The Sleep Nullifying Apparatus: A Highly Efficient Method of Sleep Depriving Drosophila
Summary
Sleep deprivation is a powerful tool to investigate sleep function and regulation. We describe a protocol to sleep deprive Drosophila using the Sleep Nullifying Apparatus, and to determine the extent of rebound sleep induced by deprivation.
Abstract
Sleep homeostasis, the increase in sleep observed following sleep loss, is one of the defining criteria used to identify sleep throughout the animal kingdom. As a consequence, sleep deprivation and sleep restriction are powerful tools that are commonly used to provide insight into sleep function. Nonetheless, sleep deprivation experiments are inherently problematic in that the deprivation stimulus itself may be the cause of observed changes in physiology and behavior. Accordingly, successful sleep deprivation techniques should keep animals awake and, ideally, result in a robust sleep rebound without also inducing a large number of unintended consequences. Here, we describe a sleep deprivation technique for Drosophila melanogaster. The Sleep Nullifying Apparatus (SNAP) administers a stimulus every 10s to induce negative geotaxis. Although the stimulus is predictable, the SNAP effectively prevents >95% of nighttime sleep even in flies with high sleep drive. Importantly, the subsequent homeostatic response is very similar to that achieved using hand-deprivation. The timing and spacing of the stimuli can be modified to minimize sleep loss and thus examine non-specific effects of the stimulus on physiology and behavior. The SNAP can also be used for sleep restriction and to assess arousal thresholds. The SNAP is a powerful sleep disruption technique that can be used to better understand sleep function.
Introduction
Sleep is near universal in animals, yet its function remains unclear. Sleep homeostasis, the compensatory increase in sleep following sleep deprivation, is a defining property of sleep, that has been used to characterize sleep states in a number of animals1,2,3,4,5.
Sleep in the fly has many similarities with human sleep, including a robust homeostatic response to sleep loss4,5. Numerous studies of sleep in the fly have used sleep deprivation both to infer sleep function by examining the adverse consequences that accrue from extended waking, and to understand sleep regulation by determining the neurobiological mechanisms controlling the homeostatic regulation of sleep. Thus sleep deprived flies were shown to exhibit impairments in learning and memory6,7,8,9,10,11,12, structural plasticity13,14,15, visual attention16, recovery from neuronal injury17,18, mating and aggressive behaviors19,20, cell proliferation21, and responses to oxidative stress22,23 to name a few. Further, investigations into the neurobiological mechanisms controlling rebound sleep have yielded critical insights into the neuronal machinery that constitutes the sleep homeostat8,9,23,24,25,26,27,28,29. Finally, in addition to revealing fundamental insights into sleep function in healthy animals, sleep deprivation studies have also informed insights into sleep function in diseased states30,31.
While sleep deprivation is undeniably a powerful tool, with any sleep deprivation experiment, it is important to distinguish phenotypes that result from extended waking, from those induced by the stimulus used to keep the animal awake. Sleep deprivation by hand deprivation or gentle handling, is generally regarded as setting the standard for minimally disruptive sleep deprivation. Here we describe a protocol for sleep depriving flies using the Sleep Nullifying Apparatus (SNAP). The SNAP is a device that delivers a mechanical stimulus to flies every 10s, keeping flies awake by inducing negative geotaxis (Figure 1). The SNAP efficiently deprives flies of >98% of night-time sleep, even in flies with high sleep drive8,32. The SNAP has been calibrated on bang sensitive flies, agitation of flies in the SNAP does not harm flies; sleep deprivation with the SNAP induces a rebound comparable with that obtained by hand deprivation7. The SNAP is thus a robust method to sleep deprive flies while controlling for the effects of the arousing stimulus.
Protocol
1. Experimental preparation
- Collect flies as they eclose into vials, separating male and female flies.
NOTE: Sleep experiments are commonly conducted with female flies. It is important to collect virgin females. Mated females will lay eggs that hatch into larvae complicating the analysis of the data. - House flies of a single sex in groups of <20.
