Applying the RatWalker System for Gait Analysis in a Genetic Rat Model of Parkinson’s Disease
Summary
Here we describe the RatWalker system, built by redesigning the MouseWalker apparatus to accommodate for the increased size and weight of rats. This system uses frustrated total internal reflection (FTIR), high-speed video capture, and open-access analysis software to track and quantify gait parameters.
Abstract
Parkinson’s disease (PD) is a progressive neurodegenerative disorder caused by the loss of dopaminergic (DA) neurons in the substantia nigra pars compacta. Gait abnormalities, including decreased arm swing, slower walking speed, and shorter steps are common in PD patients and appear early in the course of disease. Thus, the quantification of motor patterns in animal models of PD will be important for phenotypic characterization during disease course and upon therapeutic treatment. Most cases of PD are idiopathic; however, the identification of hereditary forms of PD uncovered gene mutations and variants, such as loss-of-function mutations in Pink1 and Parkin, two proteins involved in mitochondrial quality control that could be harnessed to create animal models. While mice are resistant to neurodegeneration upon loss of Pink1 and Parkin (single and combined deletion), in rats, Pink1 but not Parkin deficiency leads to nigral DA neuron loss and motor impairment. Here, we report the utility of FTIR imaging to uncover gait changes in freely walking young (2 months of age) male rats with combined loss of Pink1 and Parkin prior to the development of gross visually apparent motor abnormality as these rats age (observed at 4-6 months), characterized by hindlimb dragging as previously reported in Pink1 knockout (KO) rats.
Introduction
PD, the most common age-related neurodegenerative movement disorder, is caused by the loss of DA neurons in the substantia nigra pars compacta. This loss of nigral DA neurons and the DA inputs into the striatum lead to the observed motor function impairments seen in patients with PD1,2. The defining motor characteristics of patients with PD, known collectively as Parkinsonism, include rigidity, resting tremor, bradykinesia, postural instability, and micrographia3. Furthermore, gait disturbances, which are common in PD patients, appear early in the course of disease1,4,5. While certain lifestyles are suggested to help slow the progression of PD, such as healthy eating and regular exercise, there is currently no cure for PD, only medications to manage the symptoms. This leaves room for the need of further investigation in hopes of improved therapeutics. Thus, characterization of the gait pattern in PD animal models is a crucial tool to characterize the relevance of the model as well as how therapeutic treatments aimed at controlling PD are preventing or improving motor impairments.
There are various PD animal models that have been used to test therapeutic treatments, however each one has their limitations. For example, animal models treated with the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) have yielded a great wealth of information about processes important for nigral DA neuron loss and subsequent striatal adaptations, and pointed to the role of mitochondria in PD pathogenesis; however, the pathogenetic background of the MPTP model is of a toxic nature rather than a neurodegenerative process as in human PD6. Additional chemically inducible models include 6-hydroxydopamine (6-OHDA) and rotenone. 6-OHDA was the first agent used to induce PD by selective accumulation of the drug in the DA neurons, which eventually kills the neurons and leads to PD like symptoms. This model was first used for the tracking of DA depletion by examining the behavior in response to amphetamine and apomorphine7. This method of PD induction has proved to be useful for the screening of pharmacological agents that impact DA and its receptors8. While the 6-OHDA model is a great model for tracking quantifiable motor deficits, this model does not show how the gradual loss of neurons and formation of Lewy bodies impact the animal. The other method of induction, rotenone, has been shown to have progressive degeneration of nigrostriatal neurons with the loss of tyrosine hydroxylase and DA transporter, allowing for a better model to track loss of neurons over time9. The rotenone treated rats showed bradykinesia, postural instability, and unsteady gait10. However, this method has been found to be widely variable between different strains of rats, which has provoked questioning whether or not rotenone is a reliable PD model11,12,13. While gait analysis has been shown to be impacted by the induction of PD in rats, to date, genetically induced PD rat models have not been readily used for gait analysis by freely walking down a runway.
One way to analyze motor impairment in freely walking rodents is kinematic gait analysis, which can be performed by utilizing FTIR imaging. This established method uses an optical touch sensor based on FTIR, which records and tracks the footprints of the rodents as they move down the runway14,15,16. As compared to other methods, FTIR does not depend on any markers on the animal's body that could interfere with the paw prints. Generation of the video data produces digital paw prints of all four limbs that can be combined to create a dynamic and reproducible walking pattern for various rodent models. The principle of imaging-based gait analysis is to take each individual paw and measure the contact area over time as the rodent walks down the runway. Each stance is represented by an increase in paw area (in the braking phase) and a decrease in paw area (in the propulsion phase). This is proceeded by the swing phase, which is when there is no paw signal detected. After evaluation of the video, several parameters are generated that can be used to compare wild-type (WT) versus PD model. Some examples of the parameters are step length (distance the paw covers in one step), swing duration (duration of time the paw is not in contact with the runway), swing speed (step length as a function of swing duration), and step pattern (diagonal steps, lateral steps, or girdle steps).
