Real-Time Fluorescent Measurement of Synaptic Functions in Models of Amyotrophic Lateral Sclerosis
Summary
Two related methods are described to visualize subcellular events required for synaptic transmission. These protocols enable the real-time monitoring of the dynamics of presynaptic calcium influx and synaptic vesicle membrane fusion using live-cell imaging of in vitro cultured neurons.
Abstract
Before neuronal degeneration, the cause of motor and cognitive deficits in patients with amyotrophic lateral sclerosis (ALS) and/or frontotemporal lobe dementia (FTLD) is dysfunction of communication between neurons and motor neurons and muscle. The underlying process of synaptic transmission involves membrane depolarization-dependent synaptic vesicle fusion and the release of neurotransmitters into the synapse. This process occurs through localized calcium influx into the presynaptic terminals where synaptic vesicles reside. Here, the protocol describes fluorescence-based live-imaging methodologies that reliably report depolarization-mediated synaptic vesicle exocytosis and presynaptic terminal calcium influx dynamics in cultured neurons.
Using a styryl dye that is incorporated into synaptic vesicle membranes, the synaptic vesicle release is elucidated. On the other hand, to study calcium entry, Gcamp6m is used, a genetically encoded fluorescent reporter. We employ high potassium chloride-mediated depolarization to mimic neuronal activity. To quantify synaptic vesicle exocytosis unambiguously, we measure the loss of normalized styryl dye fluorescence as a function of time. Under similar stimulation conditions, in the case of calcium influx, Gcamp6m fluorescence increases. Normalization and quantification of this fluorescence change are performed in a similar manner to the styryl dye protocol. These methods can be multiplexed with transfection-based overexpression of fluorescently tagged mutant proteins. These protocols have been extensively used to study synaptic dysfunction in models of FUS-ALS and C9ORF72-ALS, utilizing primary rodent cortical and motor neurons. These protocols easily allow for rapid screening of compounds that may improve neuronal communication. As such, these methods are valuable not only for the study of ALS but for all areas of neurodegenerative and developmental neuroscience research.
Introduction
Modeling amyotrophic lateral sclerosis (ALS) in the laboratory is made uniquely challenging due to the overwhelmingly sporadic nature of over 80% of cases1, coupled with the vast number of genetic mutations known to be disease-causative2. Despite this, all cases of ALS share the unifying feature that before outright neuronal degeneration, there is dysfunctional communication between presynaptic motor neurons and postsynaptic muscle cells3,4. Clinically, as patients lose connectivity of the remaining upper and lower motor neurons, they present with features of neuronal hyper- and hypoexcitability throughout the disease5,6,7,8,9, reflecting complex underlying molecular changes to these synapses, which we, as ALS researchers, seek to understand.
Multiple transgenic models have illustrated that deterioration and disorganization of the neuromuscular junction occur with the expression of ALS-causative genetic mutations, including SOD110, FUS11,12, C9orf7213,14,15,16, and TDP4317,18,19 through morphological assessments, including evaluation of synaptic boutons, spine densities, and pre/postsynaptic organization. Mechanistically, since the landmark papers of Cole, Hodgkin, and Huxley in the 1930s, it has also been possible to evaluate synaptic responses through electrophysiological techniques in either in vitro cell culture or tissue slice preparations20. Through these strategies, many models of ALS have demonstrated synaptic transmission deficits. For example, a mutant variant of TDP43 causes enhanced firing frequency and decreases action potential threshold in NSC-34 (spinal cord x neuroblastoma hybrid cell line 34) motor-neuron-like cells21. This same variant also causes dysfunctional synaptic transmission at the neuromuscular junction (NMJ) before the onset of behavioral motor deficits in a mouse model22. It was previously showed that mutant FUS expression results in reduced synaptic transmission at the NMJ in a drosophila model of FUS-ALS before locomotor defects11. A recent report using induced pluripotent stem cells derived from C9orf72-expansion carriers revealed a reduction in the readily releasable pool of synaptic vesicles23. Altogether, these studies and others highlight the importance of building a more comprehensive understanding of the mechanisms underlying synaptic signaling in disease-relevant models of ALS. This will be pivotal in understanding the pathobiology of ALS and developing potential therapeutic targets for patients.
Methods of current and voltage clamping cells have been invaluable in determining membrane properties such as conductance, resting membrane potential, and quantal content of individual synapses20,24. However, one of the significant limitations of electrophysiology is that it is technically challenging and only provides insights from a single neuron at a time. Live-cell confocal microscopy, coupled with specific fluorescent probes, offers the opportunity to investigate the synaptic transmission of neurons in a spatiotemporal manner25,26,27. Although not a direct measure of neuronal excitability, this fluorescence approach can provide a relative measurement of two molecular correlations of synaptic function: synaptic vesicle release and calcium transients at synaptic terminals.
When an action potential reaches the presynaptic terminal region of neurons, calcium transients are triggered, facilitating the transition from an electrical signal to the process of neurotransmitter release28. Voltage-gated calcium channels localized to these areas tightly regulate calcium signaling to modulate the kinetics of neurotransmitter release29. The first reported fluorescence-based recordings of calcium transients were performed using either the dual-wavelength indicator Fura-2 AM or the single wavelength dye Fluo-3 AM30,31,32. While these dyes offered great new insight at the time, they suffer from several limitations such as non-specific compartmentalization within cells, active or passive dye loss from labeled cells, photobleaching, and toxicity if imaged over extended periods of time33. In the past decade, genetically encoded calcium indicators have become the workhorses for imaging various forms of neuronal activity. These indicators combine a modified fluorescent protein with a calcium chelator protein that rapidly switches fluorescence intensity after the binding of Ca2+ ions34. The application of these new indicators is vast, allowing for much easier visualization of intracellular calcium transients both in in vitro and in vivo settings. One family of these genetically encoded reporters, known as GCaMP, are now broadly utilized. These indicators contain a C-terminal calmodulin domain, followed by green fluorescent protein (GFP), and are capped by an N-terminal calmodulin-binding region35,36. Calcium-binding to the calmodulin domain triggers an interaction with the calmodulin-binding region, resulting in a conformational change in the overall protein structure and a substantial increase in the fluorescence of the GFP moiety35,36. Over the years, this family of reporters has undergone several evolutions to enable distinct readouts for particular calcium transients with specific kinetics (slow, medium, and fast), each with slightly different properties37,38. Here, the usage of the reporter GcaMP6 has been highlighted, which has been previously shown to detect single action potentials and dendritic calcium transients in neurons both in vivo and in vitro37.
Calcium transients in the presynaptic region trigger synaptic vesicle fusion events, causing neurotransmitter release into the synapse and initiation of signaling events in the postsynaptic cell28,39. Synaptic vesicles are both rapidly released and recycled, as the cell homeostatically maintains a stable cell membrane surface area and readily releasable pool of fusion capable membrane-bound vesicles40. The styryl dye used here has an affinity toward lipid membranes and specifically changes its emission properties based on the ordering of the surrounding lipid environment41,42. Thus, it is an ideal tool for labeling recycling synaptic vesicles and subsequent tracking of these vesicles as they are later released following neuronal stimulation41,42. The protocol that has been generated and optimized is an adaptation of the concepts described initially by Gaffield and colleagues, which allows us to visualize styryl dye-labeled synaptic vesicle puncta over time continuously41.
