Simplified Whole Body Plethysmography to Characterize Lung Function During Respiratory Melioidosis
Summary
This protocol presents the construction and use of a Simplified Whole Body Plethysmography apparatus to monitor bacterial respiratory disease progression non-invasively.
Abstract
Surrogate animal models of disease are subject to the 3Rs of Responsible Research. There is a frequent revisiting of refinements to animal models to ensure that both animal welfare and scientific insights advance with the availability of new technologies. This article demonstrates the use of Simplified Whole Body Plethysmography (sWBP) to non-invasively study respiratory failure in a model of lethal respiratory melioidosis. sWBP has the sensitivity to detect breathing in mice through the entirety of the course of the disease, allowing for the moribund-associated symptoms (bradypnea and hypopnea) to be measured and potentially used to develop humane endpoint criteria.
Some of the benefits of sWBP in the context of respiratory disease are that host breath monitoring comes the closest of any physiologic measurement at assessing dysfunction of the primary infected tissue, namely, the lung. In addition to biological significance, the use of sWBP is rapid and non-invasive, minimizing stress in research animals. This work demonstrates the use of in-house sWBP apparatus to monitor disease throughout the course of respiratory failure in the murine model of respiratory melioidosis.
Introduction
Respiratory bacterial pathogens are often associated with an inflammatory response in the lung leading to lung pathology1,2. In the clinical setting, diagnosis of pneumonia typically includes culture techniques from sputum, blood-oxygen saturation analysis, and chest X-ray. These techniques can be translated for small animal infection models, but only oxygen saturation analysis represents a rapid, real-time analysis in mice for disease severity. The blood oxygen saturation (SpO2) was previously investigated as a method to track disease progression in respiratory disease studies; however, moribund mice have unexpectedly high SpO2 readings both in a Pseudomonas aeruginosa model3, which are not the predictive or moribund disease, likely because mice can modulate their physiological activity. To this end, diagnostic levels of SpO2 were not found for bacterial respiratory disease in mice thus far.
Therefore, this work investigated the use of other clinically relevant methods of detecting the effects of lung disease on lung function as a rapid physiologic measurement. Simplified Whole Body Plethysmography (sWBP) offers the opportunity to investigate breath rate and depth as a rapid, non-invasive biometric analysis. Previous studies have demonstrated how to assemble WBP apparatus in a laboratory4; however, several of the components shown in such studies are not currently commercially available. Further, traditional WBP requires complex data collection and data processing based on humidity and temperature5,6. Hence, it was decided to develop a simplified WBP apparatus that is calibrated daily to room temperature/humidity and assess whether or not the temperature/humidity contribution of the subject itself has any effect on the measured breath volume. Thus, a modified sWBP apparatus has been created which sources the currently available materials. Further, it has been investigated whether this lab-sourced apparatus can detect changes in breathing associated with disease progression during the model of lethal respiratory melioidosis in mice.
The sWBP apparatus constructed for this work used commercially available equipment and software to process analog pressure sensor data into a digital readout. The pressure sensor was fitted to an airtight glass jar with bulkhead connectors. The benefit of a glass jar is the structural rigidity of the material, which will resist changes to the internal pressure of the jar, affecting measurements of volume changes during the monitoring of breathing. The sampling chamber has been engineered to have two ports on the two flat surfaces of the square jar, one to access the chamber by a Luer connector for calibration and the other to house the pressure sensor. The pressure sensor selected has a highly sensitive gauge pressure transducer with a range for small changes in pressure (25 mbar range).
This protocol is demonstrated using a murine model of respiratory melioidosis. Burkholderia pseudomallei (Bp) is the bacterial agent of melioidosis – a disease associated with tropical regions of the world7. Bp is found in the environment, specifically in wet environments of standing water and moist soil, from which it typically causes subcutaneous infections of cuts/scratches of susceptible hosts. However, Bp is also infectious when inhaled and is a potential threat for use in bioterrorism by aerosol dispersal. While fully virulent Bp requires handling in a BSL-3 laboratory, an acapsular mutant strain was previously engineered, which can be safely handled at BSL-2 and excluded from the select agent criteria8. Further, an intubation-mediated intratracheal (IMIT) infection model of respiratory melioidosis has been developed to study respiratory disease progression of Bp5,9. We have used this infection model to characterize the change in breathing that occurs during disease progression through the moribund endpoint.