To demonstrate the utility of FTIR to uncover early gait pattern changes in rats, we used a genetic rat model of PD. While most cases of PD are idiopathic; the identification of hereditary forms of PD uncovered gene mutations and variants, such as loss-of-function mutations in Pink1 and Parkin, two proteins involved in mitochondrial quality control17, that could be harnessed to create animal models18. Unfortunately, mice are resistant to neurodegeneration upon loss of these proteins (single and combined)19,20,21. In rats, Pink1 but not Parkin deficiency leads to nigral DA neuron loss and motor impairments22, but without complete penetrance. Therefore, we generated a combined Pink1/Parkin double knockout (DKO) rat model, which displays the overt visually apparent hindlimb dragging phenotype reported in male Pink1 KO rats22 but now at a higher rate: 100% versus 30-50% of males between 4-6 months.
While this method works well for analyzing motor deficits in mice14, FTIR imaging gait system specifications to accommodate the size and weight of rats was previously unavailable noncommercially. Here we explain how to build the RatWalker, a modified FTIR gait imaging system modeled after the MouseWalker14, except adapted for the size and weight of rats. This system utilizes an optical effect, FTIR, to provide a method to visualize and subsequently record animal footprints for analysis. Contact of an animal's foot with the optical waveguide (platform) causes disruption in the light path resulting in a visible scattering effect, which is captured using domestic-grade, high-speed videography and processing using open-source software. This study demonstrates the power of FTIR imaging in studying gait changes in genetic rat models of PD. For example, while overt visually apparent motor changes (i.e. hindlimb dragging) are observed in male DKO rats at 4 months at the earliest, using FTIR we are able to uncover gate abnormalities in male DKO rats at 2 months of age.
Protocol
All animal studies were approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee (IACUC).
1. Gait apparatus
NOTE: Modeled from the MouseWalker14, the RatWalker was designed with dimensions in proportion to the difference in step length between rats and mice. It consists of a side illumination backlight, walkway enclosure, optical waveguide walkway, mirror, and camera (Figure S1). LED strips, oriented in a staggered position, were used on each side of the walkway and backlight waveguides to accommodate the extra material. The materials needed to build the modified gait apparatus can be found in Table S1.
- Use a backlight (Figure S2) to create a silhouette of the animal which is utilized by the software to assign position, direction of movement, and morphometric qualities. Construction is comprised of a layered panel of an acrylic diffuser, optical waveguide, reflector, and LED light strips assembled within a stock aluminum frame (Table S1).
- Use a walkway enclosure (Figure S3) to guide the animal along the platform and direct the animal to the home-cage. Construction consists of clear acrylic sheets solvent welded with dichloromethane (Table S2).
- Use the walkway (Figure S4) to provide the medium to generate lit footprints. The walkway is constructed from clear acrylic, which is side-lit with strip LEDs and housed in aluminum angle (Table S3).
- Place a mirror (Figure S5) directly under the walkway at a 45-degree angle to reflect the underside of the walkway for videography. It is constructed from a 1/4" thick glass mirror supported by acrylic, and angled brackets arranged in a row (Table S4).
- Perform videography using a tripod-mounted, domestic-quality, high-speed action camera.
2. Equipment Setup
- Align the backlight, walkway, and mirror according to Figure S1, on top of a countertop, workbench, or stable cart. Ensure each component is centered with respect to the walkway.
- Using a level, make certain that the components are horizontally plumb.
- Place the walkway enclosure on top of the walkway.
- Clean all contact surface areas with 70% ethanol. Make sure to use a nonabrasive towel to prevent scratching of the walkway.
- Mount the high-speed camera onto a 57-inch tripod and place it mid-line to the mirror, spaced far enough to capture the entire walkway inside of the field of view. From the video settings menu, ensure that the high-speed camera is set to linear acquisition in 1080p mode at 120 frames per second (fps) with any type of auto-adjust or optimizations turned off.
- Plug in and turn on the LED strip lights for the backlight and walkway. It may be necessary to dim the backlight to reduce background capture.
3. Animal Acclimation
NOTE: One week prior to the first experiment, run the animals through the modified gait apparatus.
- Position a home-cage at the terminus of the walkway.
- With the enclosure installed and the lights off, place the rat at the end of walkway opposite the home-cage and allow it to walk across the walkway in an unforced manner.
- Run each rat through the modified gait apparatus several times, until they can smoothly cross the entire walkway.