Here, two related fluorescence-based methodologies are described, reliably reporting specific cellular events involved in synaptic transmission. Protocols have been defined to probe the dynamics of depolarization-mediated presynaptic terminal calcium influx and synaptic vesicle exocytosis in cultured neurons. Here, methods and representative results are focused on using primary rodent cortical or motor neurons as the in vitro model system, as there are published studies using these cell types43,44. However, these methods are also applicable to differentiated human i3 cortical-like neurons45, as we have also had success with both protocols in presently ongoing experimentation in our laboratory. The general protocol is outlined in a stepwise linear format, shown in Figure 1. In brief, to study calcium dynamics in neurites, mature neurons are transfected with plasmid DNA to express the fluorescent reporter GCaMP6m under a Cytomegalovirus (CMV) promoter37,46. Transfected cells have a low level of basal green fluorescence, which increases in the presence of calcium. Regions of interest are specified to monitor fluorescence changes throughout our manipulation. This allows for highly spatially and temporally localized fluctuations in calcium to be measured37,46. For evaluating synaptic vesicle fusion and release, mature neurons are loaded with styryl dye incorporated into synaptic vesicle membranes as they are recycled, reformed, and reloaded with neurotransmitters in presynaptic cells41,42,43,47,48. The current dyes used for this purpose label synaptic vesicles along neurites and are used as a proxy for these regions in live-imaging experiments, as was shown by co-staining of styryl dye and synaptotagmin by Kraszewski and colleagues49. Included here are representative images of similar staining that have also been performed (Figure 2A). Previous investigators have extensively used such dyes to report synaptic vesicle dynamics at the neuromuscular junction and hippocampal neurons48,49,50,51,52,53,54,55,56. By selecting punctate regions of dye-loaded vesicles and by monitoring decreases in fluorescence intensity following vesicle release, functional synaptic transmission capacity and temporal dynamics of release can be studied following stimulation43. For both methods, a medium containing a high concentration of potassium chloride is employed to depolarize cells to mimic neuronal activity. Imaging parameters are specified to capture sub-second intervals spanning a baseline normalization followed by our stimulation capture period. Fluorescence measurements at each time point are determined, normalized to the background, and quantified over the experimental time period. Calcium-influx mediated GCaMP6m fluorescence increase or effective synaptic vesicle exocytosis styryl dye release fluorescence decrease can be detected through this strategy. Detailed methodological setup and parameters for these two protocols and a discussion on their advantages and limitations are described below.
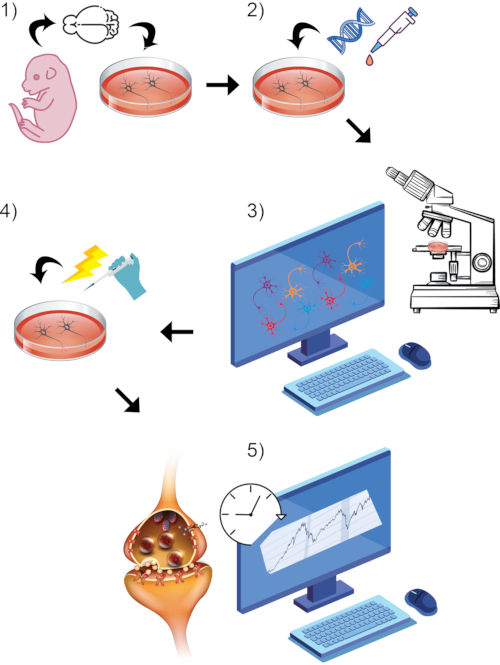
Figure 1: Visual rendering of overall general protocol process. (1) Isolate and culture primary rodent neurons in vitro to chosen maturation timepoint. (2) Introduce GCaMP DNA or styryl dye as reporters of synaptic activity. (3) Setup imaging paradigm using live-imaging equipped confocal microscope and associated software. Begin baseline recording period. (4) While cells are still undergoing live-image capture, stimulate neurons via high KCl bath perfusion. (5) Assess fluorescence intensity measurements over time to measure calcium transients or synaptic vesicle fusion. Please click here to view a larger version of this figure.
Protocol
All animal procedures performed in this study were approved by the Institutional Animal Care and Use Committee of Jefferson University.
1. Primary culture of neurons from embryonic rat cortex
NOTE: Primary cortical neurons are isolated from E17.5 rat embryos as previously described57,58. No strain bias appears to exist with the success of this culturing protocol. This method is described briefly below. The previous articles indicated should be referenced for complete details.
- Euthanize pregnant female rats by CO2 inhalation followed by secondary confirmation by cervical dislocation.
- Harvest embryos and isolate brains in ice-cold 20 mM HEPES-buffered Hank's balanced salt solution (HBSS). From the outer cortex shell, separate and then discard striatum and hippocampi. Collect cortices.
- Use fine forceps to remove meninges from cortices. To do this, hold the cortex in place with one pair of closed forceps using gentle pressure.
NOTE: With the second pair of forceps in the second hand, pinch meninges. Peel away meninges using rolling motion with pinched pair. Reposition with the closed forceps and repeat as necessary until meninges is entirely removed. - Incubate cortices with 10 µg/mL of papain in HBSS for 4 min at 37 °C.
- Wash three times in HBSS, and then triturate gently 5-10 times with a fire-polished glass Pasteur pipette to obtain a homogenous cell suspension.
NOTE: Avoid bubbles; maintain the pipette within the cell suspension. - Plate neurons on 100 µg/mL poly-D-lysine coated 35 mm glass-bottom dishes at a density of 75,000 cells/dish in neurobasal medium with B27 supplement (2%), and penicillin-streptomycin.
2. Primary culture of motor neurons from embryonic rat spinal cord
NOTE: Primary motor neurons are prepared from E13.5 rat embryos as previously described, with few modifications59,60. No strain bias appears to exist with the success of this culturing protocol. This method is described briefly below. The preceding articles indicated should be referenced for complete details.
- Euthanize pregnant female rats as in step 1.1.
- Dissect 10-20 spinal cords from embryos and break into small fragments mechanically using two pairs of forceps to pinch and pull, respectively.
- Incubate in 0.025% trypsin for 8 min, followed by addition of DNase at 1 mg/mL.
- Centrifuge through a 4% w/v BSA cushion at 470 x g for 5 min at 4 °C with no break.
- Centrifuge pelleted cells for 55 min at 830 x g at 4 °C without brake through a 10.4% (v/v) density gradient medium (see Table of Materials) cushion. Carry forward the motor neuron band at the visual interface.
NOTE: To ensure capture of all cells, use phenol-free media for the density gradient step and phenol-containing media for all the other steps. The interface of the density gradient and dissociation media is easily identifiable based on the color difference. - Spin collected bands through another 4% w/v BSA cushion at 470 x g for 5 min at 4 °C with no break.
- Resuspend purified motor neurons in complete neurobasal medium with B27 supplement (2%), glutamine (0.25%), 2-mercaptoethanol (0.1%), horse serum (2%), and penicillin-streptomycin.
- Plate neurons on 100 µg/mL of poly-lysine and 3 µg/mL of laminin-coated coverslips at a density of 50,000 cells/35 mm glass-bottom dish.
3. Neuronal transfections
NOTE: This step is carried out for neurons that will undergo GCaMP calcium imaging and/or neurons in which an exogenous DNA plasmid or RNA of interest is introduced in advance of synaptic evaluation. In contrast, styryl dye is loaded just before the imaging session and is addressed in section 6. The summary below is the transfection protocol used for primary rodent neurons in the presented examples, but it can easily be adapted and optimized to the user's needs.