Protocol
The procedures described here were reviewed and approved by the University of Louisville Institutional Biosafety Committee (protocol # 14-038) and Institutional Animal Care and Use Committee (protocol # 19567).
1. Assembly of the sampling chamber
- Create two holes using a 3/4" diamond drill bit on a drill press on the flat surfaces of a 600 mL square glass wide mouth Mason canning jar with 95 mm gasket and airtight lids, with bail and trigger clamp lids (Figure 1).
NOTE: A sampling chamber is not commercially available and must be constructed. - Assemble a brass bulkhead (1/4" NPT internal thread, 3/4-16 UNF external thread) through both the holes in the Mason jar using rubber washers (3/4" inner diameter, 1" outer diameter) on both faces of contact between the bulkhead and glass to ensure an airtight seal.
- Use one bulkhead assembly for a pressure sensor while attaching the other bulkhead by a Luer-connected syringe for calibration purposes.
- For the pressure sensor, wrap the 1/4" NPT threads of a high-performance gauge pressure transducer with Teflon tape and thread them into the bulkhead. Use a soldering iron to connect the pressure sensor wiring to an 8-pin male DIN connector, using the manufacturer wiring instructions for interface with a commercially available high-quality data acquisition device (see Table of Materials).
NOTE: This requires the use of a 150 K ohm 1/8 watt 1% metal film resistor within the DIN connector wiring. - For the calibration port, use a 1/4" male NPT to 1/8" female NPT adaptor to connect a 1/8" male NPT to a female Luer lock nickel-plated connector to the brass bulkhead wrapping threaded connections with Teflon tape. Use a polypropylene threaded male Luer cap to seal the Luer connector when not in use.
NOTE: Do not overtighten the bulkhead connectors onto the glass jar, as this will develop cracks. If desired, silicone can be added to the rubber gaskets to ensure an airtight seal of the bulkheads to the glass jar.
- For the pressure sensor, wrap the 1/4" NPT threads of a high-performance gauge pressure transducer with Teflon tape and thread them into the bulkhead. Use a soldering iron to connect the pressure sensor wiring to an 8-pin male DIN connector, using the manufacturer wiring instructions for interface with a commercially available high-quality data acquisition device (see Table of Materials).
2. System setup
- Connect the sampling chamber to a bridge amplifier using an 8-pin DIN connector and the bridge amplifier to the data acquisition device, following the manufacturer's instructions.
- Connect the data acquisition device to a power supply and a computer running physiological data analysis software using the manufacturer's cables.
NOTE: Ensure that the data acquisition device is powered on and warmed up for at least 5 min before use to ensure that the sensor stabilizes its measurements. - Initiate the software to interface with the data acquisition system.
- Download the optional Spirometry Module within the software, and modify the default unit settings from L/s to µL/s in the Spirometry > Settings window.
3. System calibration
- Within the software, create a 4-Channel window with the following data windows: Channel 1: Source data at 4 k/s sample rate and 1 mV range; Channel 2: Digital filter of Channel 1 using a high pass 1 Hz auto adjust filter; Channel 3: Smoothing of Channel 2 data by averaging of 100 samples; Channel 4: Spirometry Flow of Channel 3 data (Custom Flowhead, calibrated to Formula (µL/s) = 120,000 x Voltage).
NOTE: 120,000 is a placeholder correlation coefficient that will be modified during calibration. - Set up DataPad analysis of Channel 4 with the following Columns: Column 1: Channel 4 Data, Comments > Full Comment Text; Column 2: Channel 4 data, Cyclic Measurements > Average Cyclic Frequency; Column 3: Channel 4 data, Cyclic Measurements > Average Cyclic Height.
- Set the frame rate to 100:1 on the bottom-right corner of the chart display. Save this window configuration as a template for all future studies.