- Repeat the process two days before the experiment.
4. Gait Procedure
- Place a home-cage at the end of the walkway before the start of each run to serve as a positive cue for the rat to traverse the walkway.
- Turn off the room lights, power on the camera, and start recording several seconds before the rat is placed on the platform.
NOTE: Be certain to use a memory card that is officially recommended by the camera manufacturer. An unlisted memory card may still work but is not guaranteed to capture at the purported frame rate. - With the enclosure installed, place the rat at the end of the walkway opposite the home-cage and allow it to walk across the walkway in an unforced manner.
- Stop recording once the animal reaches the terminus of the walkway.
- Clean the walkway using 70% ethanol and a nonabrasive towel in between runs and after an animal urinates or defecates, then allow ethanol to evaporate before introducing another animal.
- Run the rats through the walkway a total of 7 times during each observation period, taking the first three runs that score as passing for analysis.
- Score a run as passing if the animal makes four or more consecutive steps in the direction of the home-cage without interruption due to grooming, pausing, or errant movements.
NOTE: It is good practice to record the mass of the animals before each round of measures. For our study, WT (n = 7) and DKO (n = 8) weighed 200.3 ± 21.67 g and 296.6 ± 3.85 g, respectively (p = 0.004, Unpaired t test with Welch's correction). We do not see an issue with rats of any age or size.
5. Video preprocessing
NOTE: The videos captured by the high-speed camera are rendered in mp4 format at 120 fps and a resolution of 1080p. To ease the burden on the analytical software downstream, first trim unnecessary footage and strip the audio from each video using LosslessCut software (version 3.23.7, https://github.com/mifi/lossless-cut), then convert the mp4 video stream into a png image sequence using the open-source software FFmpeg (version 4.2, http://ffmpeg.org/). Note: other Lossless formats such as tiff can be utilized in place of png.
- Create a directory for the videos on a PC running Windows 7 or higher, then transfer the videos from the high-speed camera's storage device to the newly created directory. In addition, copy ffmpeg.exe to the same location.
- In LosslessCut, drag the videos to the interface to open. Discard the audio, set the start and end cut points to include only the analytically relevant portion of the video, set the capture frame format to png, and export. Once the video is exported, rename the video file using any naming convention followed by "_trimmed".
- To batch convert the videos to image sequences, open a command prompt, set the working directory to the location of the videos with "cd [path to directory]", and run the following commands:
for %i in (*) do mkdir "%~ni_cropped"
for %i in (*) do mkdir "%~ni_trimmed"
for /f "tokens=1 delims=." %a in ('dir /B *_trimmed.MP4') do ffmpeg -i "%a.MP4" "%a/%a_%04d.png" - After the batch process completes, open each image sequence in ImageJ Fiji23 and crop the sequence to th e region of interest (ROI) encompassing the area of the floor within which the rat is observed.
- To reduce background from the walkway illumination, increase the color balance minimum of the cyan channel to 76.
- Save as image sequence and change the "_trimmed" suffix to "_cropped", saving the files in their respective "_cropped" folder.
6. Gait Processing
NOTE: Gait data is processed and quantified using the freely available software, MouseWalker (http://biooptics.markalab.org/MouseWalker/)14.
- Unpack and install the MouseWalker software onto a PC running a 64-bit windows environment with Microsoft Excel installed.
- After launching MouseWalker.exe, perform an initial scale calibration for each set of runs. Load an image sequence and using landmarks or a ruler captured in the video, measure two points of known distance. Calculate the number of pixels per centimeter in the video frame and enter this value into the parameters section of the settings form along with the video acquisition frame rate.
- Similarly, measure the head, tail, and feet of the rat to determine head length, maximum tail width and area, minimum and maximum foot area, and other features necessary to complete the tracking parameters section of the MouseWalker settings form. See http://biooptics.markalab.org/MouseWalker/ for the user manual and other documentation.
- To obtain the body area values, open the same image sequence in ImageJ, draw a selection outlining the rat, and perform a region of interest (ROI) pixel count.
- Parameters and settings used for this publication (Figure S6).
NOTE: Parameters are provided for illustration and are dependent on the scale of the video, acquisition hardware, and conditions. Software calibration and adjustment is required each time the camera or equipment is re-positioned. Capturing a measuring device within the acquisition improves accuracy and facilitates calibration. - Following calibration, load each image sequence. Selecting auto will start the autonomous assignment of footprints.
- Scroll through each frame of the sequence, manually correcting miss-assigned footprints. Save once this step is complete.
- Lastly, select evaluate to process the footprint position and pressure data. A series of graphs, images, and a spreadsheet with quantitative gait metrics will export into a results folder.