- Transfections for cultured primary cortical neurons occur at day 12 in vitro (DIV 12), whereas motor neurons are transfected at day 7 in vitro (DIV 7).
NOTE: All the following steps occur within an aseptic biosafety cabinet. - Transfect 500 ng of GCaMP6m using transfection reagent at a ratio of 1:2 by volume. For co-transfections, add a total of 1.25 µg of total DNA/dish, maintaining this DNA to reagent ratio.
NOTE: In the experimental examples shown, 750 ng of ALS/FTD-related plasmid of interest has been transfected to express C9orf72-ALS linked dipeptides, mutant FUS protein, etc. Ensure that the fluorescent tag of the co-transfected plasmid will not conflict with the FITC channel of GCaMP imaging. Likewise, if doing styryl dye imaging, ensure that the co-transfected plasmid will not conflict with the TRITC channel of imaging. Usage of synaptophysin-GCaMP3 is equally effective in this protocol. Therefore, transfect the same amount of this plasmid and follow all the steps as usual in section 7. - Incubate the DNA-transfection reagent complex at room temperature for 10 min.
- Gently add the incubated complex to neurons dropwise with swirling. Return the dish to CO2 incubator at 37 °C for 45-60 min.
- Remove the entire culture media containing the DNA-transfection reagent complex and replace it with a 50-50 by volume preparation of pre-conditioned and fresh neuronal media. Then, put cells back into the CO2 incubator for 48 h until imaging.
4. Preparation of buffer solutions and styryl dye stock solution
- Make low and high aCSF solutions fresh before imaging using the ingredients listed in Table 1. Filter both the buffer solutions before use.
NOTE: CaCl2 dihydrate can form Ca(OH)2 upon storage. For maximum efficacy, always use a recently opened container. If this is not possible, to remove Ca(OH)2 from the external surface and ensure maximum solubility, solutions may be carbonated with gaseous CO2 for 5 min before CaCl2 addition. If this step is taken, adjust the pH of the resulting solution to maintain a pH value of 7.4, as excessive carbonation will result in Ca(HCO3)2 formation.
| Low KCL aCSF buffer (pH 7.40 to 7.45) | |
| Reagent | Concentration |
| HEPES | 10 mM |
| NaCl | 140 mM |
| KCl | 5 mM |
| Glucose | 10 mM |
| CaCl2 2H2O | 2 mM |
| MgCl2 4H2O | 1 mM |
| High KCL aCSF buffer (pH 7.40 to 7.45) | |
| Reagent | Concentration |
| HEPES | 10 mM |
| NaCl | 95 mM |
| KCl | 50 mM |
| Glucose | 10 mM |
| CaCl2 2H2O | 2 mM |
| MgCl2 4H2O | 1 mM |
Table 1: Composition of artificial cerebrospinal fluid (aCSF) buffers. This table includes the ingredients for preparing low and high KCl artificial cerebrospinal fluid buffers used while imaging and stimulating neurons. See section 4 for preparation instructions.
- Prepare styryl dye stock solution by taking a 100 µg vial of dye and reconstituting it to a stock concentration of 10 mM with distilled water or neurobasal medium.
5. Microscope and perfusion system setup
NOTE: For imaging glass-bottom Petri dishes, an inverted confocal fluorescence microscope is preferred due to the flexibility of perfusion and for the use of a high numerical aperture oil immersion objective. Refer to the Table of Materials for the confocal microscope, camera, and objectives used for imaging of examples detailed in the Representative Results section.
- Perform all the experiments at 37 °C with constant 5% CO2 levels using an incubator system-coupled stage mount.
- Control image acquisition using confocal software. Optimize acquisition settings at the start of the imaging session to choose excitation power and gain to ensure optimal visualization of signals without photobleaching.
- Keep excitation power, exposure time, detector gain and frame rate constant across all samples. Carry out time-lapse imaging using an aspect ratio of 512 x 512 and frame rate of 2 images/s to minimize dye bleaching.
NOTE: While puncta should be clearly visible, laser intensity should be set to the minimum possible to avoid bleaching and phototoxicity. Set the confocal aperture to the narrowest setting to achieve optimal resolution of fluorescent puncta within neurites. Exposure time is set to 200 ms or less, consistent across samples. The maximum imaging speed of the camera used was 2 images/s. If using a faster camera, more images/s can be taken. Temporal imaging settings should be chosen if possible.
- Keep excitation power, exposure time, detector gain and frame rate constant across all samples. Carry out time-lapse imaging using an aspect ratio of 512 x 512 and frame rate of 2 images/s to minimize dye bleaching.
- Select the following fluorescence excitation/dichroic/emission filter combinations for imaging using confocal software: Gcamp6m/Gcamp3 with FITC and styryl dye with TRITC, respectively.
- Use the perfect focus feature of the confocal imaging acquisition software during time-lapse imaging to avoid z-drift.
NOTE: Due to the rapid speed of imaging, a single plane is imaged. Ensuring the lack of z-drift during the experiment is very important. - Select the Time tab in the image acquisition panel to set the recording periods and intervals.
- Set Phase #1 à Interval 500 ms, Duration 3 – 5 min.
- Set Phase #2 à Interval 500 ms, Duration 5 min.
NOTE: Phase #1 corresponds to baseline recording, Phase #2 to the stimulation period, respectively.
- Assemble gravity perfusion apparatus for aCSF using a valve control system and a channel manifold.
- Load high KCl (see Table 1) into a 50 mL syringe at the top of the apparatus, with tubing running through the system. Set the flow rate to 1 mL/min.
- Load a 35 mm glass dish containing neurons onto the confocal imaging stage, with the end of the perfusion tubing placed at the dish edge. Choose the field for imaging.
6. Styryl dye imaging of synaptic vesicle release
- Incubate cells in low KCl aCSF (see Table 1) for 10 min at 37 °C, 48 h post-transfection.
- Load primary cortical or motor neurons on glass-bottom Petri dishes with styryl dye.
- Remove low KCl aCSF by aspiration.
- Use a pipette to load neurons in the dark with 10 µM of styryl dye in aCSF containing 50 mM KCl for 5 min.
- Remove the loading solution and bath neurons in low KCl aCSF for 10 min to eliminate non-specific dye loading.
- Place the 35 mm glass-bottom dish onto the imaging stage, and then observe either under a 20x air objective or 40x oil immersion objective of an inverted confocal microscope.
NOTE: Use GFP fluorescence to locate transfected cells in case of cells co-transfected with a GFP-tagged plasmid. - Excite styryl dye using a 546 nm laser, collect emission using a 630-730 nm (TRITC) bandpass filter.
- Select the imaging field and engage perfect focus. Next, take a single still image with brightfield, TRITC, and fluorescence marker channels to mark neuronal boundaries.
- Initiate Run Now in the acquisition software. Carry out the basal recording for 3-5 min to exclude variations in dye intensity, if any (Phase #1).
NOTE: It is essential to ensure that any decrease in dye intensity is due to synaptic vesicle release and not passive dye diffusion or photobleaching. This 3-5 min pre-stimulation period should result in a steady and maintained dye intensity level. The final 30 s of this recording will be used to determine a mean baseline fluorescence value for each ROI. Before evaluating experimental conditions, an additional non-stimulation condition of approximately 10 min of continuous recording using the intended acquisition settings can also be performed to ensure steady fluorescence values using the laser settings for an extended period. - At the switch to Phase #2, trigger On the button for the perfusion system. Then, constantly perfuse 50 mM KCl to neurons to facilitate dye unloading (Phase #2).
