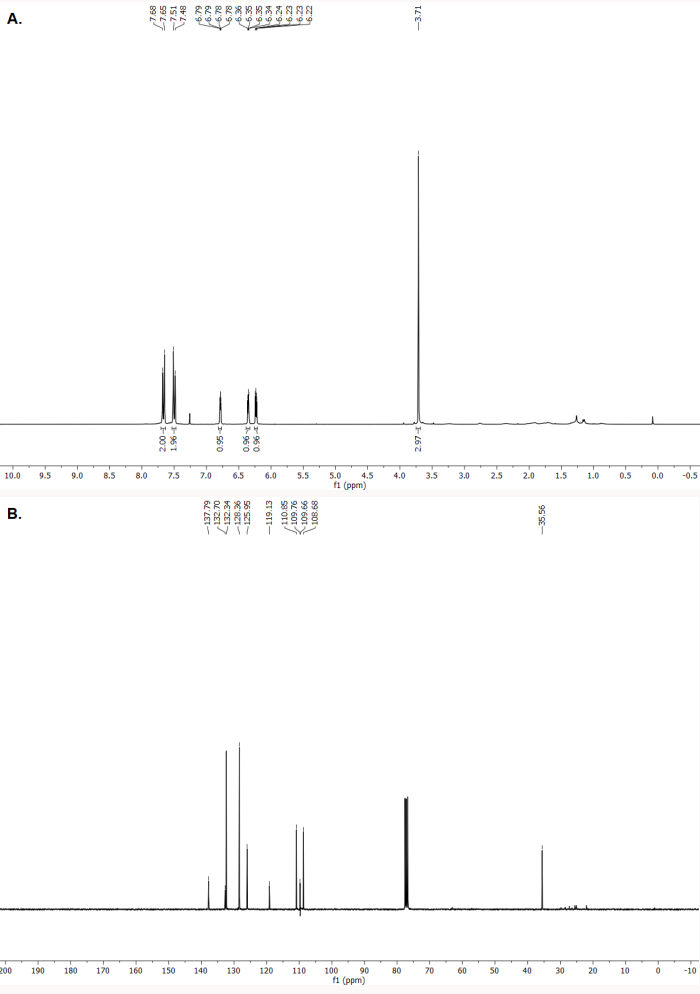
Figure 6. Characterization of 4-(1-methyl-1H-pyrrol-2-yl)benzonitrile. (A). 1H NMR spectrum (CDCl3, 400 MHz, 23 °C). (B). 13C NMR spectrum (CDCl3, 100 MHz, 23 °C). Please click here to view a larger version of this figure.
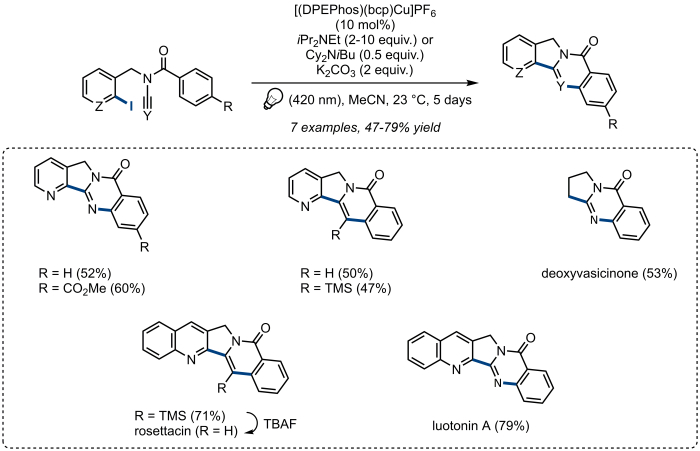
Figure 7. Photoinduced radical domino cyclization of ynamides and cyanamides. Substrate scope. Please click here to view a larger version of this figure.