7. Data Analysis
- Use the spreadsheet exported at the end of each evaluation that contains quantitative gait data for each run. Concatenate data from each run and average per rat. Plot the mean data and test for significance using GraphPad Prism version 7.0a.
Representative Results
Rat Colony Maintenance
The generation and characterization of Pink1 and Parkin single KO rats has been described previously22. The Pink1 and Parkin single KO rats were obtained from SAGE Labs (and now available from Envigo). DKO rats were generated by crossing Pink1-/- rats with Parkin-/- rats to obtain Pink1+/-/Parkin+/- rats, which were interbred to obtain Pink1-/-/Parkin-/- rats (will be available from Envigo). To confirm the deletion of 26 bp in Park6 (gene encoding Pink1), genotyping was performed using 5'-CCCTGGCTGACTATCCTGAC-3' forward and 5'-CCACCACCCACTACCACTTACT-3' reverse primers. Deletion of 5 bp in Park2 (gene encoding Parkin) was tested after DNA was amplified using a forward 5'-GGTGTCTTGGCTCAGTGTGA-3' and reverse 5'-GCCACCCAGAATAGCATCTC-3'. Amplified Polymerase Chain Reaction (PCR) samples were sent to ACGT Inc (Wheeling, IL) for sequencing (Figure S7). All rats were kept on the Long Evans Hooded (LEH) background. DKO rats were viable and fertile; however, there was a high rate of mortality among DKO dams at the time of parturition (approximately 30%). Only male rats were utilized in these experiments. Rats were kept in a temperature-controlled environment with a 12-hour light/dark cycle and free access to rat chow and water.
Outcomes
To serve as an example of the utility of the FTIR gait analysis system for rats, adapted from14, we performed gait analysis on male WT and Pink1/Parkin DKO rats at 2 months of age to determine if the use of kinematic gait analysis could uncover subtle motor impairments not observed with human visual perception prior to the appearance of gross motor problems starting at 4 months of age.
Similar to previous gait studies in mice14, the adapted FTIR system was able to display the footprint pattern created by the walking rat as well as the path created by the body center (Figure 1A). Despite the increased weight of the DKO rats as compared to WT (Figure 1B), the foot pressure applied to the walking surface (visualized as heat maps) as determined by the intensities of the FTIR signal, was unaltered (Figure 1C). Upon assessment of several gait parameters as a function of walking speed (Figure 1D-H), we observed that the walking speed and step lengths were similar between WT and DKO rats (Figure 1D). However, the variation between WT and DKO rats became apparent in stance phase and swing duration at slower walking speeds (Figure 1E, F). The fraction of the step cycle where the leg is in the stance phase (stance duration / period) is the duty factor, and this parameter highlights more time spent in the swing phase than in the stance phase as the duty factor decreases, typical of running (Figure 1G). Again, differences are highlighted at lower speeds. Further, while swing speeds increase with increased speed in WT animals, the correlation is blunted in DKO rats (Figure 1H).
The FTIR gait analysis also allowed for plots of stance phase traces of each leg relative to the body in freely walking rats (Figure 2A, B). The stance traces are normalized to body length and are defined as the position of the foot relative to the body center from paw touchdown (anterior extreme position, AEP) to the end of the stance phase (posterior extreme position, PEP). Upon comparing the paw positioning, we observed significant changes in AEP (left hindlimb) and PEP (right hindlimb) suggesting the left hindlimb is closer to the body during paw touchdown (AEP), while the right hindlimb is further away from the body during paw takeoff (PEP) in DKO as compared to WT rats (Figure 2C).
Several additional parameters were significantly changed in the DKO rats as compared to WT. In particular, changes in hindlimb swing patterns were uncovered. The swing speed of both the left and right hindlimbs was increased in DKO rats as compared to WT rats (Figure 3A), while the swing duration of both left and right hindlimbs was decreased (Figure 3B). Of note, step length was unaltered (Figure 4).
