Optimization of the Cuff Technique for Murine Heart Transplantation
Summary
We introduce an inner tube approach to the cuff technique for mouse cervical heterotopic heart transplantation to help evert the vessel over the cuff. We found that cooperation between two experienced surgeons markedly shortens the operation time.
Abstract
Murine cardiac transplantation has been performed for more than 40 years. With advancements in microsurgery, certain new techniques have been used to improve surgical efficiency. In our lab, we have optimized the cuff technique with two major steps. First, we used the inner tube technique to insert a temporary inner tube into the external jugular vein and carotid artery blood vessel to facilitate eversion of the vessel over the cuff. Second, we performed complete heterotopic cardiac transplantation through the collaboration of two experienced surgeons. These modifications effectively reduced the operation time to 25 minutes, with a success rate of 95%. In this report, we describe these procedures in detail and provide a supplemental video. We believe that this report on the improved cuff technique will offer practical guidance for murine heterotopic heart transplantation and will enhance the utility of this mouse model for basic research.
Introduction
The establishment of mouse heterotopic heart transplantation through end-to-end anastomosis within the abdomen in 1973 was a major milestone in basic transplant immunology research1. This model provided an important and valid tool for analyzing the mechanisms of ischemia reperfusion injury2, immunological rejection, and tolerance3,4. However, the complex and time-consuming nature of the surgery as well as the potential for infections can result in severe perioperative abdominal adhesions and inflammatory reactions, resulting in a low efficiency for heterotopic heart transplantation model.
The cervical heterotopic heart transplantation technique was first described by Chen in 19915. In this model, the recipient’s external jugular vein is anastomosed to the pulmonary artery of the graft and the carotid artery is anastomosed to the ascending aorta. The main advantages of this method are the convenience of monitoring and the reducing of trauma to the recipient. In the same year, Matsuura described an improved technique, in which the end of the external jugular vein and carotid artery were everted over a Teflon cuff and fixed with a circumferential silk ligature6. Some researchers also fixed the cuff to the right pulmonary artery in the donor heart before inserting the cuff into the external jugular vein of the recipient7. Thus far, the cuff technique has been widely applied in various vascular pedicle transplant models, including those for lung8, liver9, and renal10 transplantation.
To date, there are several difficulties associated with the cuff technique. For example, the carotid artery is difficult to evert over the cuff due to the additional elasticity, resulting in the tissue flipping backwards. Hence, additional practice and a microsurgical dilator may be required to complete this step. In addition, the cervical vessel preparation can take up to 25 minutes.
To resolve these issues, we introduce the inner tube technique, which is based on the cuff technique and includes fixing the cuff on the external jugular vein and carotid artery using an inner tube to helps with the eversion of the vessel wall. In addition, with simple training, the recipient preparation is reduced to 15.5 minutes. This technique reduces the complexity of the operation and does not require additional practice or the use of a vascular dilator. It can be applied in all transplantation immune research, especially for verifying third-party immune tolerance during which the recipient receives two cardiac allografts, one within the abdomen and the other in the neck11. We also recommend co-operation between two skilled surgeons to establish this model, with one surgeon preparing the recipient animal and the other harvesting and implanting the donor heart. Such collaboration can shorten the operation time to 25 minutes. Using this optimized procedure, we have established syngeneic, allogeneic12,13,14,15,16,17,18,19, and xenogeneic mouse heart transplantation models20.
The rationale for the development of the inner tube technique was to reduce the operation time for the establishment of a mouse heart transplant model with a high success rate. Optimization of the cervical heart transplantation model facilitates the acquisition of high success rates in a short period of surgery time compared to the traditional suture and cuff technique21. Further, the cooperation model can further reduce the warm ischemic time of the donor heart compared to surgeries performed with a single operator.
Protocol
Animals (BALB/c, C57BL/6, male, 8-12 weeks) are housed in a specific pathogen-free facility at the Xiamen University Laboratory Animal Center. C57BL/6 is used as recipient and BALB/c is used as donor. All the procedures are performed according to the Institutional Animal Care and Use Committee (IACUC) guidelines.