- Close the Sample Chamber lid and attach a 25 µL Gas Tight syringe to the Luer bulkhead connector. Fit the syringe with a Chaney adaptor set to repeatedly deliver a volume of 20 µL.
NOTE: An optional short piece of 1/16" tubing and Luer/barb connectors may be used to connect the syringe to Sample Chamber. However, long tubing should be avoided to avoid significant changes to the total air volume of the Sample Chamber. - Draw 20 µL of air into the syringe using the depth stop of the Chaney adaptor.
- Zero the pleth in the software (Setup > Zero All Inputs (Alt-Z)) and start a recording.
- While recording, and with a stable baseline, rapidly depress/withdraw the Syringe plunger for approximately 10 repetitions to replicate subject breathing with a measured 20 µL breath. Stop the recording.
NOTE: The frequency of the artificial breaths should exceed 2 Hz to maximize the reproducibility of the calibration. - Label the identity of the measured sample by right-clicking the beginning of numbered pleth recording and click on Add Comment.
- Reset the Syringe, Zero the input, and repeat recording measurements of 20 µL pulses two additional times (three total recording sessions).
- After completing all the measurements, use the computer mouse to select a portion of breathing pleth that accurately represents the artificial 20 µL breaths.
NOTE: Within the DataPad module, data will appear in the preview header providing a temporary readout of the breath rate (Average Cyclic Frequency, Hz) and breath depth (Average Cyclic Height, µL). The data preview can be recorded into DataPad using the Add to DataPad icon. - Review the Column 3 data (Average Cyclic Height), and calculate the average measured breath volume from the three recordings. Perform the following calculation of the average measured breath volume: Calibration coefficient = delivered volume / measured volume x 120,000.
NOTE: 120,000 was the placeholder Flow Head Calibration coefficient used in step 3.1 now modified from measured data. The system is now calibrated to typical mouse breathing using current environmental temperature and humidity. The system can now monitor the subject breathing, and calibration can be re-performed daily to account for any fluctuation in temperature/humidity.
4. Subject monitoring
- Open a master template as outlined in step 3.4, or complete steps 4.2 to 4.3.
- Within the software, create a 4-Channel window with the following data processing: Channel 1: Source data at 4 k/s sample rate and 1 mV range; Channel 2: Digital filter of Channel 1 using a High Pass 1 Hz Auto Adjust filter; Channel 3: Smoothing of Channel 2 data by Averaging of 100 Samples; Channel 4: Spirometry Flow of Channel 3 data (Custom Flowhead, calibrated to Formula (µL/s) = 120,000 x Voltage).
NOTE: 120,000 is the correlation coefficient calculated for the current pressure sensor; however, the user should perform the system calibration described in step 3 and use this user-defined correlation coefficient instead. - Set up DataPad analysis of Channel 4 with the following Columns: Column 1: Channel 4 Data, Comments > Full Comment Text; Column 2: Channel 4 data, Cyclic Measurements > Average Cyclic Frequency; Column 3: Channel 4 data, Cyclic Measurements > Average Cyclic Height.
- Place subject in sampling chamber and close lid. For this experiment a conscious 4-12 week female albino C57BL/6J mouse (B6(Cg)-Tyrc-2J/J) was used.
- Equalize the atmospheric pressure in the chamber (from sealing the lid) by briefly loosening the Luer bulkhead cap and retighten.
- Observe that the subject is not actively moving within the sampling chamber before Zeroing All Inputs (Alt-Z shortcut) and beginning a recording.
NOTE: If the subject begins to move in the sampling chamber, the baseline may move off the scale, which may be addressed by re-zeroing all inputs in the middle of a recording, which will create a fresh recording on the scale. Suppose the subject engages in exploration or grooming during the recording; note which portion of the plethysmography recording most accurately reflects normal breathing. - Label the subject's identity by right-clicking the beginning of numbered pleth recording and click on Add Comment.
- Return the subject to its cage. Limit the time spent in the sealed sampling chamber to 5 min to avoid asphyxiation and stress.