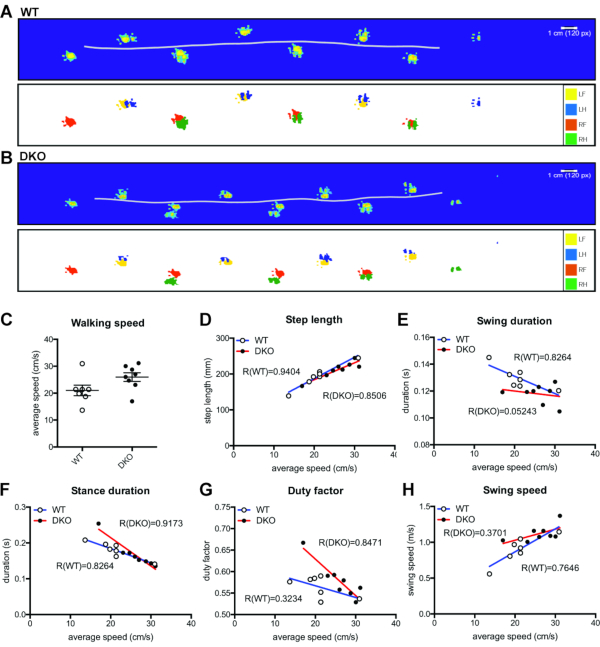
Figure 1. Footprint pattern and step parameter analysis. Representative footprint patterns for (A) WT and (B) DKO rats showing (top panel) footprint heat map representing pixel intensity and horizontal line representing the body path as well as (bottom panel) individual feet labeled with different colors: left fore (LF, yellow), left hind (LH, blue), right fore (RF, orange), and right hind (RH, green). (C) Average walking speed for each WT (n = 7) and DKO (n = 8) rat. Mean with SEM. Not significant. (D-H) Step parameters as a function of speed in WT (n = 7) and DKO (n = 8) rats. Linear regression lines and R square values included. (D) Step length increases with speed in WT and DKO rats. (E) Swing duration is inversely proportional to speed in WT rats, but not in DKO rats (R not significant). (F) Stance duration decreases with speed in WT and DKO rats. (G) Duty factor is inversely proportional to speed in DKO rats, but not WT rats (R not significant). (H) Swing speed increases linearly with speed in WT rats, but not DKO rats (R not significant). Please click here to view a larger version of this figure.
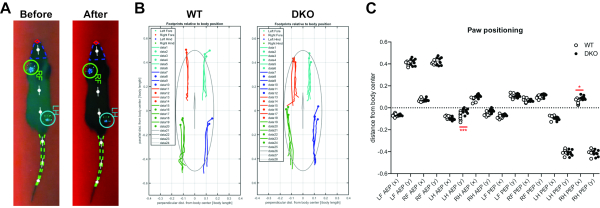
Figure 2. Stance traces and paw positioning analysis. (A) Representative walking track analysis for a WT rat (before and after background correction) visualized with nose (red solid), head contour (blue dashed), tail contour (green dashed), body center (white dashed), and footprints (circles: green, RF and light blue, LH). (B) Representative plots of stance traces for freely walking WT and DKO rats. (C) Paw positioning in WT (n = 7) and DKO (n = 8) rats are shown. AEP, anterior extreme position; PEP, posterior extreme position; L, left; R, right; F, forepaw; H, hindpaw. Mean with SEM. Significant compared to WT (p < 0.05*, 0.001***) using two-way ANOVA and Sidak's multiple comparison test. Please click here to view a larger version of this figure.
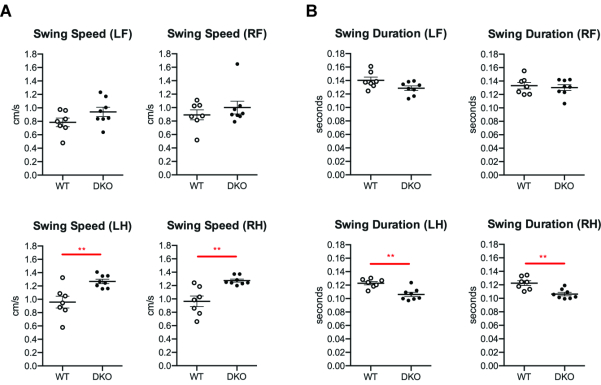
Figure 3. Hindpaw swing parameters altered in DKO rats. Forepaw and hindpaw measurements of the (A) speed at which paws are moving and the (B) time paws are airborne in WT (n = 7) and DKO (n = 8) rats. L, left; R, right; F, forepaw; H, hindpaw. Mean with SEM. Significant compared to WT (p < 0.01**) using Student's unpaired two-tailed t-test with Welch's correction. Please click here to view a larger version of this figure.