NOTE: A set of microsurgical instruments, including a micro scissor, micro straight forceps, micro curved forceps and micro needle holders, are necessary for the operation (Table and Materials, Figure 1B, C, D, E). One pair of single-use bulldog clamps (Figure 1F) is needed. Two cuffs for the external jugular vein and carotid artery are prepared by cutting the customized polyamide tubes with a No. 10 scalpel under a microscope. The diameter of the vein and artery cuff is 0.9 mm and 0.55 mm, respectively. In addition, the diameter of the inner tube for the corresponding vein cuff is 0.6 mm, and these of the inner tube for the corresponding artery cuff is 0.28 mm (Figure 1G).
1. Recipient preparation
- Anesthetize the recipient mouse with pentobarbital (60 mg/kg, i.p). Use atraumatic mechanical clippers to remove the hair at the right lateral cervical region.
- Use a sterile cotton tip applicator to wipe the surgical area with iodine antiseptic followed by 70% ethanol.
- Place the mouse in the supine position on the operation platform. Cover the mouse with sterile gauze.
- Use an ophthalmic scissor to make a transverse incision from the lower one-third neck midline to the right shoulder-clavicle joint.
- Isolate the right external jugular vein with micro curved forceps to expose enough length, cut off the branches via electrocoagulation, and ligate the vessel at the distal end using a 6-0 silk suture.
- Clamp the external jugular vein proximally using a bulldog clamp and then transect the vein proximally to the ligature using a micro scissor.
- Wash the vessel lumen with 100 U/mL 0-4 °C heparinized saline to remove any residual blood.
- Pull the external jugular vein through the vein cuff using micro straight forceps; insert the vein inner tube into the lumen as a stent, and evert the vessel wall over the cuff with micro straight forceps (Figure 2A).
- Fix the everted vessel endothelium at the proximal end of the cuff using a circumferential 8-0 silk suture (Figure 2B).
- Use micro straight forceps to withdraw the vein inner tube from the vein vessel.
- Perform blunt dissection with micro curved forceps to isolate the right carotid artery adjacent to the inner edge of the sternocleidomastoid.
- Clamp the right carotid artery proximally using a bulldog clamp, ligate the carotid artery distally using a 6-0 silk suture, and use a micro scissor to transect the carotid artery proximally to the ligature.
- Wash the carotid artery with 100 U/mL 0-4 °C heparinized saline to remove any residual blood.
- Pass the carotid artery through the artery cuff and insert the artery inner tube into the artery vessel using micro straight forceps (Figure 2C).
- Evert the vessel over the cuff using micro straight forceps; fix the everted vessel endothelium using a circumferential 8-0 silk suture (Figure 2D).
- Withdraw the artery inner tube from the artery vessel with micro straight forceps.
NOTE: Preserve the submandibular gland of the recipient.
2. Donor preparation
- Anesthetize the donor mouse with pentobarbital (60 mg/kg, i.p). Use atraumatic mechanical clippers to remove the hair at the abdominal region.
- Place the mouse in the supine position on the operation platform. Cover the mouse with sterile gauze.
- Use a sterile cotton tip applicator to wipe the surgical area with iodine antiseptic followed by 70% ethanol.
- Make an abdominal midline incision with an ophthalmic scissor and expose the abdominal cavity.
- Use micro curved forceps to expose the inferior vena cava, and then intravenously inject 200 μL of 100 U/mL 0-4 °C heparinized saline per 20 g of bodyweight through the inferior vena cava.
- Perform thoracotomy with ophthalmic scissors, cut off the ribs through the bilateral midaxillary line incisions, flip the anterior chest wall outwards to expose the thoracic cavity.
- Excise the thymus with micro curved forceps.
- Expose the aorta, and then perfuse 200 µL of 100 U/mL 0-4 °C heparinized saline to the coronary artery through the aortic arch.
NOTE: Avoid perfusing any gas bubbles into the donor heart. - Use a micro scissor to transect the ascending aorta at the beginning of the aortic arch.
- Transect the pulmonary artery at the beginning of the two main branches with a micro scissor.
- Ligate the superior vena cava and inferior vena cava proximally using a 6-0 silk suture and use a micro scissor to transect vein distally to the ligature.
- Ligate the pulmonary veins together, circumferentially, using a single 6-0 silk suture, and cut off the vein branches distally to the ligature using a micro scissor.
- Remove the heart graft from the surrounding soft tissues; preserve it in 0-4 °C heparinized saline.
3. Heart implantation
- Place the donor heart upside down into the right neck region of the recipient.
- Input the pulmonary artery of the donor heart to a 6-0 silk loop with micro straight forceps.