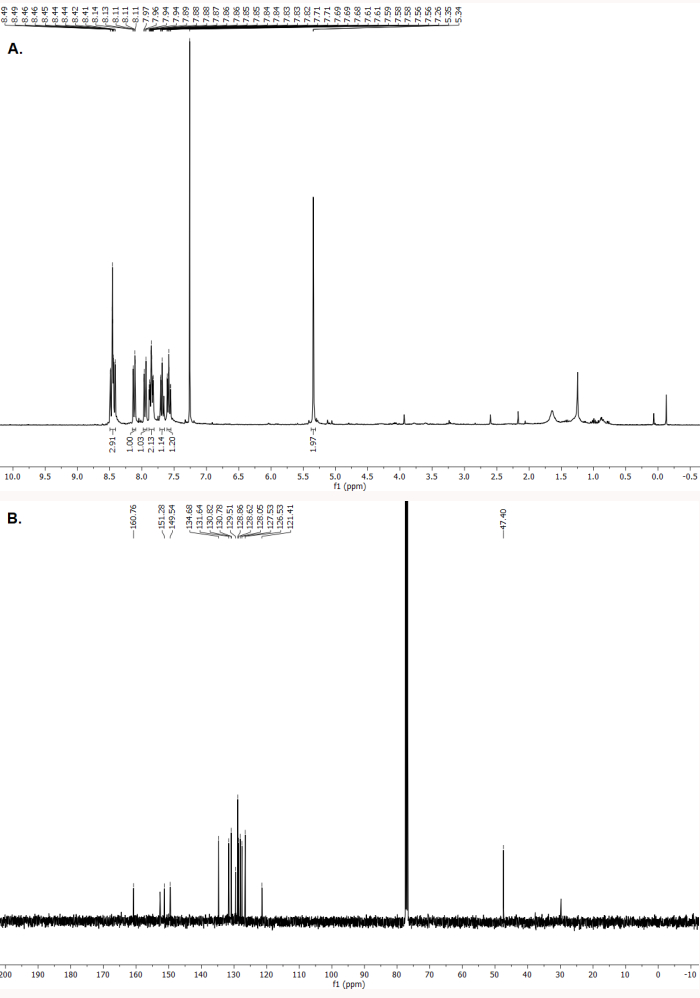
Figure 8. Characterization of luotonin A. (A). 1H NMR spectrum (CDCl3, 300 MHz, 23 °C). (B). 13C NMR spectrum (CDCl3, 75 MHz, 23 °C). Please click here to view a larger version of this figure.
Discussion
Synthesis of [(DPEPhos)(bcp)Cu]PF6
The synthesis of [(DPEPhos)(bcp)Cu]PF6 is typically performed using dry dichloromethane (distilled prior to use) and under argon to ensure the highest yield, purity and good reproducibility. As mentioned in the protocol, the synthesis of [(DPEPhos)(bcp)Cu]PF6 can be performed with regular dichloromethane (99.8%) and/or under air with variable efficiencies. Indeed, while the use of regular dichloromethane under argon afforded the same efficiency (89% yield), performing the reaction with distilled dichloromethane under air only provides the desired complex in a 75% yield. Lastly, performing the reaction with non-distilled dichloromethane under air affords the desired complex with a noticeably lower purity (ca 70% as estimated by 1H NMR using an internal standard). As a consequence, one may conclude that the quality of the dichloromethane used does not have a strong impact on the efficiency of the reaction (high yield and purity), providing that the reaction is conducted under argon. On the other hand, performing the reaction under air is not recommended, as it affords the desired complex with reduced efficiency and, even more importantly, with lower purity when combined with non-distilled dichloromethane.