NOTE: Housing flies in a socially enriched environment (groups of >50) modulates sleep drive6,13 potentially confounding measurements of rebound sleep. Further, following social enrichment, sleep will decline over a few days6. Thus, baseline sleep is not stable complicating analysis of rebound sleep. Keeping flies in groups of <20 avoids this potential confound. - Keep flies in vials for 3-5 days in a light and humidity controlled environment.
NOTE: Age and maturity of flies strongly influence sleep. Sleep is high in one day old flies and stabilizes by 3-5 days of age4. Flies are typically maintained on a 12 h light: 12 h dark schedule at 50% humidity.
2. Preparation of tubes for sleep recording
NOTE: Sleep is monitored using locomotor activity monitors. A monitor can hold 32 flies housed individually in 5 mm diameter tubes. Typically, genotypes are analyzed in groups of 16 or 32 flies.
- Prepare an appropriate number of tubes with fly food at one end.
NOTE: Diet and metabolism are known to influence sleep33,34, hence it is particularly important to place flies on the same food on which they were reared. - Seal the end of the tubes with wax.
NOTE: Sleep deprivation and rebound is a five day experiment, and food can dry out if not properly sealed. In properly sealed tubes, food can be maintained for 10 days or more. Thus, it is critical to ensure that the ends of the tubes are sealed well. Flies can also get stuck to wet food, however. Thus, it helps to make tubes 1-2 days before the start of the experiment. - Individually place wake, behaving flies into 65 mm long glass tubes for sleep recording using an aspirator and plug the end of the tubes with a foam stopper.
NOTE: Flies are never re-exposed to CO2 anesthesia when placing flies into tubes for sleep recording. The aspirator is made from rubber tubing with one end covered with cheesecloth and inserted into a 1 mL pipette tip.
3. Recording sleep
- Load flies in tubes into activity monitors to monitor sleep.
NOTE: The SNAP rocks monitors back and forth from -60° to +60° every ~10 s. The monitors are held at -60° for ~5.9s ; it takes ~2.9 s for the tray holding the monitors to move from -60° to +60° and ~1 s to move back from +60° to -60°. The cycle length can be altered as needed by adjusting the voltage supplied to the motor.- Take care to ensure that tubes are placed in activity monitors in the correct orientation. In the correct orientation, the end of the tube with food is at the top of the SNAP to ensure that flies do not get pushed into the food. In addition, the end with food is on the side of the monitor with the sleep recording jack. This allows activity monitors to be oriented correctly in the SNAP for efficient sleep deprivation while simultaneously monitoring activity.
- Place activity monitors in the recording chamber to monitor sleep.
- Monitor sleep for at least two full days to estimate baseline sleep.
NOTE: The day flies are loaded into activity monitors is typically excluded as an adaptation day to allow flies to adapt to being housed in tubes. Baseline sleep is recorded for at least two full days (48hrs) beginning with the morning following the day flies are loaded. - Save locomotor activity counts of flies in 1 min bins from the time of lights on a given day to lights on the previous day using activity recording software (e.g., from 8 AM to 8 AM).
- Estimate sleep from the locomotor activity data with custom macros using 5 min of inactivity as the threshold for a bout of sleep35.
NOTE: A number of sleep metrics are computed from the locomotor activity counts. These include sleep in min/h over 24 h, total sleep time in 24 h, average and maximum daytime and nighttime sleep bout lengths36.
4. Sleep deprivation and recovery
- As flies can be sleep deprived for variable lengths of time (e.g., 12 h, 24 h and 36 h) and recovery sleep can also be evaluated at various intervals (e.g., 6 h, 12 h, 24 h and 48 h), determine the duration of recovery by experimental need. Sleep recovery can be visualized using a sleep gain/loss plot or by examining percent sleep recovered over a predetermined interval (e.g., 6 h).
- If sleep is stable over the two baseline days, on the third day, place activity monitors into the SNAP for overnight sleep deprivation.