NOTE: The risk for asphyxiation is low given that the 600 mL air volume of the Sample Chamber will not be rapidly spent by a healthy mouse breathing at <15 mL/min. - Use the computer mouse to select a portion of breathing pleth that accurately represents the subject breathing.
NOTE: Within the DataPad module, data will appear in the preview header providing a temporary readout of the breath rate (Average Cyclic Frequency, Hz) and breath volume (Average Cyclic Height, µL). The data preview can be recorded into DataPad using the Add to DataPad icon. - Continue measuring subject mice one at a time and recording representative sections of breathing pleth to DataPad.
- After data recording, export DataPad data to Excel. Calculate the minute volume as follows: Minute Volume (mL/min) = Breath Rate (Hz) x Breath Volume (µl) x 0.06.
Representative Results
System calibration
The data analysis software allows for direct calibration of a custom flowhead, such as the one described herein. This is performed when setting the Spirometry Flow. As described in step 3.1, an option exists to input the known calibration air volume, which calculates the voltage to volume correlation coefficient within the system. This, however, generates a correlation coefficient based on a single read, and it has been observed that the inherent variation of calibration from an n = 1 standard has poor utility. The current approach can address this shortcoming and allows a user to perform a daily calibration using multiple readings averaged to calculate a calibration coefficient. Calibration with 20 µL of injected air was demonstrated herein, representing a typical high-end breath volume in a typical mouse. The software assumes an origin intercept (0,0) and is thus calibrated from 0-20 µL using this approach.
The methodology proposed here for sWBP calibrates daily, thus accounting for any fluctuations in environmental humidity/temperature. The original methods used for specific WBP, date back to the methodology of Drorbaugh and Fenn from 1955, who developed WBP for measuring ventilation in human infants5. The Drorbaugh and Fenn calculations account for variations in temperature and humidity of the environment and the subject. The current approach corrects for environmental fluctuations by calibrating each sWBP session. Still, it was decided to address whether the heating and humidification of breathing across the nasal cavity/lung of a mouse affect the measurement of a known volume of air. Thus, an artificial apparatus was created to mimic the subject's effect on heating and humidifying calibrated air measurements. Luer connectors were attached to a 15 mL conical tube and placed this sealed conical in-line between the sample chamber and the gas-tight calibration syringe. A 20 µL calibration was performed using an empty conical tube held at room temperature (23 °C). The conical tube was then partially filled with distilled water to just below the Luer connectors, allowing time to equilibrate the headspace of the conical; the calibration volume was then re-measured to investigate the effect of humidity. The conical tube was placed in a heating block and equilibrated at 37 °C in a humid environment, and finally equilibrated to 37 °C with no water to assess the effect of subject heating and with no additional contribution of humidity. Figure 2 demonstrates that all conditions tested did not significantly impact the calibrated 20 µL measurement delivered by the gas-tight syringe. From this finding, it was concluded that sWBP offers an accessible approach to monitor breathing in research animals without the need for complex calculations predicated on the temperature and humidity of the animal subject, as these make no significant impact on measured breath volume.