- Carry out recordings for 300 s after KCl addition. After this, acquisition stops; trigger the Off switch for the perfusion system.
- Save the experiment and analyze data using confocal software as described in section 8 below.
NOTE: The experiment may be stopped at this point, and analysis can be performed later. In the case where cells do not require transfection for the expression of proteins of interest, this protocol may be performed at any timepoint in vitro when seeking to examine the functionality of synaptic unloading. Following a test of control cells to ensure proper synaptic unloading, the experimenter should be blinded to the genetic or pharmacologic conditions of each dish tested to minimize bias.
7. Fluorescence imaging of Gcamp6m calcium transients
- Transfect primary rodent cortical neurons cultured on 35 mm glass-bottom dishes with 500 ng of Gcamp6m as indicated in section 3.
NOTE: If desired, co-transfect neurons with a plasmid of interest containing a fluorescent tag in the red or far-red range. - Incubate neurons with low KCl aCSF for 15 min 48 h post-transfection and then mount the dish on the imaging platform.
- Visualize GCaMP6m fluorescence using a FITC filter (488 nm) and a 20x or 40x objective.
- Select the imaging field and engage perfect focus. Next, take a single still image with brightfield, FITC, and fluorescence marker channels to mark neuronal boundaries.
- Initiate Run Now in the acquisition software. Carry out the baseline recording for 5 min, and then perfuse with aCSF containing 50 mM KCL in the same manner as described in section 6 for styryl dye experiments.
NOTE: The goal of this imaging is to measure evoked calcium transients. Should a neuron have basal firing activity and calcium fluxes during the pre-stimulation period, it is not used in data analysis. Instead, only cells with stable background fluorescence are used. Post-stimulation periods can also be extended to 60 min for calcium transients, with or without additional continuous high KCl perfusion. - Save the experiment and analyze data using confocal software as described in section 8 below. The experiment may be stopped at this point, and the analysis can be performed later.
NOTE: If the cells do not require transfection for expression of proteins of interest, this protocol may be performed at any timepoint in vitro when seeking to examine calcium transients. Following a test of control cells to ensure measurement of calcium transients, the experimenter should be blinded to the genetic or pharmacologic conditions of each dish tested to minimize bias.
8. Image analysis
- Open time-lapse images with confocal software.
- Align images in time-lapse series by the command series : Image | Processing | Align Current Document. Select Align to the First Frame.
- Select regions of interest (ROIs) along neurites using the ROI selection tool, a bean-shaped icon on the right of the image frame (Figure 3B). Also, mark an ROI representing background fluorescence intensity.
NOTE: ROIs are selected by choosing areas of distinctly separated puncta along neuronal tracks indicated by the brightfield still image. At least five ROIs per neuron are chosen for analysis. The background ROI is chosen in a region of the field of view that does not contain neurites. - Measure raw fluorescence over time for selected ROIs using the following command series: Measure | Time Measurement.
- Initiate the Measure function at the upper portion of the Time Measurement panel. A graphical representation of raw fluorescence over time (Figure 3C) and quantitative data are both generated.
NOTE: Each data point represents the raw fluorescence for that ROI for the frame associated with that specific measured time point.
- Initiate the Measure function at the upper portion of the Time Measurement panel. A graphical representation of raw fluorescence over time (Figure 3C) and quantitative data are both generated.
- Export raw fluorescence intensities to the spreadsheet software.
- Analyze each ROI independently. First, normalize data by subtracting the background ROI intensity from the ROI of interest intensity at each time point.
- Take the raw fluorescence value from the background ROI at each specific time point and subtract this from the ROI of interest raw intensity value at that time point.
NOTE: This is done for the entire recording period, both basal and stimulation phases.
- Take the raw fluorescence value from the background ROI at each specific time point and subtract this from the ROI of interest raw intensity value at that time point.
- Determine the average ROI of interest intensity for the last 30 s of baseline.
- Average the normalized raw basal values generated in step 8.6 from the final 30 s of the 3-5 min basal recording period.
NOTE: The entire period of basal recording could be used to generate this value. However, as this value stabilizes and is maintained, the 60-time points of the final 30 s are of sufficient sampling size to represent the whole.
- Average the normalized raw basal values generated in step 8.6 from the final 30 s of the 3-5 min basal recording period.
- Compare this baseline value to the normalized intensity value at each time point, generating a change in fluorescence (ΔF) value.
- Take the value from step 8.7 and subtract the average baseline fluorescent value. Do this and subsequent steps for the entire recording period, both basal and stimulation phases.
- Calculate this change concerning the baseline fluorescence value, generating a change in fluorescence/baseline fluorescence (ΔF/F). To do this, take the value from step 8.8 and divide it by the average baseline fluorescent value.
- Finally, set the starting point value to 1 so that increases or decreases can be easily visualized graphically over time.
- Take the value generated in step 8.9 and add 1.
NOTE: When this is done correctly, the 30 s of baseline period values should hover near the value of 1. In cells effectively releasing synaptic content, this value should trend from 1 to 0 following the start of stimulation. An example of steps 6-9 is presented in Figure 3E.
- Take the value generated in step 8.9 and add 1.
9. Data analysis
NOTE: The experimenter can be unblinded to experimental conditions to pool data for analysis appropriately. Use a sample size of at least 10 neurons per condition from each of three independent experiments. Only consider neurons for inclusion if at least five ROI regions can be designated. This level of experimental replication was sufficient in published studies to demonstrate a profound loss of synaptic unloading in ALS-related poly-GA-containing cells versus GFP controls (see Representative Results). However, if a more subtle phenotype is observed, the number of biological and/or technical replicates may require optimization by the user.
- Using all calculated ΔF/F values over time from ROIs of a given experimental condition, determine a mean ΔF/F value for each time point along with an SEM.
- Plot data using a graphing and statistics software program in xy-format, where x is the time elapsed, and y is the ΔF/F value calculated in step 9.1. Present all the values as mean ± SEM.
- To determine statistical significance, use the Student's t-test for comparing two groups and one-way analysis of variance (ANOVA) followed by Tukey's posthoc analysis for comparing three or more groups.
Representative Results
Following the successful implementation of the above protocol, representative results are shown for a typical styryl dye synaptic vesicle release experiment. Cultured rat primary cortical neurons were loaded with dye using the method described in section 6. The specificity of dye loading was determined by co-labeling with synaptic vesicle marker synaptophysin. A majority of styryl dye positive puncta are co-positive for this marker (Figure 2A). To determine whether the settings used for styryl dye imaging cause photobleaching, typically, an untreated well is loaded with dye and imaged for an extended 10 min period without stimulation to make sure fluorescence values remain constant.
Additionally, results are again shown using co-labeling of synaptophysin and styryl dye as in Figure 2A. These fluorophores were co-imaged using the paradigm indicated in section 6 for TRITC styryl dye imaging, plus dual capture of synaptophysin fluorescence using high-intensity laser power on the DAPI channel to visualize the mTurquoise-synaptophysin. Shown are representative images of synaptic regions pre- and post-stimulation (Figure 2B). While this method is an indirect inference for lack of photobleaching, analysis of the raw intensity values over the entire imaging period reveals that synaptophysin intensity remains constant, while styryl dye intensity goes down following stimulation (Figure 2C). Thus, taking this experiment and our other controls of stable fluorescence before stimulation and over extended periods in non-stimulation wells, we have established that decreases in styryl fluorescence can be attributed to synaptic unloading through KCl depolarization rather than photobleaching effects.