Copper-catalyzed photoredox direct arylation of (hetero)arenes with aryl halides
As illustrated in Figure 5, the reaction was found to be rather general with a series of 2-arylated pyrroles being obtained in fair to good yields. Noteworthy, aryl iodides substituted with a bromide or a boronate are convenient starting materials and therefore afford a starting point for further functionalization and valorization of the corresponding biaryls. Boc-protected pyrroles were also shown to be readily arylated with a variety of aryl iodides while the reaction could also be extended to the arylation of some electron-rich benzene rings such as 2,4,6-mesitylene, 2,4,6-trimethoxybenzene and 1,3-dimethoxybenzene. Finally, more challenging electron-poor aryl bromides could also be used for the direct arylation of N-methylpyrrole while unactivated aryl bromides and aryl chlorides are not reactive under the reaction conditions, which probably represent the main limitation of this procedure. Altogether, these results show that [(DPEPhos)(bcp)Cu]PF6 efficiently activates the carbon – halogen bond of several aryl halides to promote the direct arylation of some (hetero)arenes which have to be used in large excess to achieve good efficiency. This limitation is, however, common to most procedures reported to date promoting the same transformation.
As mentioned in the protocol section, the copper-catalyzed photoredox direct arylation of (hetero)arenes with aryl halides is mainly performed using a photoreactor with irradiation at 420 nm. Alternatively, the use of simpler and more readily available experimental setups has also been demonstrated, with the use of commercially available blue LEDS strips, as well as of a photochemistry device with a blue LED lamp (440 nm, 34W). Interestingly, small but noticeable differences in efficiency are observed using these three devices. While the photoreactor and blue LEDs strips afford the desired arylated product in comparable yields (72% and 76%, respectively), the photochemistry device with a blue LED lamp affords the highest yield in desired product (86%). This is most probably due to the amount of light that actually penetrates into the reaction mixture, as it is well known that visible light penetration in solution containing photoactive species is quite low. As a consequence, the amount of photoredox catalyst that is actually activated in solution, and that is responsible for catalysis, can also be somehow limited and become the limiting factor. Among the three experimental setups used for the direct arylation of N-methylpyrrole with 4-iodobenzonitrile, the photochemistry device with a blue LED lamp certainly is the most intense, which leads to the highest amount of excited complex and thus, to a higher efficiency. Such considerations are also critical for scaling up such photoinduced processes which are probably more conveniently performed under continuous flow conditions on larger scales34,35.
Photoinduced radical domino cyclization of ynamides and cyanamides
As illustrated in Figure 7, [(DPEPhos)(bcp)Cu]PF6 was shown to be an effective promoter under visible light irradiation for the radical domino cyclization of various ynamides and cyanamides, affording tri-, tetra- or pentacyclic nitrogen heterocycles, in fair to good yields from readily available precursors, at the core structures of various natural products. This strategy permitted the synthesis of rosettacin, luotonin A, and deoxyvasicinone, natural products of great interest that possess anticancer, antimicrobial, anti-inflammatory and antidepressant activities. Noteworthy, the cyclization of ynamides and cyanamides had already been described by Malacria using more classical tin-based conditions33,36, or under photoredox conditions by Yu using the highly reducing fac-Ir(ppy)3 as photoredox catalyst37. When compared to those previously reported methods, the new copper-based photoredox system we reported appears to be an attractive alternative as it provides similar substrate scope and yields while avoiding the use of highly toxic Bu3SnH (Malacria) or expensive fac-Ir(ppy)3 (Yu). Overall, this system has proven its efficiency for the activation of various unactivated aryl- or alkyl- carbon - halogen bonds leading to key structures in organic synthesis, as well as in drug discovery and natural product synthesis.
Altogether, the results discussed above highlight the efficiency of [(DPEPhos)(bcp)Cu]PF6 as an alternative to iridium- and ruthenium- based photocatalysts. Similar levels of efficiency can be obtained with a copper-based catalyst that is both much easier to prepare and much less expensive. The synthesis of the copper-based photocatalyst is in addition highly modular, which paves the way for the design and development of second generation catalysts addressing the main limitations met with [(DPEPhos)(bcp)Cu]PF6 (i.e., the rather high catalyst loading still required in most cases and its unability to activate some aryl bromides and aryl chlorides).