Subject monitoring
sWBP was used to monitor breathing during the disease of lethal respiratory infections with the bacterial pathogen B. pseudomallei. One challenge of monitoring breathing in conscious animals is the curiosity of normal healthy animals moving within the sample chamber. The movement of the mouse creates a constantly moving baseline that can be mitigated in part by pre-conditioning subjects to the chamber over a period of several days prior to measurement. This issue primarily affects the baseline measurement in healthy mice, as the subjects become lethargic during infection, making sWBP much more manageable with reduced subject activity. It may be tempting to attempt to use some form of restraint, whether physical or anesthesia. The use of physical restraint may affect natural breathing by causing stress. Further, the use of anesthetics is known to have pronounced effects on breath rate and depth10; thus, it was decided to investigate the impact of anesthesia with the in-house sWBP apparatus. Isoflurane is commonly used to perform in vivo diagnostic imaging during the infection models, and therefore, a C57BL/6 mouse was anesthetized and monitored progression until recovery out of anesthesia using sWBP. This trial was conducted with a juvenile 4-week old albino C57BL/6J mouse to prolong the window of recovery from anesthesia. Figure 3 demonstrates that the preferred anesthetic causes mice to exhibit a slow breath rate with a large tidal volume of air. As mice begin to recover from sedation, their breath rate increases and breath volume decreases, with the net effect that total inspired air slowly increases. In this trial, it was found that the breath volume is restored to pre-anesthesia levels within the first 30 s of recovery. The breath rate steadily increases until the baseline breathing is restored to 2-2.5 min post-removal from anesthesia. The minute volume closely followed the effects of breath rate, reaching baseline minute volume by 2.5 min post-removal from anesthesia. This finding supports that anesthesia should not be employed in the sWBP approach. It dramatically affects baseline breathing, not surprisingly, as anesthesia will slow the host metabolism, creating a reduced demand for inspired oxygen. Sanitation of the sample chamber should also be considered between subjects to address study-specific infection control as well as the impact of pheromones from urine or feces which could impact the stress between subjects.
WBP is an attractive strategy for monitoring lung function in respiratory disease models in a non-invasive manner. sWBP was used to study how breathing changes during lethal respiratory melioidosis infections (Figure 4), with time points mirroring bioluminescence monitoring in the lung. It was observed that this model is associated with an early onset of lethargy, which persists in a slowly progressive manner until the development of the moribund disease at approximately 3 days post-infection. It was also observed that the breath rate and total inspired air (minute volume) of the mice decrease rapidly during the first day of infection and remain low for the remainder of the course of infection (Figure 4A,C). This pattern is consistent with the early onset lethargy, which persists for the next 2 days of the infection. In contrast, the breath volume does not steeply drop during the first 24 h and instead has a slight and steady decrease, which approaches a linear decline over the 3-day course of the disease (Figure 4B).

Figure 1: sWBP apparatus. A custom Sample Chamber was constructed from a sealable square glass jar with bulkhead connectors on two flat faces. One bulkhead was used to mount a gauge pressure sensor connected to a bridge amplifier and data acquisition device digitizer via an 8-pin DIN connection. The second bulkhead was fitted with a Luer connector for calibration by a gas-tight syringe. The device was connected to a PC running the software. Please click here to view a larger version of this figure.
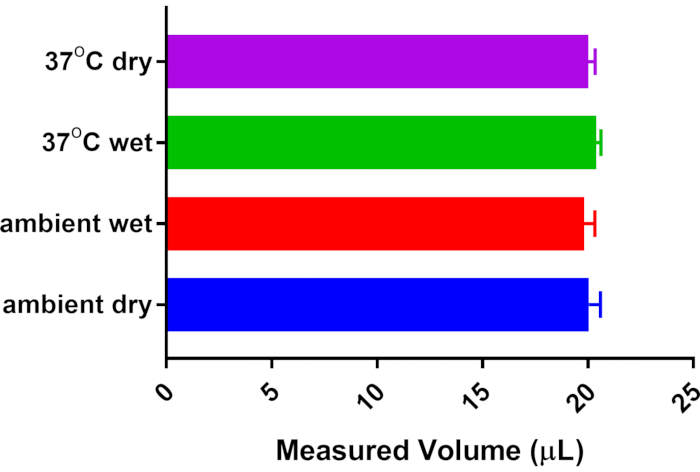
Figure 2: Effect of subject temperature and humidity on breath volume. A 15 mL conical tube with Luer connectors was installed in-line between the 20 µL calibration syringe and the Sample Chamber. The system was calibrated to 20 µL with no additional temperature/humidity contribution from the conical tube. Other measurements were collected post-equilibration with saturated humidity from distilled water and/or warming of the conical tube from room temperature (23 °C) to body temperature (37 °C). No significant difference was detected from n = 5 measurements of each condition by One-way ANOVA with Tukey's Multiple Comparison post-test. Please click here to view a larger version of this figure.