- Wrap the vessel lumen around the vein cuff, and then tighten the 6-0 silk suture loops around the cuff to band the vessel joint.
- Perform anastomosis of the aorta of the graft and the artery cuff by following the steps described in step 3.2.
- Release the clamped jugular vein followed by the clamped jugular artery. Keep the vessel joint untwisted and ensure that the blood flow is unobstructed.
NOTE: The sinus rhythm returning to more than 200 times within 1 min is considered normal. - Moisten the donor heart using warm (37 °C) saline and inspect whether the graft is bleeding. Set the pulsing heart graft into the subcutaneous space, and then suture the incision.
4. Postoperative Care and Graft Assessment
- Record the time to normal sinus rhythm and the preservation of normal sinus rhythm for at least 5 minutes after clamp release to monitor the post-operative graft function.
- Place the recipient alone on a warm blanket until the recipient wakes up from anesthesia. Administer buprenorphine analgesia, 0.05 mg/kg, s.c, at the end of the surgery and every 12 hours for 72 hours post-surgery.
- Record the weight and postoperative recovery status of the recipient daily. In case of >15% weight loss relative to that on the surgery date, hemiplegic paralysis, or infection, euthanize the recipient via terminal isoflurane inhalation21.
- Monitor graft survival by palpation daily. The surgery is considered to be successful if the murine allograft survives for >72 hours. Grade the graft function, as previously reported22: Scale 3 – vigorously pulsate and frequency; Scale 2 – less pulsate; Scale 1- fibrillation and imminent rejection; or Scale – 0, loss of heart beat and complete rejection.
Representative Results
Surgical Operation Time
After training, a skilled surgeon can successfully perform the operation within 35 minutes using the inner tube technique, wherein approximately 15.5 minutes are required for recipient preparation, 10.9 minutes are required for donor preparation, and 4.4 minutes are required for donor heart anastomoses. The cold and warm ischemia time (from donor preparation to heart implantation) is reduced to 15.3 minutes compared to the operation using the cuff technique without the inner tube technique and suture technique (Table 1)21.
We designed a cooperation model to further improve the efficiency of the operation. As shown in the schematic (Figure 3), one surgeon begins performing recipient preparation first, followed by initiation of the donor preparation by a second surgeon after 4-5 minutes. After 15-16 minutes, the first surgeon should have finished the recipient preparation, at which point the second surgeon having finished harvesting the donor heart, should begin anastomosing the donor heart within the recipient. This cooperation model requires each surgeon to be trained in only a single part of the cuff technique and further shortens the total operation time to approximately 25 minutes. An analysis of >600 heterotopic murine transplantations performed via cooperation between two surgeons over the past two years at the Organ Transplantation Institute of Xiamen University indicates a success rate for cardiac transplantation using this technique of up to 95%.
Survival of Major Histocompatibility Complex Cardiac Mismatched and Matched Cardiac Grafts
Major Histocompatibility Complex (MHC), designated “H-2”, has been used to determine genetic disparities and similarities. Donor-mismatched MHC antigens can trigger graft rejection by interacting directly with the recipient T cells or indirectly as donor MHC-derived peptides expressed on recipient MHC molecules23. A fully MHC mismatched BALB/c (H-2d) allograft heart can be rejected, with a median survival time of 7.5 days after transplantation into C57BL/6 (H-2b) recipient mice (Figure 4A). In our studies, syngeneic heart transplants survived more than 100 days, except in one rare case due to a 15% weight loss compared to normal weight before the operation.
Heart allografts can be harvested for histopathological examination at the time of rejection. Figure 4B shows the appearance of marked cell-mediated rejection characteristics, such as inflammatory cell infiltration, tissue edema, and microvascular occlusion. Syngeneic grafts are near normal with no evidence of myocyte necrosis or inflammatory cell infiltration.
Effect of Inner Tube on Vascular Endothelium
To evaluate the damage on the vascular endothelium after inserting the inner tube into the lumen, 100 days after syngeneic heart transplant, the vascular endothelium of anastomosis site can be collected and stained by immunofluorescent. In this analysis, no obvious narrowing of vascular wall, thrombosis, or thickening of the intima were observed (Figure 4C). Electron microscopic imaging revealed that a smooth endothelium and a regular longitudinal crest formation, with the endothelial cells arranged neatly and closely, with no obvious sediments on the surface (Figure 4D).