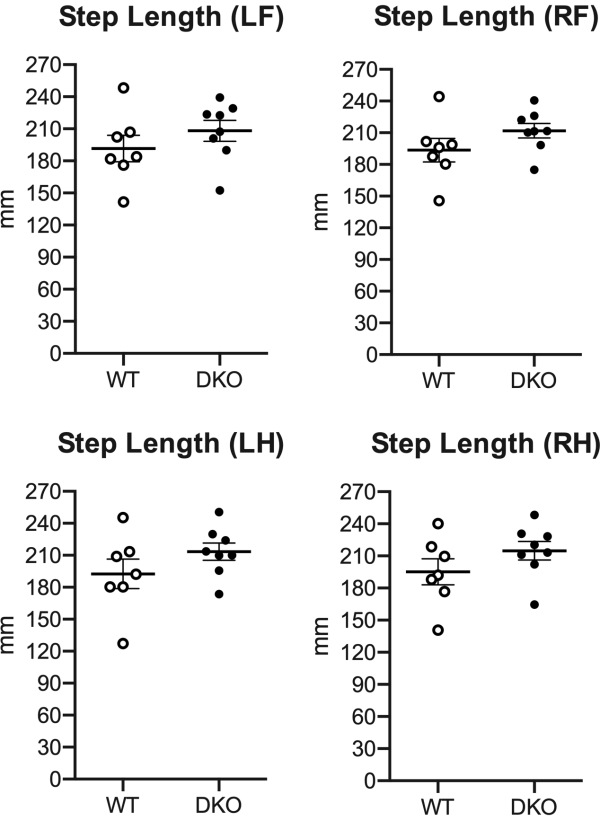
Figure 4. Step length unaltered in DKO rats. Forepaw and hindpaw measurements of the step length in WT (n = 7) and DKO (n = 8) rats. L, left; R, right; F, forepaw; H, hindpaw. Mean with SEM. Significant compared to WT (not significant) using Student's unpaired two-tailed t-test with Welch's correction. Please click here to view a larger version of this figure.
Supplemental Figure. Please click here to download this file.
Supplemental Table. Please click here to download this table.
Discussion
Gait disturbances, including decreased arm swing, slower walking speed, and shorter steps, are a defining feature of PD, and occur early during disease course1,5. Several methods have been developed over the years to observe and record footfalls for gait analysis in rodent models of PD, with manual techniques for quantifying footfall position leading to automated approaches that are more sensitive and capable of capturing dynamic parameters. Some static approaches involve "inking" rodent's paws with an indicator to be tracked onto media placed on a walkway24,25,26. The residual footfalls are later quantified by hand. These methods are often used in conjunction with videotaping for manual kinematic scoring. Autonomous methods of capturing and quantifying gait patterns were introduced more recently15,16, and are available commercially. Autonomous gait quantification adds temporal attributes to otherwise static footprints, allowing investigators to look for abnormalities attributed to velocity and time in addition to distance and angle27. Pressure is also quantifiable when coupled with the FTIR method.
Gait pattern abnormalities have also been reported in toxin-induced rat models of PD. In particular, gait changes have been reported in the 6-OHDA lesion model using a commercial gait analysis platform28,29,30. The most prominent alteration in this model has been declines in walking speed and cadence. Further, the commercial gait analysis platform has been used to assess a number of dynamic and static gait parameters of PD models, such as the impact of unilateral 6-OHDA-induced lesions and how transplantation of dopaminergic neurons rescues the gait alterations31. Additionally, a study has analyzed the ratio of parameters from 6-OHDA lesions in injured versus uninjured sides of the brain, while correcting for speed in related parameters such as hindlimb step cycle, hindlimb print area, and step sequence, all of which are significantly altered when comparing different types of lesions to saline controls32. However, it is important to note that the pathogenetic background of toxin-induced models is of a toxic nature rather than a neurodegenerative process as in human PD6, and as such gait assessment following lesion induction might mimic advanced PD when neurons are lost but make studies into early motor changes more difficult.
Gait geometry has been measured in Pink1 KO, Parkin KO, and DJ-1 KO rats22, models of hereditary PD, that exhibit a progressive nigral dopaminergic neuron loss with aging. Gait was measured using the commercial NeuroCube apparatus, where the rats are allowed to walk in a circle, looking at geometry and dynamic features. Pink1 and DJ-1 KO rats showed shorter duration in stride, swing, and stance when compared to WT at 4 and 8 months.
Because commercial gait analysis systems are costly and come with proprietary analysis pipelines, we sought an open alternative for our study of hereditary rat models of PD. The MouseWalker system14, which comes with build instructions and open-access software, captures all of the parameters of commercial equipment designed for small rodents. Since the platform was too small to consistently achieve passable results with adult rats, i.e. four uninterrupted steps during locomotion at walking speed, we re-scaled the hardware to accommodate rats. In addition, we utilized a domestic action camera in place of a commercial high-speed video solution.
Lower frame density was a potential pitfall of using an action camera in place of a high-speed camera. However, the quality threshold in domestic videography is rapidly rising and is capable of recording at 120 fps in high resolution. Furthermore, lens distortion can be corrected during recording by camera software producing a consistently linear field of view (FOV).