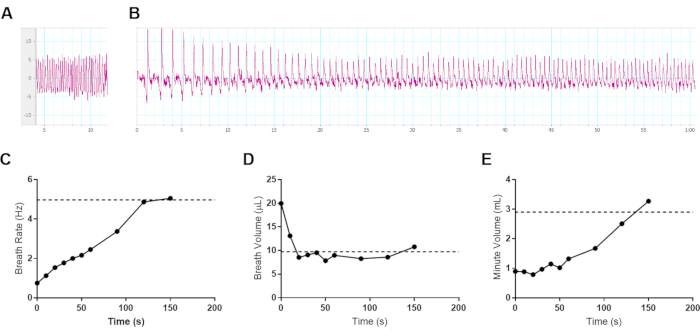
Figure 3: Effect of gas anesthesia on breathing in mice. Representative data from a 4-week old female albino C57BL/6J mouse (8.6 g) was sedated for 5 min with 3% isoflurane in oxygen and transferred to an sWBP Sample Chamber. Pleth data was collected for 150 s following removal from anesthesia. The subject began initial ambulation by 100 s following removal from anesthesia. (A) Baseline breathing before anesthesia, measuring a 4.97 Hz breath rate, 9.74 µL breath volume, and 2.91 mL minute volume. (B) The first 60 s of changes to breathing during recovery from anesthesia. (A–B) Vertical axis measuring µL per breath and horizontal axis in seconds. (C–E) Ventilation data was collected during the 150 s of recovery from anesthesia, averaged from ≥3 breath cycles per time point for (C) Breath Rate, (D) Breath Volume, and the (E) calculated Minute Volume. The pre-anesthesia baseline values are indicated with a horizontal dotted line in each respective graph. Please click here to view a larger version of this figure.
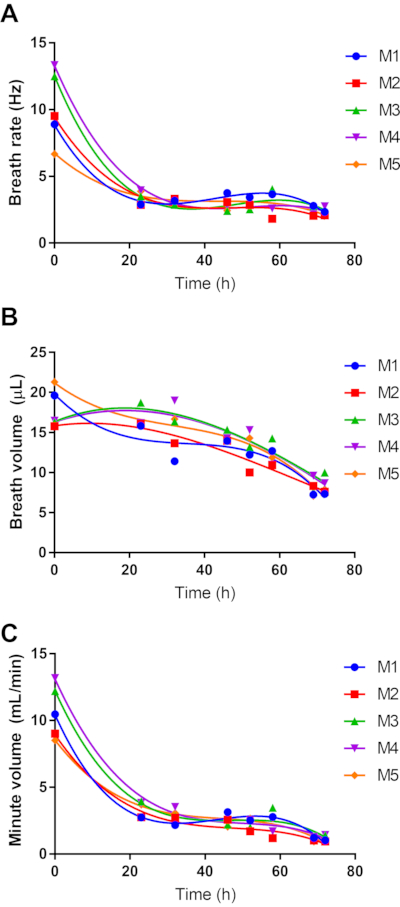
Figure 4: Effect of respiratory melioidosis on host breathing. Five 8-week female C57BL/6 mice were infected with 4.9 log CFU of bioluminescent B. pseudomallei strain JW270. sWBP was conducted throughout the 3-day course of infection, measuring Breath Rate (A) and Breath Volume (B). The total inspired air was calculated as the Minute Volume (C). Data for each of the five subjects are independently plotted with third-order polynomial regression. Please click here to view a larger version of this figure.
Discussion
sWBP is an attractive approach for enhancing the understanding of respiratory infection in small animal models. Importantly, it is a non-invasive approach, and as such, it does not pose a significant risk of causing undue stress to research animals during an infection challenge. Indeed, the procedure of monitoring subject breathing is a rapid test requiring several minutes and minimal subject handling. The scientific benefit is the high-resolution understanding of how microbial pathogens influence lung function during disease. This approach will provide benefit to basic research, facilitating the understanding of how a pathogen causes disease, as well as providing a translational utility to understand how a novel therapeutic restores a research subject to a state of respiratory health.