NOTE: Flies will exhibit a robust sleep rebound over a range of sleep times8,32,37,38, but sleep has to be stable to reliably evaluate rebound sleep. Sleep is stable when the difference in sleep between baseline days is ± 100 min. - Make sure activity monitors are secured in place with monitor holder pins, monitor cords plugged in, and monitors oriented correctly with the end with food at the back, and plastic barriers in front (Figure 1).
NOTE: The SNAP is designed so the cam rotates once every 10 s (Figure 1). The plastic insert resets the tubes by pushing the tubes back when the apparatus is in the "up" position. Resetting the tubes is important to ensure that all tubes have the full range of motion at the beginning of each cycle. - Unplug activity monitors, and take monitors out of the SNAP immediately upon lights on following overnight sleep deprivation.
NOTE: It is critical that sleep deprivation is terminated, and flies are placed in recovery immediately upon lights on following 12 h of overnight sleep deprivation. Even a 20-30 min delay in placing flies into recovery can interfere with the extent of rebound sleep. - Place flies in a recording chamber where they will be undisturbed for two days (48 h) to monitor recovery sleep.
NOTE: If the recording chamber is being used for other experiments, extra care must be taken to avoid stimulating recovering flies. - Calculate the amount of sleep lost. For each individual fly, calculate the hourly difference between sleep obtained during sleep deprivation and the corresponding hour during baseline; sum the hourly differences to calculate total sleep lost.
- Calculate the amount of sleep recovered. For each individual fly, calculate the hourly difference between sleep obtained during recovery and the corresponding hour during baseline; sum the hourly differences to calculate total sleep gained.
NOTE: Whether a fly is actually sleep deprived is empirical. Thus, the experimenter should examine percent sleep lost. If the fly has not lost a sufficient amount of sleep it can be excluded from the analysis. Although this might be required for other sleep deprivation approaches, it is rarely if ever required for the SNAP. More commonly, sleep may not be stable in a given fly prior to the initiation of sleep deprivation. If sleep is not stable, homeostasis cannot be calculated. We accept a maximum difference of ± 100 min of sleep calculated prior to the initiation of sleep deprivation as candidates for inclusion. On occasion, an individual fly's sleep is distributed unevenly across the 24 h day (e.g., some individuals may obtain 60-70% of their sleep quota during the day and thus only lose a small proportion of their 24 h sleep quota when deprived for 12 h at night). These flies can be evaluated separately. - Calculate the average percentage of sleep recovered (relative to baseline) over 12 h, 24 h and 48 h of the recovery period for each genotype.
- From sleep data, compute the average and maximum daytime sleep bout length on baseline, and recovery days for each genotype.
NOTE: Rebound sleep in flies is characterized by increased sleep amount, and increased sleep depth in the recovery days. Sleep consolidation is used as measure of sleep depth. Arousal thresholds could also be used as a measure of sleep depth.
Representative Results
Canton S (Cs) was used as a wild-type strain. Flies were maintained on a 12 h light: 12 h dark schedule, and sleep deprived for 12 hours overnight. Inspection of the sleep profiles of Cs flies on the baseline day (bs), sleep deprivation day (sd), and two recovery days (rec1 and rec2) (Figure 2A) suggests that flies were effectively sleep deprived in the SNAP, and recovered sleep during the day consistent with observed reports in the literature4,5. The effectiveness of the SNAP in keeping flies awake is also seen in the high activity (300-350 counts/h) exhibited by flies during sleep deprivation (Figure 2B). Indeed, monitoring the activity counts of flies during sleep deprivation can be a useful barometer of the effectiveness of the deprivation protocol and/or an indirect measure of sleep drive. When the sleep deprivation is ineffective, flies are not as active during the period of deprivation. Flies that are under high sleep drive quickly fall asleep after each stimulus and do not traverse the tube as much35. Both the angle of tilt of the apparatus, and the speed of the drop are critical to ensuring that flies are effectively kept awake without harming them. Each lab can optimize the angle and velocity by adjusting the spring (Figure 1B) and/or the size and shape of the cam (Figure 1C and Figure 1D, right).