We were initially concerned about the pressure dynamics of using a similar thickness of acrylic for the walkway with a wider base and heavier animals, and the ability of the software to process video of larger animals in a wider FOV. We speculate that the range of mass between mice and rats falls within the range of sensitivity of the acrylic FTIR platform allowing us to measure rats during their entire life cycle. Furthermore, it is possible that potential pixel dilution may be offset by the higher surface area of the rats' pawprints relative to the area captured in the FOV, if there is any significant difference at all. With proper calibration, as documented here, the freely available software14 was able to process rat gait video as described.
With this protocol, we were able to demonstrate that the FTIR mouse gait system14 modified here for rats can detect alterations in gait prior to the visual observation of hindlimb dragging in male DKO rats. Of note, the overt visually apparent motor impairment (hindlimb dragging) observed in DKO rats has been previously reported in male Pink1 single KO rats22. While male DKO rats exhibit visually observable hindlimb dragging starting at 4-6 months of age, the gait analysis uncovered locomotion changes at 2 months of age. In particular, changes in hindlimb gait parameters were found. DKO rats exhibit increased hindlimb (both left and right) swing speed and an associated decrease in hindlimb (both left and right) swing duration. Further, the DKO rats place their left hindpaw closer to their body during paw touchdown, while the right hindpaw is further away from their body during paw takeoff. While we did not uncover changes in stride or stance duration in DKO rats at 2 months, the swing duration was shorter in DKO rats as is similarly reported in Pink1 and DJ-1 KO rats22. Overall, these changes suggest the hindlimb gait parameters are altered prior to the development of hindlimb dragging in male DKO rats. Future longitudinal gait studies tracking the development of locomotion alterations will help pinpoint the age at which gait changes become significant.
In this study, we showed that a FTIR mouse gait system14 modified here for the study of rats can be used to distinguish changes in gait parameters in 2-month-old male DKO rats, a model for hereditary PD, as compared to age-matched WT rats. Previous studies in PD patients revealed reduced stride length and lower average swing time, as well as increased stride time variability and swing time variability4. Thus, our findings of changes in swing time in DKO rats, and previous reports of swing time alterations in Pink1 KO and DJ-1 KO rats22, appear relevant to PD progression.
Divulgations
The authors have nothing to disclose.
Acknowledgements
KS and HF thank the Michael J Fox Foundation for Parkinson's Research for support of their work on Parkinson's disease.
Materials
| Aluminum | |||
| 1.5” Aluminum Angle (1/8” – 6063) | Dimensions: 8' Qty: 8 |
||
| 1” Aluminum Square Tube (1/16” – 6063) | Dimensions: 8' Qty: 4 |
||
| 32 Gauge Aluminum Sheet | Dimensions: 10' Qty: 1 |
||
| 1” Aluminum Tube (1/8” – 6063) | Dimensions: 8' Qty: 1 |
||
| Acrylic | |||
| 7/32” Clear Acrylic Sheet | Dimensions: 4'x8' Qty: 2 |
||
| 1/8” White Acrylic Sheet 55% (2447) | Dimensions: 4'x8' Qty: 1 |
||
| Mirror | |||
| 7/32” Glass Mirror | Dimensions: 60"x12" Qty: 1 |
||
| LED | |||
| 5050 LED Tape Light (Green) | Dimensions: 16.4' Qty: 1 |
||
| 5050 LED Tape Light (Red) | Dimensions: 16.4' Qty: 1 |
||
| Camera | |||
| GoPro Hero 6 Black | Qty: 1 | ||
| Tripod | Dimensions: 57" Qty: 1 |
References
- Behari, M., et al. Parkinson’s disease. Annals of Indian Academy of Neurology. 14, 2-6 (2011).
- Kalia, L. V., Lang, A. E. Parkinson’s disease. Lancet. 386 (9996), 896-912 (2015).
- Fahn, S. Description of Parkinson’s disease as a clinical syndrome. Annals of the New York Academy of Sciences. 991, 1-14 (2003).
- Frenkel-Toledo, S., et al. Effect of gait speed on gait rhythmicity in Parkinson’s disease: variability of stride time and swing time respond differently. Journal of NeuroEngineering and Rehabilitation. 2, 23 (2005).
- Shulman, J. M., De Jager, P. L., Feany, M. B. Parkinson’s disease: genetics and pathogenesis. Annual Review of Pathology. 6, 193-222 (2011).
- Klemann, C., Martens, G. J. M., Poelmans, G., Visser, J. E. Validity of the MPTP-Treated Mouse as a Model for Parkinson’s Disease. Molecular Neurobiology. 53 (3), 1625-1636 (2016).
- Ungerstedt, U. Striatal dopamine release after amphetamine or nerve degeneration revealed by rotational behaviour. Acta Physiologica Scandinavian Supplementum. 367, 49-68 (1971).