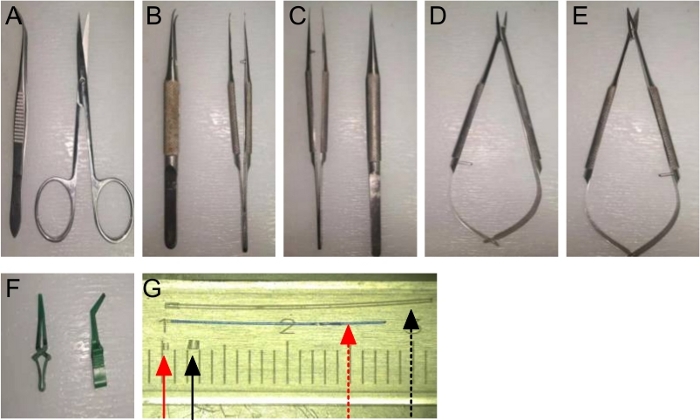
Figure 1: A set of Sterile Surgical Instruments:
(A) Fine forceps and ophthalmic scissor; (B) Micro curved forceps; (C) Micro straight forceps; (D) Micro needle holders; (E) Micro scissors; (F) Bulldog clamps; (G) An artery inner tube (red dotted arrow) and cuff (red solid arrow), along with a vein inner tube (black dotted arrow) and cuff (black solid arrow). Please click here to view a larger version of this figure.
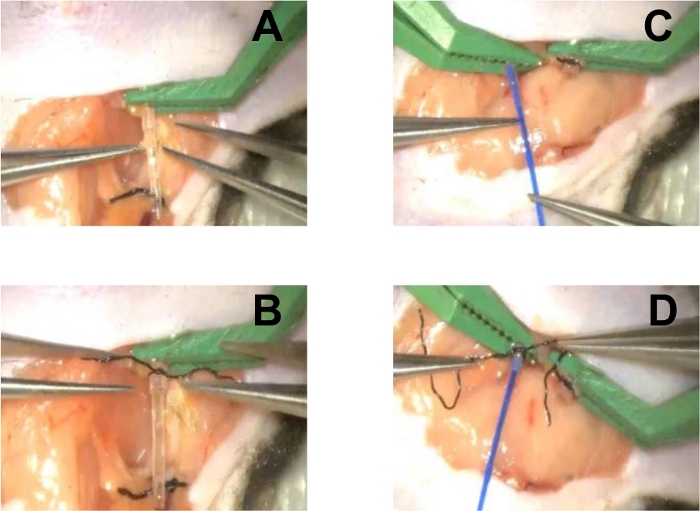
Figure 2: Recipient Preparation.
(A) Insert the vein inner tube into the external jugular vein vessel; (B) Evert the vein vessel over the cuff and fix it using a circumferential 8-0 silk suture; (C) Insert the artery inner tube into the lumen of the artery vessel; (D) Evert the vein vessel over the cuff and fix it using a circumferential 8-0 silk suture. Please click here to view a larger version of this figure.
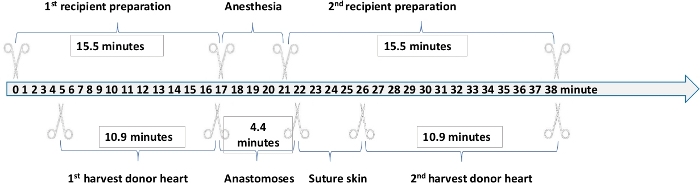
Figure 3: Operation Time of Each Step in Heterotopic Murine Transplantation. Please click here to view a larger version of this figure.
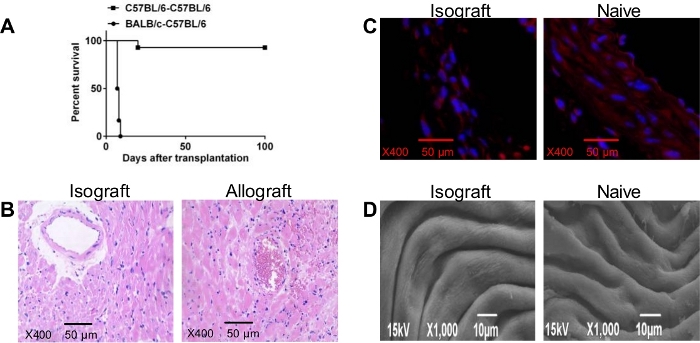
Figure 4: (A) Cardiac graft Survival Time. Kaplan-Meier plot displaying the survival of heart allografts (BALB/c) and syngeneic grafts (C57BL/6) from donor mice transplanted into C57BL/6 recipient mice (n=12 mice/group); (B) Microscopic Examination of C57BL/6 (left) Isograft and Wild Type BALB/c Allograft (right) on Day 7 after Transplantation (Scale bars, 50 um; magnification ×400); (C) Immunofluorescence (Scale bars, 50 µm; magnification ×400) (D) Electron Microscopy Scanning of Vascular Endothelium in Transplant (left) and Naive (right) Recipient. Please click here to view a larger version of this figure.