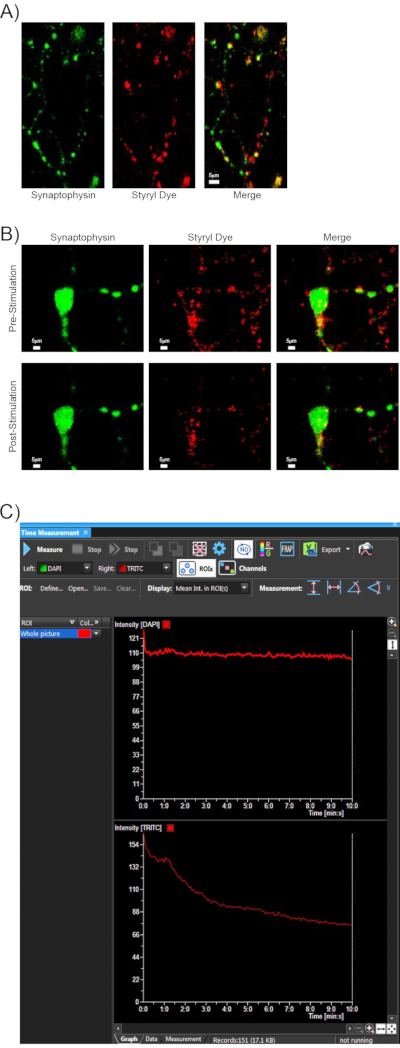
Figure 2: Assessment of specificity and imaging parameters of styryl dye labeling. (A) A representative 40x image of a styryl dye-labeled rat cortical neuron (red), which was transfected 24 h previously with a plasmid to express mTurquoise2-synaptophysin61 driven off the synapsin promoter (green). Colocalization of these two fluorophores indicates that a large majority of styryl dye positive puncta are co-stained with synaptic vesicle marker synaptophysin. Scale bar indicates 5 μm. (B) Cortical neurons were transfected and styryl dye loaded as in (A) and imaged according to the styryl dye paradigm along with dual fluorescence capture of the mTurquoise-synaptophysin on the DAPI channel. Representative images are showing synaptophysin and styryl dye puncta regions pre- and post-stimulation. Scale bar indicates 5 μm. (C) Fluorescence was monitored over time for synaptophysin (DAPI channel top) and styryl dye (FITC channel bottom). Synaptophysin intensity was maintained at a steady fluorescence over the imaging and stimulation period, while styryl dye fluorescence decreased as the dye was unloaded at stimulation. Please click here to view a larger version of this figure.
Representative images show the loading of styryl dye during imaging, with the selection of ROIs (Figure 3A–B). The raw fluorescence intensity of selected ROIs and a background ROI are plotted using the confocal software (Figure 3C). The neurons shown here were also transfected with plasmids to study the effects of dipeptide proteins produced in the context of C9ORF72-ALS, namely, FLAG-eGFP and FLAG-GA50-eGFP driven off of the T7 promoter. Successful synaptic vesicle release results in striking loss of dye fluorescence upon high KCl depolarization (Figure 3D, top two panels) for a GFP-transfected control neuron. A representative video included here demonstrates how this appears during imaging. In addition, a neuron selectively unloads styryl dye in a neurite region following stimulation (Video 1).
On the other hand, impaired synaptic transmission is represented by retained dye fluorescence even after high KCl depolarization in neurons transfected with a C9ORF72-linked dipeptide repeat construct (GA50) (Figure 3D, bottom two panels)43. Following quantitative data analysis (Figure 3E), data are represented as dye intensity throughout imaging for the control (GFP) and ALS/FTD (GA50) groups (Figure 3F)43. This method can also parse intermediate effects and is not simply an "all or nothing" binary measure. In experiments designed to rescue the synaptic deficits mediated by GA50, exogenous synaptic vesicle-associated protein 2 (SV2) was introduced by lentiviral transduction using the rSV2a-eGFP-pRRL plasmid driven off of the human PGK promoter. Following imaging and analysis as outlined above, synaptic firing was rescued in neurons co-expressing GA50 and SV2 (Figure 3G).
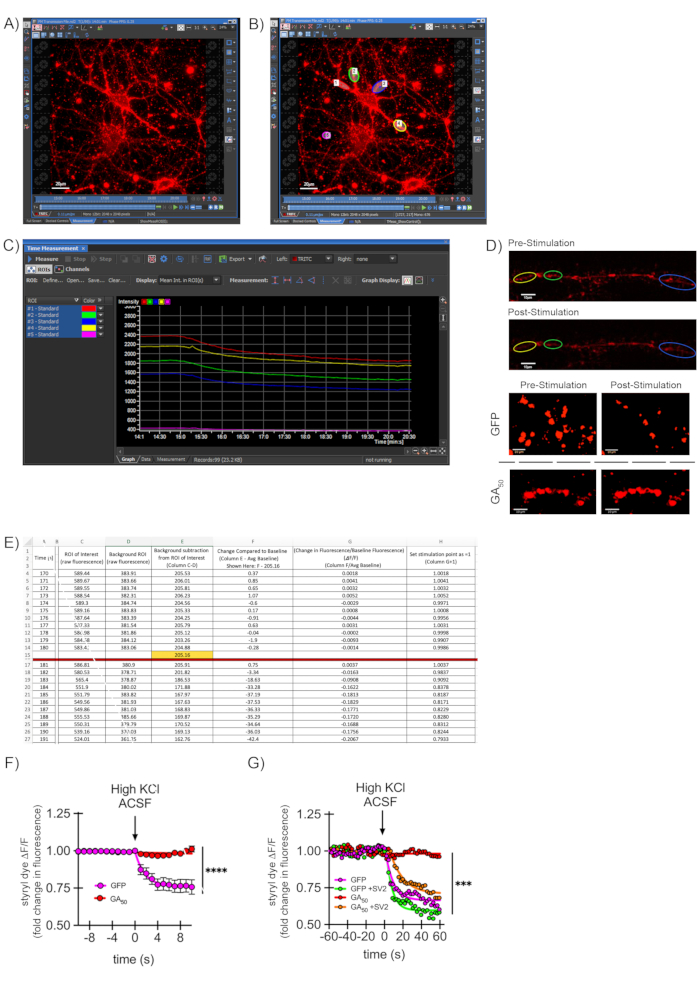
Figure 3: Evaluating synaptic vesicle release through styryl dye labeling. (A) A representative 40x image of a styryl dye-labeled neuron in low KCl aCSF media at the start of an imaging experiment. Scale bar indicates 20 μm. (B) Depiction of ROI generation on the image from (A). Specific puncta regions along neurites (#1-4) are chosen using the ROI tool, the bean-shaped button highlighted at the right. A final ROI (#5) is selected in a blank region to capture background signal intensity. Areas with non-specific, non-neuronal bright spots are avoided. Scale bar indicates 20 μm. (C) Representation of the signal intensities for ROIs #1-5 from (B) over time as graphed/depicted by confocal software. (D) Visual representations of ROI regions pre/post-stimulation for a neuron that effectively undergoes synaptic transmission (top two panels). The bottom two panels are zoomed images with an unbiased threshold applied to isolate puncta from the background to show distinct regions visually. GFP-containing neurons effectively undergo synaptic release, whereas expression of C9ORF2-ALS related peptide GA50 prevents this effect. Scale bars indicate 10 μm-Reprinted from Jensen et al. 202043. (E) Example of a quantification file using spreadsheet software, showing normalization of values to baseline and generation of ΔF/F values over time. Data are first normalized to the background ROI intensity value at each time point. This value is then compared to the average ROI of interest baseline value for the last 30 s pre-stimulation (shown here as 10 s in 1 s intervals for simplicity) (ΔF). This change is next calculated as a fraction of the baseline fluorescence (ΔF/F). Finally, the starting point value is set to 1 so that increases or decreases can be easily visualized graphically over time. At least five puncta regions per neuron and at least ten neurons per experimental condition are collected, with data such as this combined to generate average measurements at each time point. (F) Data from a spreadsheet file such as in (E) are moved into a graphing and statistics software program. For the graph shown here, the ΔF/F value at each timepoint averaged over all puncta regions of an experimental condition is plotted, along with its standard error of the mean. The graph here is data from the GFP versus GA50 experiments indicated in (D), where the last 10 s of baseline and first 10 s of the stimulation period are shown-reprinted from Jensen et al. 202043. (G) This assay can produce curves for intermediate synaptic release and is not simply an "all or nothing" response. GA50 was co-expressed with exogenous synaptic protein SV2 (synaptic vesicle-associated protein 2), and styryl dye imaging and analysis were performed as indicated in the previous steps. As in Figure 3F, the graph shown here represents the ΔF/F value at each timepoint averaged over all puncta regions of an experimental condition is plotted, along with its standard error of the mean. The graph shows the last minute of baseline and the first minute of the stimulation period from Jensen et al. 202043. Please click here to view a larger version of this figure.