- Beal, M. F. Experimental models of Parkinson’s disease. Nature Reviews Neuroscience. 2 (5), 325-334 (2001).
- Betarbet, R., et al. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nature Neuroscience. 3 (12), 1301-1306 (2000).
- Cannon, J. R., et al. A highly reproducible rotenone model of Parkinson’s disease. Neurobiology of Disease. 34 (2), 279-290 (2009).
- Quary, S., et al. Major strain differences in response to chronic systemic administration of the mitochondrial toxin 3-nitropropionic acid in rats: implications for neuroprotection studies. Neurosciences. 97 (3), 521-530 (2000).
- Cicchetti, F., Drouin-Ouellet, J., Gross, R. E. Environmental toxins and Parkinson’s disease: what have we learned from pesticide-induced animal models. Trends in Pharmacological Sciences. 30 (9), 475-483 (2009).
- Greenamyre, J. T., Cannon, J. R., Drolet, R., Mastroberardino, P. G. Lessons from the rotenone model of Parkinson’s disease. Trends in Pharmacological Sciences. 31 (4), 142-143 (2010).
- Mendes, C. S., et al. Quantification of gait parameters in freely walking rodents. BMC Biology. 13, 50 (2015).
- Hamers, F. P., Lankhorst, A. J., van Laar, T. J., Veldhuis, W. B., Gispen, W. H. Automated quantitative gait analysis during overground locomotion in the rat: its application to spinal cord contusion and transection injuries. Journal of Neurotrauma. 18 (2), 187-201 (2001).
- Hamers, F. P., Koopmans, G. C., Joosten, E. A. CatWalk-assisted gait analysis in the assessment of spinal cord injury. Journal of Neurotrauma. 23 (3-4), 537-548 (2006).
- Pickrell, A. M., Youle, R. J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron. 85 (2), 257-273 (2015).
- Dawson, T. M., Ko, H. S., Dawson, V. L. Genetic animal models of Parkinson’s disease. Neuron. 66 (5), 646-661 (2010).
- Gispert, S., et al. Parkinson phenotype in aged PINK1-deficient mice is accompanied by progressive mitochondrial dysfunction in absence of neurodegeneration. PLoS One. 4 (6), 5777 (2009).
- Goldberg, M. S., et al. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. Journal of Biological Chemistry. 278 (44), 43628-43635 (2003).
- Kitada, T., Tong, Y., Gautier, C. A., Shen, J. Absence of nigral degeneration in aged parkin/DJ-1/PINK1 triple knockout mice. Journal of Neurochemistry. 111 (3), 696-702 (2009).
- Dave, K. D., et al. Phenotypic characterization of recessive gene knockout rat models of Parkinson’s disease. Neurobiology of Disease. 70, 190-203 (2014).
- Schindelin, J., et al. Fiji: an open-source platform for biological-image analysis. Nature Methods. 9 (7), 676-682 (2012).
- Hruska, R. E., Kennedy, S., Silbergeld, E. K. Quantitative aspects of normal locomotion in rats. Life Sciences. 25 (2), 171-179 (1979).
- de Medinaceli, L., Freed, W. J., Wyatt, R. J. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Experimental Neurology. 77 (3), 634-643 (1982).
- Kunkel-Bagden, E., Dai, H. N., Bregman, B. S. Methods to assess the development and recovery of locomotor function after spinal cord injury in rats. Experimental Neurology. 119 (2), 153-164 (1993).
- Jacobs, B. Y., Kloefkorn, H. E., Allen, K. D. Gait Analysis Methods for Rodent Models of Osteoarthritis. Current Pain and Headache Reports. 18 (10), 456 (2014).
- Boix, J., von Hieber, D., Connor, B. Gait Analysis for Early Detection of Motor Symptoms in the 6-OHDA Rat Model of Parkinson’s Disease. Frontiers in Behavioral Neuroscience. 12, 39 (2018).
- Zhou, M., et al. Gait analysis in three different 6-hydroxydopamine rat models of Parkinson’s disease. Neuroscience Letters. 584, 184-189 (2015).
- Vandeputte, C., et al. Automated quantitative gait analysis in animal models of movement disorders. BMC Neuroscience. 11, 92 (2010).
- Chuang, C. S., et al. Quantitative evaluation of motor function before and after engraftment of dopaminergic neurons in a rat model of Parkinson’s disease. Journal of Biomedical Science. 17, 9 (2010).
- Baldwin, H. A., Koivula, P. P., Necarsulmer, J. C., Whitaker, K. W., Harvey, B. K. Step Sequence Is a Critical Gait Parameter of Unilateral 6-OHDA Parkinson’s Rat Models. Cell Transplantation. 26 (4), 659-667 (2017).