To quantitatively estimate the effectiveness of sleep deprivation and of recovery, sleep lost during deprivation and then regained in the recovery days was calculated for each individual fly (Figure 2C). Importantly, there was no significant change in baseline sleep between the deprivation day and the baseline day (see 0-12 h in Figure 2C) indicating that sleep is stable in these flies. A large difference in sleep in this 12 hour period (e.g., ± 100 min) would suggest that sleep was not stable. The SNAP effectively deprived flies of >98% of their night-time sleep. Flies recovered ~20% of their sleep in the first 12 h and did not recover additional sleep during the night, as previously reported. However, flies began to recover sleep the following day such that they recovered ~36% of their sleep over 48 h of recovery (Figure 2D). 30 – 40% recovered sleep over 48 h is fairly typical for wild-type flies sleep deprived using the SNAP.
Sleep homeostasis is characterized both by increased sleep duration and by increased sleep depth during the recovery period following deprivation. Daytime sleep consolidation is commonly used as a readout of sleep depth. Sleep consolidation can be assessed as the average sleep bout duration over the entire day (Figure 2E). However, as sleep pressure is dissipated during recovery, the average sleep bout duration will be reduced as the day progresses. Thus, it is frequently helpful to also examine changes in the maximum sleep bout duration which can provide a more sensitive metric (Figure 2F).
| Method of Sleep Deprivation | Total # of papers | % papers / technique | Avg recovery evaluated |
| SNAP | 52 | 37.14% | 33 ± 3 |
| Vortexer/Random Shaking | 49 | 35.00% | 18 ± 3 |
| Hand-Deprivation | 9 | 6.43% | 36 ± 11 |
| Thermogenetic SD | 15 | 10.71% | 36 ± 12 |
| Unspecified | 15 | 10.71% | 29 ± 10 |
Table 1: Survey of different methods of sleep deprivation used in the literature. Only 116 /254 papers used sleep deprivation. The number of papers using each method = "Total # of papers". The fraction of papers using each method = "% papers / technique". The mean length of recovery evaluated for each method = "Avg recovery evaluated". SD – Sleep deprivation. SNAP – Sleep Nullifying Apparatus
| Length of SD | Total studies |
| < 6 h | 12 |
| 6 h | 23 |
| >6 h & < 12 h | 17 |
| 12 h | 69 |
| >12 h & <24 h | 7 |
| 24 h | 19 |
| > 24 h | 9 |
| Chronic SD | 4 |
| Any SD | 160 |
Table 2. Length of sleep deprivation performed in different studies. SD – Sleep deprivation
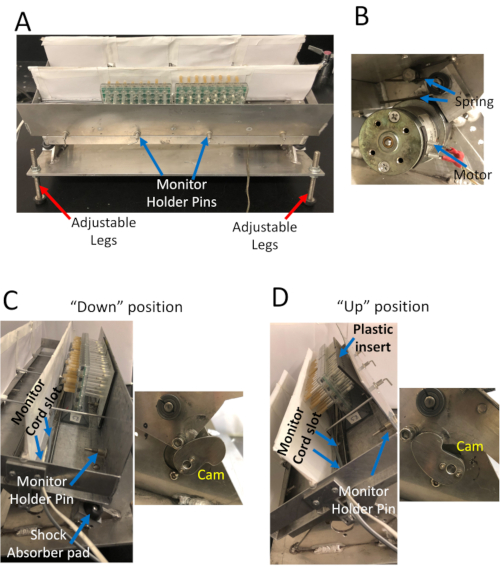
Figure 1. The Sleep Nullifying APparatus (SNAP). A) Front view of the apparatus. The SNAP can accommodate 8 activity monitors in two rows; holder pins restrain the monitors in place. The legs can be adjusted to help position the apparatus at the correct orientation. B) Closeup view of the motor and spring that rock the apparatus back and forth. The motor turns a cam that tilts the apparatus back to the "up" position and compresses the spring. Release of the spring from compression snaps the apparatus back to the "down" position. C) Left – Side view of the apparatus in the "down" position. Holder pins restrain monitors; a monitor cord slot ensures that monitor cords are held in place. Pads help cushion the impact of the apparatus snapping to the 'down' position. Right – Close view up of the cam. D) Left Side view of the apparatus in the "up" position. Right – The counter-clockwise rotation of the cam tilts the apparatus into the 'up' position. Please click here to view a larger version of this figure.