Video 1: Representative video of styryl dye imaging experiment. Shown is a representative video of a cortical neuron loaded with styryl dye. The video shows the period beginning at the time of bath perfusion of high KCl aCSF. The boxed region highlights the neurites, with observable loss of puncta fluorescence over time. Please click here to download this Video.
Following the method described in section 7, shown are representative fluorescence images of cortical neurons transfected with GcaMP6m before and after KCl depolarization (Figure 4A). Raw intensity graphs show increased fluorescence values that fluctuate, indicating calcium entry into neurites following KCl-induced depolarization (Figure 4B). This second representative video shows neurons expressing GcaMP6m with low fluorescence at the end of the recording baseline period to demonstrate this effect. At the start of the stimulation, the neurons display a dramatic fluorescence increase (Video 2). This approach has been successfully utilized to demonstrate calcium-entry alterations in a mutant FUS-ALS model. Astrocytes in this model were transduced with adenovirus containing CMV-promoter-driven eGFP-FUS plasmids. When conditioned medium from mutant FUS-expressing astrocytes was placed on motor neurons, increased calcium influx was observed compared to non-mutant FUS conditions following α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) +cyclothiazide stimulation (Figure 4C)44. The sensitivity of this assay have been tested by performing experiments with and without calcium included in the perfusion medium. In the setting of GFP versus GA50 mentioned above, increased peak calcium transients was observed in GA50 containing motor neurons. Notably, this depended on calcium coming into the cell and not the release of internal calcium stores. When calcium was removed from the stimulating aCSF medium, no calcium transient responses were noted in either experimental condition (Figure 4D)43.
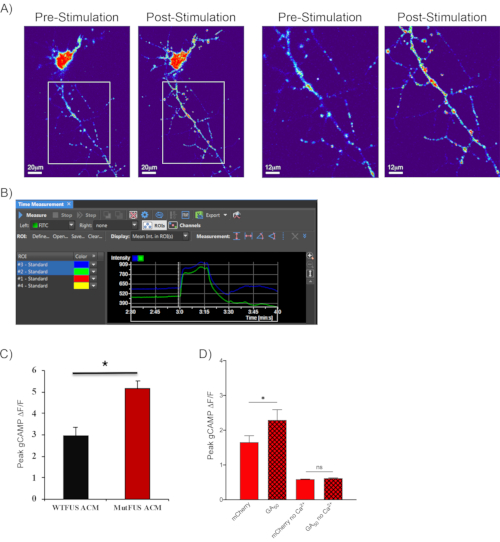
Figure 4: Observing calcium transients in real-time using GCaMP reporters. (A) A 40x image of a GCaMP6m expressing neuron, pseudo-colored to show the intensity of GFP fluorescence (low in blue to high in red). Left-hand panels show the whole 40x field of view pre- and post-stimulation. Scale bar indicates 20 μm. The images to the right are zoomed versions of the boxed areas revealed. Through these images, it is evident by eye that there is a robust increase in focal neuritic calcium levels following stimulation. Scale bar indicates 12 μm. (B) ROI selection and fluorescence intensity monitoring throughout the experiment is performed as in Figure 3. The final 30 s of baseline and 1 min stimulation for two selected ROIs of a neuron undergoing GCaMP6m fluorescence monitoring is shown here. In contrast to styryl dye imaging, the fluorescence intensity increases following neuronal stimulation. Additionally, as is noted here, calcium transients fluctuate in neurites over time; therefore, results are typically represented as the averaged peak normalized fluorescence change value for each ROI region. (C) Representative quantification of such an experiment is shown as was published in Kia-McAvoy et al. 201844, where primary motor neurons which received supernatant derived from mutant FUS-ALS astrocytes displayed significantly increased influx of calcium following AMPA receptor stimulation, compared to neurons receiving supernatant from wild-type FUS expressing astrocytes. (D) Representative quantification of calcium transient imaging where calcium was not included in the stimulating aCSF. Shown are conditions of mCherry versus GA50-mCherry stimulated with high KCl aCSF with or without calcium as published in Jensen et al. 202043. GA50 containing neurons displayed increased peak calcium influx compared with mCherry containing cells. There was no elevation of internal calcium levels in either experimental condition when calcium was removed from stimulating high KCL aCSF. Please click here to view a larger version of this figure.
Video 2: Representative video of GCaMP calcium transient experiment. Shown is a representative video of a field of cortical neurons transfected with GCaMP6m. Following a baseline period, a large calcium influx is noted at the 6 s mark when high KCl aCSF was applied. Calcium entry can be measured either for the whole cell, or at specific neurite regions as described. Please click here to download this Video.
| Potential problems | Possible reasons/solution | |||
| 1 | Non-specific loading | Be precise with the loading time. Longer loading times result in non-specific loading of FM4-64 into endosomes and lysosomes | ||
| 2 | No appreciable exocytosis in control neurons | Check the pH of high KCl aCSF solution. | ||
| 3 | Loss of focus and lateral drift | Make sure that perfect focus is on. Align the images post imaging using Nikon software or ImageJ plugin. | ||
| 4 | Bleaching of FM4-64 fluorescence | Check laser intensity used for imaging. Always try to use the lowest possible intensity. Confirm the laser intensity used does not result in bleaching by running trial runs. | ||
Table 2: Possible problems and troubleshooting for styryl dye experiments. Typical scenarios for issues and general troubleshooting for synaptic unloading experiments.
| Potential problems | Possible reasons/solution | |||
| 1 | Transfection efficiency too low | Check neuronal health. If neurons are hard to transfect one can switch to AAV or lentiviral transduction of GCaMP. | ||
| 2 | Accumulation of GCaMP6 fluorescence in the nucleus | Compromised neuronal health. Check transfection protocol. | ||
| 3 | Loss of focus and lateral Image drift | Make sure that perfect focus is on. Align the images post imaging using Nikon software or ImageJ plugin. | ||
| 4 | No calcium response upon KCL stimulation | Make sure the pH of aCSF is correct. | ||
| 5 | Bleaching of GCaMP6 fluorescence | Check laser intensity used for imaging. Always try to use the lowest possible intensity. Confirm the laser intensity used does not result in bleaching by running trial runs. | ||
| 6 | Blebbing of neurites | Compromised neuronal health due to transfection. Use a fresh batch of neuronal cultures. | ||
Table 3: Possible problems and troubleshooting for imaging calcium transients in neurites. Typical scenarios for issues and general troubleshooting for GCaMP calcium transient experiments.