| Anastomosis technique | Recipient preparation time | Donor preparation time | Heart implantation time | Cold and warm ischemia time | Total operation time | |
| 5Cervical Heterotopic | Suture | N | N | N | < 45 min | N |
| 6Cervical Heterotopic | Cuff | 45 min | 15 min | 10 min | N | N |
| 24Cervical heterotopic | Cuff | 15 min | 20 min | 15 min | 25-40 min | < 60 min |
| 25Abdomen Heterotopic | Suture | N | N | N | N | 35 min |
| 7Cervical Heterotopic | Cuff | N | 20 min | N | 30 min | 35 min |
| 26Cervical Heterotopic | Cuff | N | 20 min | 20 min | < 35 min | N |
| 4Abdomen Heterotopic | Suture | 60–70 min | 6–7 min | N | N | 75 min |
| 21Cervical Heterotopic | Cuff | N | N | 7 min | 20 min | 45 min |
| 27Abdomen Heterotopic | Suture | N | 10-15 min | N | N | 45-60 min |
| 28Cervical Heterotopic | Cuff | 25 min | 20 min | 15 min | 20 min | 60 ± 8 min |
| 29Cervical Heterotopic | Cuff | 31.9 min | 21.1 min | 5.1 min | 28.5 min | 57.8±3.9 min |
| 29Cervical Heterotopic | Suture | 25.2 min | 20.5 min | 30.8 min | 51.3 min | 83.9±2.9 min |
| Cervical Heterotopic in Our Protocol | Cuff | 15.5 min | 10.9 min | 4.4 min | 15.3 min | 35 min |
| (Single Operation) | ||||||
| 23 min | ||||||
| (Cooperation) |
Table 1: Comparison of Time of Different Phases in Different Mouse Heart Transplantation Techniques.
Discussion
Mouse heart transplants models are important tools for transplant immunology research, as tools and materials for evaluating the immune mechanisms of this model and a large number of gene-modified mice are available. However, microsurgical technical challenges, such as vessels suture and eversion, have limited its widespread use. In the present study, we have investigated certain core technical challenges of murine heart transplantation and have obtained good outcomes. A critical step of the protocol the insertion of an inner tube into the lumen as a stent to evert the vessel wall over the cuff. This optimization step resolves the technical challenge of everting the artery vessel due to the need for an extensive stretch. Individuals with no microsurgical skill can begin performing the technique after two months training, which will also aid in the broad application of this model.
In our experience, an appropriate inner tube will enhance transplantation outcomes. The outside diameter of the inner tube should be slightly smaller than the inside diameter of the recipient blood vessel. Moreover, blunt polypropylene tubes or cylinders with a slippery surface should be used as the temporary inner cores to avoid damaging the vascular endothelium. In our hands, no thrombosis occurred in the 5% of models that failed, even though thrombus is a major factor for failure after anastomosis using the suture technique. Using these mature models, our labs have published several basic research articles that have been recognized by peer reviewers14,15,16,17,18,19.
Surgeries performed within 35 minutes were not significantly different compared to the traditional cuff technique, but the cold and warm ischemia time were significantly lower than other techniques (Table 1). Using the cooperation model further reduces the average operation time to 23-25 minutes, which is reflected in the anesthesia time of the recipient mouse and in the donor heart implantation time. Another advantage of the cuff technique that it limits the warm ischemic time (Table 1). As no ice bag is used to protect the heart graft from the warm body temperature of the mouse recipient, the warm ischemic time is equivalent to the anastomosis time. The optimized cuff technique involves the preparation of both cuffs on the recipient to simplify the anastomosis procedure and accordingly shortens the anastomosis time. Therefore, the cuff technique limits the warm ischemic time to only 4.4 minutes on average.