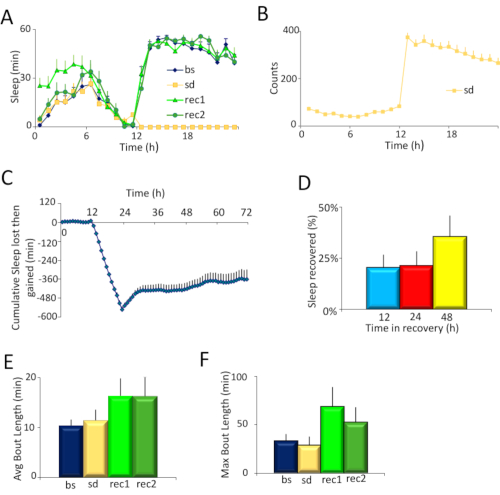
Figure 2. Experimental results. A) Sleep plots of Cs flies for the four days of the experiment: the baseline day (bs), sleep deprivation day (sd), and two days of recovery (rec1 and rec2). B) Average locomotor activity counts of flies on the day of sleep deprivation. Flies were sleep deprived from hours 12-24. C) Time course of sleep deprivation and recovery. Cs flies were sleep deprived from hours 12 – 24, and allowed to recover from hour 24 – 72. The SNAP effectively deprived flies of >98% sleep, which was partially recovered over 48 h (n = 12 flies, Repeated Measures ANOVA for time, F [70,1470]=12.97, p < 10-15). D) Percentage of sleep recovered over 48 h. Flies recovered ~20% of their sleep over 12 h, and ~36% of their sleep over 48 h. E) Sleep consolidation for each day of the experiment as measured by average sleep bout duration during the day. Sleep is more consolidated on the first recovery day compared to baseline (p <0.05, t-test). F) Sleep consolidation for each day of the experiment as measured by maximum sleep bout duration during the day. Sleep is more consolidated on the first recovery day compared to baseline (p <0.05, t-test). Please click here to view a larger version of this figure.
Discussion
Sleep in Drosophila was independently characterized in 2000, by two groups4,5. In these pioneering studies, flies were deprived of sleep by gentle handling (i.e., hand deprivation) and shown to exhibit a robust homeostatic response to overnight sleep deprivation. Importantly, with any sleep deprivation experiment it is crucial to control for potential confounding effects of the method used to keep the animal awake. Hand deprivation studies set the benchmark for studies of fly homeostasis as a minimally disruptive means of sleep depriving flies. The SNAP efficiently deprives flies of sleep of >98% of nighttime sleep, and importantly induces a sleep rebound comparable to that obtained with hand deprivation4,7.
Since the foundational studies defining sleep in flies, a number of methods have been developed to evaluate sleep homeostasis in flies in a high throughput manner7,9,39,40,41. We surveyed ~250 papers on sleep in flies and found ~46% of these published articles reported using sleep deprivation to evaluate sleep regulation or function (Table 1). A number of different methods effectively induced a sleep rebound in flies. Interestingly, of the studies that have evaluated sleep rebound, the protocols used for sleep deprivation and sleep rebound differed. Specifically, both the duration of sleep deprivation (Table 2) and duration for which rebound was evaluated (Table 1) varied substantially, potentially complicating comparisons of results obtained with different protocols. Sleep rebound in flies is known to persist for up to 48hours following sleep deprivation5. Accordingly, we think a thorough description of the effects of a given sleep manipulation on homeostasis are best obtained when homeostatic rebound is evaluated over a 48 h recovery period.