Discussion
Three steps common to both methods described are of crucial importance for experimental success and quantifiable outcomes. First, preparation of fresh aCSF before each round of experiments is essential, following the attached instructions. Failure to do so may prevent appropriate neuronal depolarization. A sample of untreated control neurons should constantly be tested before stimulation of any experimental groups to ensure proper cellular depolarization and provide a benchmark for positive results obtained in that imaging session. Second, to successfully track fluorescence over time for specific synaptic regions, particular care should also be taken when setting up imaging parameters and areas of ROIs for monitoring. The baseline period recording should immediately transition to the stimulation recording period. A sufficiently rapid frame rate (2 images/s) is required to capture the single-decay dynamics of styryl dye release. Finally, a critical step in the analysis of experimentation is the normalization of fluorescence measurements first to the imaging background and then to the pre-stimulated baseline. As with other imaging protocols, subtraction of a blank imaging region is first performed across all paired time-fluorescence measurements to remove any background autofluorescence that the addition of KCl media may introduce. It is also essential to measure and calculate the average fluorescence reading for each ROI from the last 30 s baseline before stimulation. This average starting value is then used across all time measurements for that ROI as a defined starting point to quantify the "change from baseline" intensity.
Primary neuronal culture is notoriously difficult to transfect with exogenous DNA, and even optimized protocols frequently yield low efficiencies of total cells in culture. 20%-25% of transfection efficiency is commonly achieved in our neuronal cultures, with no evidence of transfection-induced toxicity. While fairly consistent expressions are seen across cells containing GCaMP, subtly differing levels of probe expression within individual cells are considered based on the method of individual region quantification of change in baseline fluorescence compared to that baseline. However, with this relatively low efficiency, using transfection-based methods of introducing the GCaMP reporter is not of sufficient robustness for large-scale biological replicates for high throughput screening studies. To overcome this, several variant constructs are commercially available for lentiviral or adenoviral packaging or cell-lineage specific expression, which will allow for higher transduction efficiencies. A primary modification that may be desired when using these protocols is to use optogenetic stimulation of neurons instead of bath application of KCl62. Transduction with lentiviral constructs designed to express one of the families of now available channelrhodopsins, followed by activation with specific wavelengths of light, allows for spatial control for depolarization of subsets of neurons and also for the evaluation of the effect of trains of action potentials on repetitive calcium influx levels and depletion of synaptic vesicle stores63. However, it is essential to keep in mind that usage of this method is presently somewhat constrained, as the user must ensure that their optogenetic reporter, synaptic reporter, and any additional exogenously introduced proteins do not have spectral overlap between utilized fluorophores. A common troubleshooting issue using the styryl dye is passive intensity reduction during the baseline recording period. Before experimental studies, it is critical to optimize laser intensity and photo capture intervals to minimize photobleaching effects. For experiments to be considered, stabilization of intensity for at least the last 30 s of the baseline period is necessary. Refer to Tables 2 and Table 3 for common problems and their solutions for styryl dye and calcium transient experiments, respectively.
The primary limitation of these two methods is that cultures can only be stimulated and examined in a single instance. As bath application is used to introduce the depolarizing agent KCl, all neurons to be examined from that condition in that dish must be within the initial field of view of the microscope objective. If functionality over time is of interest, paired cultures are required. However, the optogenetic method mentioned above will allow calcium dynamics to be observed over time if desired. Additionally, if neurons have a defect in the recycling of synaptic vesicles, the initial loading of the styryl dye will be impaired. While internal intensity normalization allows for comparison across conditions, slight differences in magnitudes of responses may be difficult to detect. Finally, new iterations of these reporters have rapidly become available and can be switched out for faster detection times or more robust results. For example, GCaMP6m is no longer considered the quickest reporter of calcium transients at synaptic terminals. Instead, one can utilize the latest generation jGCaMP864.
The methods described here are a fast and reliable way to determine whether genetic or pharmacological manipulation of neurons perturbs functional synaptic signaling and release. The detailed experiments are less technically challenging and less costly than traditional voltage/current clamp electrophysiology and electron microscopy. Additionally, while electrophysiology is by nature low throughput and on the individual cell level, the protocols here described are higher throughput and rapid, allowing several neurons to be imaged simultaneously and many iterations to be performed in a single imaging session. It is proposed that these calcium dynamics and synaptic release paradigms be used as the first indicator of synaptic transmission alterations in ALS models and the study of other forms of neuronal degeneration. Although the conclusions derived from Gcamp6m and styryl dye imaging cannot precisely tease out a specific mechanism of synaptic dysfunction, based on such findings, researchers can then undertake targeted, evidence-based complementary studies using traditional methods to determine channels involved, synaptic vesicle number, or quantal content.
The utility in these protocols is the rapid and straightforward assessment of alterations in proper depolarization-mediated calcium influx and/or synaptic vesicle fusion in specific ALS models. We have recently demonstrated this in a publication showing increased calcium influx and abrogation of synaptic unloading in cortical neurons expressing the dipeptide protein (glycine-alanine)50 resulting from the C9ORF72 hexanucleotide repeat expansion43. As shown in our published work, these methods can be performed easily in tandem with co-transfection of mutant RNAs or proteins of interest or in cells cultured directly from transgenic animal models43. Additionally, the reliability and robustness of these protocols have successfully tested in neuronally-differentiated human induced pluripotent stem cells45, enabling future studies to be done directly in patient-derived disease-relevant cells. In another recent manuscript, we provide evidence that wild-type motor neurons undergo high calcium influx following stimulation when in the presence of a soluble factor released from mutant FUS expressing astrocytes44. Astrocytic calcium signaling events are also deeply intertwined with the synaptic transmission in neighboring neurons65. Calcium dynamics protocol have been applied to investigate whole-cell calcium transients in models of astrocytic culture, which has proven effective in both rodents primary and human IPS-derived cells. Further understanding of astrocytic calcium dynamics and signaling in ALS models will provide valuable insight into how synaptic transmission maybe consequently affected. Ultimately, employing cell-type-specific GCaMP reporters will also be a powerful tool for investigating neuronal and astrocytic calcium dynamics in mixed co-culture settings and specifically evaluating non-cell-autonomous effects originating from each cell population.