In this manuscript, representative results are provided for the pathogen B. pseudomallei, which causes an early lethargic response. Not all bacterial lung infections present in the same manner in mouse infection models. Previous experience with other infection models has demonstrated that the bacterial pathogen Klebsiella pneumoniae presents as an asymptomatic infection up to the point at which mice succumb to infection, also at approximately Day 3 post-infection11. It is hypothesized that the host demand for inspired air (i.e., minute volume) may closely relate to the degree of lethargy with which a given disease presents. Future studies will be required to examine how different bacterial pathogens affect lung function during respiratory disease. It is understood that different pathogens have unique approaches to evade host defense, including differences in, (1) propensity to be intracellular or extracellular pathogens, (2) the ability to cause early/late hypothermic response, and (3) use of different repertoires of virulence determinants3,12,13. Therefore, it is likely that different disease strategies will result in unique effects on lung function and breathing during infection.
The recommended settings described in this protocol can be modified to accommodate unique challenges present during sWBP. One of the common issues experienced during an sWBP recording session is the subject’s movement within the Sample Chamber. As mentioned, this movement modifies the baseline and can affect the accuracy of breathing measurements. A Digital filter was used to normalize the shifting baseline, allowing viable breath measurements despite small movements. Excessive movement can push a baseline measurement out of the range of a zeroed input. Recordings are recommended at 1 mV range (Channel 1 setting), which provides a compromise of still observing the peaks of the plethysmography while avoiding loss of data outside of range. For exceptionally active subjects, it may be necessary to extend the recording range >1 mV to avoid persistent out of range signals.
The recommended procedure calls for daily calibration (or at each session) to accommodate environmental humidity/temperature fluctuations. Traditional WBP uses complex calculations that factor in the temperature/humidity of both environment and subject5,6. It has been demonstrated that in the present sWBP apparatus, the effects of the host temperature/humidity do not significantly alter the measured breath volume of a calibration source. Therefore, this approach in sWBP fundamentally differs from the >50-year-old approach of Drorbaugh and Fenn. Here, sWBP directly relates pressure changes to a measured breath volume with no further correction from the host.
It is essential to contrast research animal WBP to that of clinical WBP. The types of biometric data that were attempted to collect by sWBP are breath volume and frequency. Such measurements are collected clinically using simple spirometry equipment in which a patient holds a breath monitor to their mouth and breaths normally into a device monitoring airflow. Similar spirometry in research animals requires restraint, thus contributing to stress and an inherent disruption in breathing. Therefore, simple spirometry is functional clinically but not for research animals. WBP serves an essential purpose in the clinic to collect advanced data, including such measurements as residual lung volume. Such data can only be contained in the context of a subject being able to follow instructions on how they breathe, including Forced Expiration (emptying of their lung by a deep exhale). Research animals cannot be relied upon to follow breathing instructions from a researcher. Many of the advanced measurements collected clinically during WBP cannot be reproduced in research animals. WBP in research animals is fundamentally different from clinical WBP. Animal WBP seeks to collect simple ventilation data (breath rate and volume) in a non-restrained fashion to avoid animal stress and breathing perturbation. Thus far, the use of WBP in research animals appears to replicate the techniques used in clinical WBP, including complex calculations based on environmental and subject temperature and humidity, but without the ability to collect the advanced data from a subject that can follow instructions on how to perform a forced expiration. With this in mind, it was sought to demonstrate whether a simplified version of WBP would suffice to collect the pertinent breathing frequency and volume relevant to respiratory disease studies. A calibration session was employed, which compensated for any variation in environmental temperature and humidity. Further, it was demonstrated with an artificial mouse that subject temperature, and humidity to a measured breath volume have no significant effect on accurately measuring breath volume. It was concluded that sWBP has excellent application to research animal studies, without the requirement of the user to employ cumbersome mathematical treatment of data.
Divulgations
The authors have nothing to disclose.
Acknowledgements
These studies were supported by the National Institutes of Health COBRE grant P20GM125504-01 Sub-Project 8246.