It is important to note that depriving flies of sleep during the day does not consistently increase sleep drive4. Hence, starting a 24 h sleep deprivation protocol at lights-on and continuing until the next day would not additionally enhance recovery sleep compared to a 12 h sleep deprivation protocol beginning at lights-off. In fact, the calculated sleep rebound may be lower since it will include non-homeostatically regulated daytime sleep in addition to nighttime sleep. The observation that daytime sleep deprivation does not induce a homeostatic rebound can however be used to control for potential confounding effects of the method of sleep deprivation. Thus, flies sleep deprived overnight in the SNAP are compared to flies that receive a comparable stimulus in the daytime7.
In addition to being used for total sleep deprivation, by changing the frequency of the stimulus, the SNAP can also be used to chronically restrict and fragment sleep7,42, thus mimicking conditions of chronic sleep loss in humans. Further, by delivering stimuli in steps of increasing frequency, the SNAP can also be used to measure arousal thresholds8. The SNAP is thus a facile way to effectively deprive and restrict sleep of flies, evaluate the homeostatic response, and measure other sleep characteristics.
The SNAP can fit in a standard laboratory fly incubator, but will definitely disturb flies in the incubator that are not part of the experiment. Fortunately, the SNAP can be placed in an isolated location to sleep deprive flies without disturbing other ongoing experiments. Since recovery sleep is fragile, care should be taken to ensure that recovery sleep takes place in a quiet location.
Complementing studies of sleep deprivation, genetic and pharmacological tools have been developed to enhance sleep in flies8,43,44. Thus, the ability to readily modulate sleep bidirectionally will allow fly sleep research to continue to provide deep insights into sleep regulation and function.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by NIH grants 5R01NS051305-14 and 5R01NS076980-08 to PJS.
Materials
| Locomotor activity tubes | |||
| Fisher Tissue Prep Wax | Thermo Fisher | 13404-122 | Wax used for sealing tubes |
| Glass tubes | Wale Apparatus | 244050 | We cut 5mm diameter Pyrex glass tubes into 65mm long tubes to record sleep. Pre-cut tubes can also be purchased. |
| Nutri Fly Bloomington Formulation fly food | Genesee Scientific | 66-113 | Labs might use their own fly food recipe. It is important that sleep be recorded on the same food that flies were reared in. |
| Rotary glass cutting tool | Dremel Multi Pro | 395 | Used to cut 65mm long glass tubes |
| Monitoring Sleep | |||
| DAM System and DAMFileScan software | Trikinetics | Software used to acquire data from DAM monitors and save the acquired data in an appropriate format | |
| Data acquisition computer | Lenovo | Idea Centre AIO3 | A equivalent computer from any manufacturer can substitute |
| Drosophila Activity Monitors | Trikinetics | DAM2 | These monitors are used to record flies' locomotor activity |
| Environment Monitor | Trikinetics | DEnM | Not essential, but an easy way to monitor environmental conditions in the chamber where sleep is recorded |
| Light Controller | Trikinetics | LC4 | A convenient way to control the timing of when the SNAP is turned on and off |
| Power Supply Interface Unit for DAM | Trikinetics | PSIU-9 | Required for data acquisition computers to record Trikinetics locomotor acitvity data |
| RJ11 connector | 7001-64PC | Multicomp | DAM monitors accept RJ11 jacks |
| Splitters | Trikinetics | SPLT5 | Used to connect upto 5 DAM monitors |
| Telephone cable wire | Radioshack | 278-367 | Phone cables to acquire data from DAM monitors |
| Sleep Deprivation | |||
| Power supply | Gw INSTEK | GPS-30300 | Power supply for the SNAP |
| Sleep Nullifying Apparatus | Washington University School of Medicine machine shop |
References
- Nath, R. D., et al. The Jellyfish Cassiopea Exhibits a Sleep-like State. Current Biology. 27 (19), 2984-2990 (2017).
- Vorster, A. P., Krishnan, H. C., Cirelli, C., Lyons, L. C. Characterization of sleep in Aplysia californica. Sleep. 37 (9), 1453-1463 (2014).