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Université libre de Bruxelles (ULB), the Fédération Wallonie-Bruxelles (ARC Consolidator 2014-2019), Innoviris (project PhotoCop), and the COST action CM1202. H.B. acknowledges the Fonds pour la formation à la Recherche dans l’Industrie et dans l’Agriculture (F.R.I.A.) for graduate fellowship. C.T. acknowledges the Fonds de la Recherche Scientifique (FNRS) for research fellowship.
Materials
| Material | |||
| Bathocuproine (bcp) | Acros | 161340010 | |
| Acetonitrile, 99.9+ | Acros | 326811000 | |
| Celite 545 | Acros | 349670025 | |
| Bis[(2-diphenylphosphino)phenyl] ether (DPEphos) | Acros | 383370050 | |
| Calcium hydride | Acros | C/1620/48 | |
| Dichloromethane, 99.8% | Fisher Chemical | D/1852/25 | |
| Dietyl ether, >= 99% | Fisher Chemical | D/2400/MS21 | |
| Ethyl acetate | Fisher Chemical | E/0900/25 | |
| N-Methylpyrrole, 99% | Sigma Aldrich | M78801 | |
| 4-Iodobenzonitrile, 98% | Combi-Blocks | OR-3151 | |
| Petroleum ether (40-60 °) | Fisher Chemical | P/1760/25 | |
| Potassium carbonate, anhydrous | Fisher Chemical | P/4120/60 | |
| Tetrakisacetonitrile copper(I) hexafluorophosphate, 97% | Sigma Aldrich | 346276 | |
| Equipment | |||
| 1H and 13C NMR spectrometer | Bruker | Avance 300 Spectrometer | |
| 1H and 13C NMR spectrometer | Varian | VNMRS 400 Spectrometer | |
| 420 nm light tubes | Luzchem | LZC-420 | |
| Blue LEDs lamp | Kessil | H150-Blue | |
| Blue LEDs strips | Eglo | 92065 | |
| Photochemistry Device PhotoRedOx Box | Hepatochem | HCK1006-01-016 | |
| Photoreactor | Luzchem | CCP-4V | |
| Spectrofluorimeter | Shimadzu | RF-5301PC | |
| UV/Vis spectrometer | Perkin Elmer | Lambda 40 |
References
- Chatgilialoglu, C., Studer, A. . Encyclopedia of Radicals in Chemistry, Biology and Materials. , (2012).
- Narayanam, J. M. R., Stephenson, C. R. J. Visible light photoredox catalysis: applications in organic synthesis. Chemical Society Reviews. 40, 102-113 (2011).
- Prier, C. K., Rankic, D. A., MacMillan, D. W. C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chemical Reviews. 113 (7), 5322-5363 (2013).
- Romero, N. A., Nicewicz, D. A. Organic Photoredox Catalysis. Chemical Reviews. 116 (17), 10075 (2016).
- Paria, S., Reiser, O. Copper in Photocatalysis. ChemCatChem. 6 (9), 2477-2483 (2014).
- Reiser, O. Shining Light on Copper: Unique Opportunities for Visible-Light-Catalyzed Atom Transfer Radical Addition Reactions and Related Processes. Accounts of Chemical Research. 49 (9), 1990-1996 (2016).
- Boyer, C., et al. Copper-Mediated Living Radical Polymerization (Atom Transfer Radical Polymerization and Copper(0) Mediated Polymerization): From Fundamentals to Bioapplications. Chemical Reviews. 116 (4), 1803-1949 (2016).
- Paria, S., Reiser, O., Stephenson, C. R. J., Yoon, T. P., MacMillan, D. W. C. Visible Light and Copper Complexes: A Promising Match in Photoredox Catalysis. Visible Light Photocatalysis in Organic Chemistry. , 233-252 (2018).