However, there are certain key steps to note with the new technique discussed. Be sure to preserve the submandibular gland of the recipient mouse in cervical heterotopic heart transplantation30, as the submandibular gland can be used to fix the heart graft and avoid twisting of the vessels. Avoid damaging the vagus nerve when isolating the external jugular vein and carotid artery, as injury can lead to neck hemiplegia in the recipient. The pressure of the bulldog clamps should be maintained at 20-25 grams to avoid clip injury or vessel leakage. Wash the lumen of the vessels and the cuffs with 0-4 °C heparinized saline to remove any residual blood and gas bubbles; this prevents embolism in grafts after reperfusion. Use a 1 mL syringe for perfusing the donor with 0-4 °C heparinized saline and increase the speed to 50 μL per second to maintain an appropriate pressure.During anastomosis, do not band the circumferential 8-0 sutures (used to fix the everted vascular endothelium to the cuffs) into the lumen of the graft arteries.
Although there are limitations to the cooperation model, which include the need for microsurgical techniques, the availability of two simultaneous microscopes, and double the number of skilled surgeons, it has nevertheless shown to be a successful approach to performing vascularized organ transplantation. Its broader application may further contribute to developing novel immunosuppressive protocols and study of the mechanisms of acute and chronic rejection in the transplant area.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Fujian Provincial Health Education Joint Research Project (WKJ2016-2-20), the National Natural Science Foundation of China (81771271 and 81800664), the National Key R&D Program of China (2018YFA0108304) and the Education and Scientific Research Project for Young and Middle-aged Teachers in Fujian Province (JAT170714), Natural Science Foundation of Hunan Province of China (2019JJ50842) and Huxiang Young Talents of Hunan Province (2019RS2013).
Materials
| Artery cuff | Self-made | Polyamide tube. diameter: 0.55 mm,length: 1.0 mm | |
| Artery inner tube | Self-made | Polyamide tube. Diameter: 0.28mm | |
| Micro curved forceps | Shanghai Medical Instruments (Group) Ltd., Corp. Surgical Instruments Factory | WA3050 | 1/8 arc, 0.3-mm tip without a hook |
| Micro needle holders | Shanghai Medical Instruments (Group) Ltd., Corp. Surgical Instruments Factory | WA2050 | 0.2-mm tip |
| Micro scissors | Shanghai Medical Instruments (Group) Ltd., Corp. Surgical Instruments Factory | WA1050 | Straight, blade length: 10 mm |
| Micro straight forceps | Shanghai Medical Instruments (Group) Ltd., Corp. Surgical Instruments Factory | WA3060 | 0.15-mm tip without a hook |
| Scanlan Vascu-Statt Bulldog Clamps | Scanlan International Inc | 1001-531 | Clamping pressure 20–25 grams |
| Vein cuff | Self-made | Polyamide tube. diameter: 0.9 mm,length: 1.2 mm | |
| Vein inner tube | Self-made | Polyamide tube. Diameter: 0.6 mm |
References
- Corry, R. J., Winn, H. J., Russell, P. S. Heart transplantation in congenic strains of mice. Transplantation Proceedings. 5 (1), 733-735 (1973).
- Que, W., et al. Prolonged cold ischemia time in mouse heart transplantation using supercooling preservation. Transplantation. , (2019).
- Wang, C. Y., et al. Suppression of murine cardiac allograft arteriopathy by long-term blockade of CD40-CD154 interactions. Circulation. 105 (13), 1609-1614 (2002).
- Hasegawa, T., Visovatti, S. H., Hyman, M. C., Hayasaki, T., Pinsky, D. J. Heterotopic vascularized murine cardiac transplantation to study graft arteriopathy. Nature Protocols. 2 (3), 471-480 (2007).
- Chen, Z. H. A technique of cervical heterotopic heart transplantation in mice. Transplantation. 52 (6), 1099-1101 (1991).
- Matsuura, A., Abe, T., Yasuura, K. Simplified mouse cervical heart transplantation using a cuff technique. Transplantation. 51 (4), 896-898 (1991).
- Wang, Q., Liu, Y., Li, X. K. Simplified technique for heterotopic vascularized cervical heart transplantation in mice. Microsurgery. 25 (1), 76-79 (2005).
- Li, W., et al. Surgical technique for lung retransplantation in the mouse. Journal of Thoracic Disease. 5 (3), 321-325 (2013).