- Zhdanova, I. V., Wang, S. Y., Leclair, O. U., Danilova, N. P. Melatonin promotes sleep-like state in zebrafish. Brain Research. 903 (1-2), 263-268 (2001).
- Shaw, P. J., Cirelli, C., Greenspan, R. J., Tononi, G. Correlates of sleep and waking in Drosophila melanogaster. Science. 287 (5459), 1834-1837 (2000).
- Hendricks, J. C., et al. Rest in Drosophila is a sleep-like state. Neuron. 25 (1), 129-138 (2000).
- Ganguly-Fitzgerald, I., Donlea, J., Shaw, P. J. Waking experience affects sleep need in Drosophila. Science. 313 (5794), 1775-1781 (2006).
- Seugnet, L., Suzuki, Y., Vine, L., Gottschalk, L., Shaw, P. J. D1 receptor activation in the mushroom bodies rescues sleep-loss-induced learning impairments in Drosophila. Current Biology. 18 (15), 1110-1117 (2008).
- Donlea, J. M., Thimgan, M. S., Suzuki, Y., Gottschalk, L., Shaw, P. J. Inducing sleep by remote control facilitates memory consolidation in Drosophila. Science. 332 (6037), 1571-1576 (2011).
- Seidner, G., et al. Identification of Neurons with a Privileged Role in Sleep Homeostasis in Drosophila melanogaster. Current Biology. 25 (22), 2928-2938 (2015).
- Li, X., Yu, F., Guo, A. Sleep deprivation specifically impairs short-term olfactory memory in Drosophila. Sleep. 32 (11), 1417-1424 (2009).
- Melnattur, K., et al. A conserved role for sleep in supporting Spatial Learning in Drosophila. Sleep. , 197 (2020).
- Seugnet, L., Suzuki, Y., Donlea, J. M., Gottschalk, L., Shaw, P. J. Sleep deprivation during early-adult development results in long-lasting learning deficits in adult Drosophila. Sleep. 34 (2), 137-146 (2011).
- Donlea, J. M., Ramanan, N., Shaw, P. J. Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science. 324 (5923), 105-108 (2009).
- Bushey, D., Tononi, G., Cirelli, C. Sleep and synaptic homeostasis: structural evidence in Drosophila. Science. 332 (6037), 1576-1581 (2011).
- Huang, S., Piao, C., Beuschel, C. B., Götz, T., Sigrist, S. J. Presynaptic Active Zone Plasticity Encodes Sleep Need in Drosophila. Current Biology. 30 (6), 1077-1091 (2020).
- Kirszenblat, L., et al. Sleep regulates visual selective attention in Drosophila. Journal of Experimental Biology. 221, (2018).
- Singh, P., Donlea, J. M. Bidirectional Regulation of Sleep and Synapse Pruning after Neural Injury. Current Biology. 30 (6), 1063-1076 (2020).
- Stanhope, B. A., Jaggard, J. B., Gratton, M., Brown, E. B., Keene, A. C. Sleep Regulates Glial Plasticity and Expression of the Engulfment Receptor Draper Following Neural Injury. Current Biology. 30 (6), 1092-1101 (2020).
- Kayser, M. S., Yue, Z., Sehgal, A. A critical period of sleep for development of courtship circuitry and behavior in Drosophila. Science. 344 (6181), 269-274 (2014).
- Kayser, M. S., Mainwaring, B., Yue, Z., Sehgal, A. Sleep deprivation suppresses aggression in Drosophila. Elife. 4, 07643 (2015).
- Szuperak, M., et al. A sleep state in Drosophila larvae required for neural stem cell proliferation. Elife. 7, (2018).
- Vaccaro, A., et al. Sleep Loss Can Cause Death through Accumulation of Reactive Oxygen Species in the Gut. Cell. 181 (6), 1307-1328 (2020).
- Kempf, A., Song, S. M., Talbot, C. B., Miesenböck, G. A potassium channel β-subunit couples mitochondrial electron transport to sleep. Nature. 568 (7751), 230-234 (2019).