- Grutsch, P. A., Kutal, C. Photobehavior of copper(I) compounds. Role of copper(I)-phosphine compounds in the photosensitized isomerization of norbornadiene. Journal of the American Chemical Society. 101 (15), 4228-4233 (1979).
- Mitani, M., Kato, I., Koyama, K. Photoaddition of alkyl halides to olefins catalyzed by copper(I) complexes. Journal of the American Chemical Society. 105 (22), 6719-6721 (1983).
- Kern, J. -. M., Sauvage, J. -. P. Photoassisted C-C coupling via electron transfer to benzylic halides by a bis(di-imine) copper(I) complex. Journal of the Chemical Society, Chemical Communications. , 546-548 (1987).
- Creutz, S. E., Lotito, K. J., Fu, G. C., Peters, J. C. Photoinduced Ullmann C-N coupling: demonstrating the viability of a radical pathway. Science. 338 (6107), 647-651 (2012).
- Kainz, Q. M., Matier, C. D., Bartoszewicz, A., Zultanski, S. L., Peters, J. C., Fu, G. C. Asymmetric copper-catalyzed C-N cross-couplings induced by visible light. Science. 351 (6274), 681-684 (2016).
- Matier, C. D., Schwaben, J., Peters, J. C., Fu, G. C. Copper-Catalyzed Alkylation of Aliphatic Amines Induced by Visible Light. Journal of the American Chemical Society. 139 (49), 17707-17710 (2017).
- He, J., Chen, C., Fu, G. C., Peters, J. C. Visible-Light-Induced, Copper-Catalyzed Three-Component Coupling of Alkyl Halides, Olefins and Trifluoromethylthiolate to Generate Trifluoromethyl Thioethers. ACS Catalysis. 8 (12), 11741-11748 (2018).
- Pirtsch, M., Paria, S., Matsuno, T., Isobe, H., Reiser, O. [Cu(dap)2Cl] As an Efficient Visible-Light-Driven Photoredox Catalyst in Carbon-Carbon Bond-Forming Reactions. Chemistry – A European Journal. 18 (24), 7336-7340 (2012).
- Paria, S., Pirtsch, M., Kais, V., Reiser, O. Visible-Light-Induced Intermolecular Atom-Transfer Radical Addition of Benzyl Halides to Olefins: Facile Synthesis of Tetrahydroquinolines. Synthesis. 45 (19), 2689-2698 (2013).
- Knorn, M., Rawner, T., Czerwieniec, R., Reiser, O. [Copper(phenanthroline(bisisonitrile)]+-Complexes for the Visible-Light-Mediated Atom Transfer Radical Addition and Allylation Reactions. ACS Catalysis. 5 (9), 5186-5193 (2015).
- Bagal, D. B., Kachkovskyi, G., Knorn, M., Rawner, T., Bhanage, B. M., Reiser, O. Trifluoromethylchlorosulfonylation of Alkenes: Evidence for an Inner-Sphere Mechanism by a Copper Phenanthroline Photoredox Catalyst. Angewandte Chemie International Edition. 54 (24), 6999-7002 (2015).
- Hossain, A., et al. Visible-Light-Accelerated Copper(II)-Catalyzed Regio- and Chemoselective Oxo-Azidation of Vinyl Arenes. Angewandte Chemie International Edition. 57 (27), 8288-8292 (2018).
- Hernandez-Perez, A. C., Vlassova, A., Collins, S. K. Toward a Visible Light Mediated Photocyclization: Cu-Based Sensitizers for the Synthesis of [5]Helicene. Organic Letters. 14 (12), 2988-2991 (2012).
- Baralle, A., Fensterbank, L., Goddard, J. -. P., Ollivier, C. Aryl Radical Formation by Copper(I) Photocatalyzed Reduction of Diaryliodonium Salts: NMR Evidence for a CuII/CuI Mechanism. Chemistry – A European Journal. 19 (23), 10809-10813 (2013).