Materials
| 1/8" NPT Luer adaptor | Amazon | B07DH9MY8W | Calibration port |
| 1/8" NPT to 1/4" NPT adaptor | Amazon | B07T6CR6FS | Bulkhead to luer adaptor |
| 150 kohm resistor | Amazon | B07GPRYL81 | Pressure transducer excitation voltage selection |
| 3/4" diamond drill bit | Drilax | DRILAX100425 | To drill bulkhead mounts in glass jar |
| Bridge Amp | AD Instruments | FE221 | One channel option |
| Bulkhead fitting | Legines | 3000L-B | 1/4" NPT, 3/4-16 UNF brass bulkhead coupling |
| Chaney adaptor | Hamilton | 14725 | Gas tight syringe adaptor for set volume |
| DIN connector | AD Instruments | SP0104 | To connect pressure sensor to Bridge Amp |
| Gastight syringe, 25 uL | Hamilton | 80201 | Calibration syringe |
| LabChart | AD Instruments | Life Science Data Acquisition Software | |
| Luer plug | Cole Parmer | 45513-56 | Calibration port closure |
| PowerLab 4/26 | AD Instruments | PL2604 | Digital interface to computer |
| Pressure transducer | Omega Engineering | PX409-10WGV | High accuracy oil filed gage pressure sensor |
| Rubber gasket | Amazon | B07LH4C8LS | To mount bulkheads (4 required per chamber) |
| Square glass jar | Amazon | B07VNSPR8P | 600 ml with 95 mm silicone gasket |
References
- Warawa, J. M., Long, D., Rosenke, R., Gardner, D., Gherardini, F. C. Role for the Burkholderia pseudomallei capsular polysaccharide encoded by the wcb operon in acute disseminated melioidosis. Infection and Immunity. 77 (12), 5252-5261 (2009).
- West, T. E., Myers, N. D., Liggitt, H. D., Skerrett, S. J. Murine pulmonary infection and inflammation induced by inhalation of Burkholderia pseudomallei. International Journal of Experimental Pathology. 93 (6), 421-428 (2012).
- Lawrenz, M. B., et al. Development and evaluation of murine lung-specific disease models for Pseudomonas aeruginosa applicable to therapeutic testing. Pathogens and Disease. 73 (5), (2015).
- Lim, R., et al. Measuring respiratory function in mice using unrestrained whole-body plethysmography. Journal of Visualized Experiments: JoVE. (90), e51755 (2014).
- Drorbaugh, J. E., Fenn, W. O. A barometric method for measuring ventilation in newborn infants. Pediatrics. 16 (1), 81-87 (1955).
- Simon, G., Pride, N. B., Jones, N. L., Raimondi, A. C. Relation between abnormalities in the chest radiograph and changes in pulmonary function in chronic bronchitis and emphysema. Thorax. 28 (1), 15-23 (1973).
- Gassiep, I., Armstrong, M., Norton, R. Human melioidosis. Clinical Microbiology Reviews. 33 (2), 06-19 (2020).
- Gutierrez, M. G., Warawa, J. M. Attenuation of a select agent-excluded Burkholderia pseudomallei capsule mutant in hamsters. Acta Tropica. 157, 68-72 (2016).
- Gutierrez, M. G., Pfeffer, T. L., Warawa, J. M. Type 3 secretion system cluster 3 is a critical virulence determinant for lung-specific melioidosis. PLoS Neglected Tropical Diseases. 9 (1), 3441 (2015).
- Rocco, P. R. M., Zin, W. A., Gullo, A. . Anaesthesia, Pain, Intensive Care and Emergency Medicine. , (2002).
- Fodah, R. A., et al. Correlation of Klebsiella pneumoniae comparative genetic analyses with virulence profiles in a murine respiratory disease model. PLoS One. 9 (9), 107394 (2014).
- Gotts, J. E., et al. Clinically relevant model of pneumococcal pneumonia, ARDS, and nonpulmonary organ dysfunction in mice. American Journal of Physiology-Lung Cellular and Molecular Physiology. 317 (5), 717-736 (2019).
- Galan, J. E. Common themes in the design and function of bacterial effectors. Cell Host & Microbe. 5 (6), 571-579 (2009).