- Donlea, J. M., Pimentel, D., Miesenböck, G. Neuronal machinery of sleep homeostasis in Drosophila. Neuron. 81 (4), 860-872 (2014).
- Liu, S., Liu, Q., Tabuchi, M., Wu, M. N. Sleep Drive Is Encoded by Neural Plastic Changes in a Dedicated Circuit. Cell. 165 (6), 1347-1360 (2016).
- Pimentel, D., et al. Operation of a homeostatic sleep switch. Nature. 536 (7616), 333-337 (2016).
- Sitaraman, D., et al. Propagation of Homeostatic Sleep Signals by Segregated Synaptic Microcircuits of the Drosophila Mushroom Body. Current Biology. 25 (22), 2915-2927 (2015).
- Foltenyi, K., Greenspan, R. J., Newport, J. W. Activation of EGFR and ERK by rhomboid signaling regulates the consolidation and maintenance of sleep in Drosophila. Nature Neuroscience. 10 (9), 1160-1167 (2007).
- Seugnet, L., et al. Notch signaling modulates sleep homeostasis and learning after sleep deprivation in Drosophila. Current Biology. 21 (10), 835-840 (2011).
- Seugnet, L., Galvin, J. E., Suzuki, Y., Gottschalk, L., Shaw, P. J. Persistent short-term memory defects following sleep deprivation in a Drosophila model of Parkinson disease. Sleep. 32 (8), 984-992 (2009).
- Tabuchi, M., et al. Sleep interacts with aβ to modulate intrinsic neuronal excitability. Current Biology. 25 (6), 702-712 (2015).
- Melnattur, K., Zhang, B., Shaw, P. J. Disrupting flight increases sleep and identifies a novel sleep-promoting pathway in Drosophila. Science Advances. 6 (19), 2166 (2020).
- Thimgan, M. S., Suzuki, Y., Seugnet, L., Gottschalk, L., Shaw, P. J. The perilipin homologue, lipid storage droplet 2, regulates sleep homeostasis and prevents learning impairments following sleep loss. PLOS Biology. 8 (8), (2010).
- Keene, A. C., et al. Clock and cycle limit starvation-induced sleep loss in Drosophila. Current Biology. 20 (13), 1209-1215 (2010).
- Shaw, P. J., Tononi, G., Greenspan, R. J., Robinson, D. F. Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature. 417 (6886), 287-291 (2002).
- Andretic, R., Shaw, P. J. Essentials of sleep recordings in Drosophila: moving beyond sleep time. Methods Enzymol. 393, 759-772 (2005).
- Seugnet, L., et al. Identifying sleep regulatory genes using a Drosophila model of insomnia. Journal of Neuroscience. 29 (22), 7148-7157 (2009).
- Bushey, D., Huber, R., Tononi, G., Cirelli, C. Drosophila Hyperkinetic mutants have reduced sleep and impaired memory. Journal of Neuroscience. 27 (20), 5384-5393 (2007).
- Geissmann, Q., et al. Ethoscopes: An open platform for high-throughput ethomics. PLOS Biology. 15 (10), 2003026 (2017).
- Faville, R., Kottler, B., Goodhill, G. J., Shaw, P. J., van Swinderen, B. How deeply does your mutant sleep? Probing arousal to better understand sleep defects in Drosophila. Scientific Reports. 5, 8454 (2015).
- Huber, R., et al. Sleep homeostasis in Drosophila melanogaster. Sleep. 27 (4), 628-639 (2004).
- Klose, M., Shaw, P. Sleep-drive reprograms clock neuronal identity through CREB-binding protein induced PDFR expression. bioRxiv. , (2019).
- Dissel, S., et al. Sleep restores behavioral plasticity to Drosophila mutants. Current Biology. 25 (10), 1270-1281 (2015).
- Gerstner, J. R., Vanderheyden, W. M., Shaw, P. J., Landry, C. F., Yin, J. C. Fatty-acid binding proteins modulate sleep and enhance long-term memory consolidation in Drosophila. PLoS One. 6 (1), 15890 (2011).