- Kamada, N., Calne, R. Y. A surgical experience with five hundred thirty liver transplants in the rat. Surgery. 93 (1), 64-69 (1983).
- Chen, H., Zhang, Y., Zheng, D., Praseedom, R. K., Dong, J. Orthotopic kidney transplantation in mice: technique using cuff for renal vein anastomosis. PLoS One. 8 (10), 77278 (2013).
- Miller, M. L., et al. Spontaneous restoration of transplantation tolerance after acute rejection. Nature Communications. 6, 7566 (2015).
- Lin, Y., et al. Overexpression of Jagged-1 combined with blockade of CD40 pathway prolongs allograft survival. Immunology and Cell Biology. 93 (2), 213-217 (2015).
- Xie, B., et al. Combined costimulation blockade inhibits accelerated rejection mediated by alloantigen-primed memory T cells in mice. Immunological Investigations. 38 (7), 639-651 (2009).
- Shao, W., et al. Combination of monoclonal antibodies with DST inhibits accelerated rejection mediated by memory T cells to induce long-lived heart allograft acceptance in mice. Immunology Letters. 138 (2), 122-128 (2011).
- Dai, H., et al. Blockade of CD27/CD70 pathway to reduce the generation of memory T cells and markedly prolong the survival of heart allografts in presensitized mice. Transplant Immunology. 24 (4), 195-202 (2011).
- Yan, G., et al. Inhibition of accelerated rejection mediated by alloreactive CD4(+) memory T cells and prolonged allograft survival by arsenic trioxide. Immunological Investigations. 42 (5), 438-454 (2013).
- Yan, G., et al. Inhibiting accelerated rejection mediated by alloreactive CD4(+) memory T cells and prolonging allograft survival by 1alpha,25-dihydroxyvitamin D(3) in nude mice. Immunology Letters. 149 (1-2), 54-61 (2013).
- Lin, Y., et al. Arsenic trioxide is a novel agent for combination therapy to prolong heart allograft survival in allo-primed T cells transferred mice. Transplant Immunology. 25 (4), 194-201 (2011).
- Shao, W., et al. CD44/CD70 blockade and anti-CD154/LFA-1 treatment synergistically suppress accelerated rejection and prolong cardiac allograft survival in mice. Scandinavian Journal of Immunology. 74 (5), 430-437 (2011).
- Li, Y., et al. A highly reproducible cervical cuff technique for rat-to-mouse heterotopic heart xenotransplantation. Xenotransplantation. , (2017).
- Oberhuber, R., et al. Murine cervical heart transplantation model using a modified cuff technique. Journal of Visualized Experiments. (92), e50753 (2014).
- Blanchard, J. M., Pollak, R. Techniques for perfusion and storage of heterotopic heart transplants in mice. Microsurgery. 6 (3), 169-174 (1985).
- Felix, N. J., et al. H2-DMalpha(-/-) mice show the importance of major histocompatibility complex-bound peptide in cardiac allograft rejection. Journal of Experimental Medicine. 192 (1), 31-40 (2000).
- Tomita, Y., et al. Improved technique of heterotopic cervical heart transplantation in mice. Transplantation. 64 (11), 1598-1601 (1997).
- Niimi, M. The technique for heterotopic cardiac transplantation in mice: experience of 3000 operations by one surgeon. Journal of Heart and Lung Transplantation. 20 (10), 1123-1128 (2001).
- Wang, K., Zhang, N., Li, H. Improved technique of mouse heterotopic heart graft retransplantation. Microsurgery. 26 (3), 200-202 (2006).
- Plenter, R. J., Grazia, T. J. Murine heterotopic heart transplant technique. Journal of Visualized Experiments. (89), (2014).
- Ratschiller, T., et al. Heterotopic Cervical Heart Transplantation in Mice. Journal of Visualized Experiments. (102), e52907 (2015).
- Zhou, Y., Gu, X., Xiang, J., Qian, S., Chen, Z. A comparative study on suture versus cuff anastomosis in mouse cervical cardiac transplant. Experimental and Clinical Transplantation. 8 (3), 245-249 (2010).
- Fukunaga, N., Bissoondath, V., Rao, V. Submandibular Gland-preserving Technique for Heterotopic Cervical Heart Transplantation in Mice. Transplantation. 102 (11), 464-465 (2018).