- Hernandez-Perez, A. C., Collins, S. K. A Visible-Light-Mediated Synthesis of Carbazole. Angewandte Chemie International Edition. 52 (48), 12696-12700 (2013).
- Tang, X. -. J., Doldier, W. R. Efficient Cu-catalyzed Atom Transfer Radical Addition Reactions of Fluoroalkylsulfonyl Chlorides with Electron-Deficient Alkenes Induced by Visible Light. Angewandte Chemie International Edition. 54 (14), 4246-4249 (2015).
- Fumagalli, G., Rabet, P. T. G., Boyd, S., Greaney, M. F. Three-Component Azidation of Styrene-Type Double Bonds: Light-Switchable Behavior of a Copper Photoredox Catalyst. Angewandte Chemie International Edition. 54 (39), 11481-11484 (2015).
- Demmer, C. S., Benoit, E., Evano, G. Synthesis of Allenamides by Copper-Catalyzed Coupling of Propargylic Bromides and Nitrogen Nucleophiles. Organic Letters. 18 (6), 1438-1441 (2016).
- Theunissen, C., Wang, J., Evano, G. Copper-catalyzed direct alkylation of heteroarenes. Chemical Science. 8, 3465-3470 (2017).
- Michelet, B., Deldaele, C., Kajouj, S., Moucheron, C., Evano, G. A General Copper Catalyst for Photoredox Transformations of Organic Halides. Organic Letters. 19 (13), 3576-3579 (2017).
- Baguia, H., Deldaele, C., Romero, E., Michelet, B., Evano, G. Copper-Catalyzed Photoinduced Radical Domino Cyclization of Ynamides and Cyanamides: A Unified Entry to Rosettacin, Luotonin A, and Deoxyvasicinone. Synthesis. 50 (15), 3022-3030 (2018).
- Deldaele, C., Michelet, B., Baguia, H., Kajouj, S., Romero, E., Moucheron, C., Evano, G. A General Copper-based Photoredox Catalyst for Organic Synthesis: Scope Application in Natural Product Synthesis and Mechanistic Insights. CHIMIA. 72 (9), 621-629 (2018).
- Luo, S. -. P., et al. Photocatalytic Water Reduction with Copper-Based Photosensitizers: A Noble-Metal-Free System. Angewandte Chemie International Edition. 52 (1), 419-423 (2013).
- Gryko, D. T., Vakuliuk, O., Gryko, D., Koszarna, B. Palladium-Catalyzed 2-Arylation of Pyrroles. The Journal of Organic Chemistry. 74 (24), 9517-9520 (2009).
- Servais, A., Azzouz, M., Lopes, D., Courilon, C., Malacria, M. Radical Cyclization of N-Acylcyanamides: Total Synthesis of Luotonin A. Angewandte Chemie International Edition. 46 (4), 576-579 (2007).
- Cambié, D., Bottecchia, C., Straathof, N. J. W., Hessel, V., Noël, T. Applications of Continuous-Flow Photochemistry in Organic Synthesis, Material Science, and Water Treatment. Chemical Reviews. 116 (17), 10276-10341 (2016).
- Straathof, N. J. W., Noël, T., Stephenson, C. R. J., Yoon, T. P., MacMillan, D. W. C. Accelerating Visible-Light Photoredox Catalysis in Continuous-Flow Reactors. Visible Light Photocatalysis in Organic Chemistry. , 389-413 (2018).
- Marion, F., Courillon, C., Malacria, M. Radical Cyclization Cascade Involving Ynamides: An Original Access to Nitrogen-Containing Heterocycles. Organic Letters. 5 (26), 5095-5097 (2003).
- Han, Y. -. Y., Jiang, H., Wang, R., Yu, S. Synthesis of Tetracyclic Quinazolinones Using a Visible-Light-Promoted Radical Cascade Approach. The Journal of Organic Chemistry. 81 (16), 7276-7281 (2016).