Finally, the potential usage of these methods extends not just beyond the study of ALS, but also to the broader fields of neurodegenerative and even developmental neuroscience. Detailed information about the specific plasmids used to generate the representative results is provided and successful at transfecting plasmids containing many different genetic variants of FUS, SOD1 and C9ORF72 hexanucleotide repeats and dipeptides43,44,66,67,68. Provided that plasmids contain a promoter used in neurons, there is no limitation on what models of ALS or degenerative disease could be studied using these methods. Furthermore, transfection to express mutant proteins is an entirely optional step. Cultures generated from transgenic animals or human patient-derived cells eliminate the need to identify cells containing the mutant protein of interest. Presently, these systems are constrained in the fashion that if styryl dye will be used, the TRITC channel is occupied, and if GcaMP6 is used, the FITC channel is occupied. Techniques that allow for the examination of neuronal and astrocytic activity in real-time broaden the possibilities for understanding time-course analysis for synaptic maturation/degeneration, as well as rapid tests for the efficacy of pharmacological compounds in promoting or suppressing neuronal communication. These two methods are amenable to high throughput scaling for screening. Rather than plating neurons in individual 35 mm dishes, the imaging parameters of these two protocols could be employed in an automated fashion across 96-well plates. Using such a platform, compound libraries can be quickly tested for therapeutics, which modulate calcium entry or improve synaptic release using a genetic model of disease or even cells derived directly from patients. This method could fast-track the possibility of subgroup-specific or even personalized therapeutics by rapidly identifying candidate molecules for further investigation.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We would like to acknowledge the present and former members of the Jefferson Weinberg ALS Center for critical feedback and suggestions for optimizing these techniques and their analyses. This work was supported by funding from the NIH (RF1-AG057882-01 and R21-NS0103118 to D.T), the NINDS (R56-NS092572 and R01-NS109150 to P.P), the Muscular Dystrophy Association (D.T.), the Robert Packard Center for ALS Research (D.T.), the Family Strong 4 ALS foundation and the Farber Family Foundation (B.K.J., K.K, and P.P).
Materials
| 20x air objective | Nikon | For imaging | |
| 40x oil immersion objective | Nikon | For imaging | |
| B27 supplement | Thermo Scientific | 17504044 | Neuronal growth supplement |
| BD Syringes without Needle, 50 mL | Thermo Scientific | 13-689-8 | Part of gravity perfusion assembly |
| Biosafety cell culture hood | Baker | SterilGARD III SG403A | Asceptic cell culturing, transfection, and dye loading |
| b-Mercaptoethanol | Millipore Sigma | M3148 | For culturing and maintenance of neuronal cultures |
| Bovine Serum Albumin | Millipore Sigma | A9418 | For preparing neuronal cultures |
| Calcium chloride dihydrate | Millipore Sigma | 223506 | Component of aCSF solutions |
| Cell culture CO2 incubator | Thermo Scientific | 13-998-123 | For culturing and maintenance of neurons |
| Centrifuge | Eppendorf | 5810R | For neuronal culture preparation |
| Confocal microscope | Nikon | Eclipse Ti +A1R core | For fluorescence imaging |
| CoolSNAP ES2 CCD camera | Photometrics | For image acquisition | |
| D-Glucose | Millipore Sigma | G8270 | Component of aCSF solutions |
| DNase | Millipore Sigma | D5025 | For neuronal culture preparation |
| Female, timed-pregnancy Sprague Dawley rats | Charles river | 400SASSD | For preparing embryonic cortical and spinal motor neuron cultures |
| FITC Filter cube | Nikon | 77032509 | For imaging Gcamp calcium transients |
| FM4-64 styryl dye | Invitrogen | T13320 | For imaging synaptic vesicle release |
| Glass bottom petri dishes (Thickness #1.5) | CellVis | D35-10-1.5-N | For growth of neurons on imaging-compatible culture dish |
| Glass Pasteur pipette | Grainger | 52NK56 | For preparing neuronal cultures |
| Hank's Balanced Salt Solution (HBSS) | Millipore Sigma | H6648 | For preparing neuronal cultures |
| HEPES | Millipore Sigma | H3375 | Component of aCSF solutions |
| High KCl artifical cerebrospinal fluid (aCSF) | For imaging. Please see recipes* | ||
| horse serum | Millipore Sigma | H1138 | For culturing and maintenance of neurons |
| Laminar flow dissection hood | NUAIRE | NU-301-630 | For preparing neuronal cultures |
| Laminin | Thermo Scientific | 23017015 | For preparing neuronal cultures |
| Leibovitz's L-15 Medium | Thermo Scientific | 11415064 | For preparing neuronal cultures |
| Leibovitz's L-15 Medium, no phenol red | Thermo Scientific | 21083027 | For preparing neuronal cultures |
| L-Glutamine (200 mM) | Thermo Scientific | 25030149 | Neuronal culture supplement |
| Lipofectamine 2000 Transfection Reagent | Thermo Scientific | 11668019 | For neuronal transfections |
| Low KCl artifical cerebrospinal fluid (aCSF) | For imaging. Please see recipes* | ||
| Magnesium chloride | Millipore Sigma | 208337 | Component of aCSF solutions |
| Microsoft Excel | Microsoft | Software for data analysis/normalization | |
| Nalgene Filter Units, 0.2 µm PES | Thermo Scientific | 565-0020 | Filter unit for aCSF solution |
| Neurobasal medium | Thermo Scientific | 21103049 | For culturing and maintenance of neuronal cultures |
| NIS-Elements Advanced Research | Nikon | Software for image capture and analysis | |
| Nunc 15 mL Conical tubes | Thermo Scientific | 339650 | For preparing neuronal culture and buffer solutions |
| Nunc 50 mL conical tubes | Thermo Scientific | 339652 | For preparing neuronal culture and buffer solutions |
| Optiprep | Millipore Sigma | D1556 | For preparing neuronal cultures |
| Papain | Millipore Sigma | P4762 | For preparing neuronal cultures |
| Penicillin-Streptomycin (10,000 U/mL) | Thermo Scientific | 15140122 | To prevent bacterial contamination of neuronal cultures |
| Perfusion system | Warner Instruments | SF-77B | For exchange of aCSF |
| Perfusion tubing | Cole-Parmer | UX-30526-14 | Part of gravity perfusion assembly |
| pGP-CMV-Gcamp6m plasmid | Addgene | 40754 | For imaging calcium transients |
| Poly-D-lysine hydrobromide | Millipore Sigma | P7886 | Coating agent for glass bottom petri dishes |
| Potassium chloride | Millipore Sigma | P3911 | Component of aCSF solutions |
| Sodium bicarbonate | Millipore Sigma | S5761 | Component of aCSF solutions |
| Sodium Chloride | Millipore Sigma | S9888 | Component of aCSF solutions |
| Stage Top Incubator | Tokai Hit | For incubation of live neurons during imaging period | |
| TRITC Filter cube | Nikon | 77032809 | For imaging FM4-64 |
| Trypsin Inhibitor | Millipore Sigma | T6414 | For preparing neuronal cultures |
| Trypsin-EDTA (0.25%), phenol red | Thermo Scientific | 25200056 | For preparing neuronal cultures |
| Vibration Isolation table | New Port | VIP320X2430-135520 | Table/stand for microscope |
References
- Gibson, S. B., et al. The evolving genetic risk for sporadic ALS. Neurology. 89 (3), 226-233 (2017).
- Kim, G., Gautier, O., Tassoni-Tsuchida, E., Ma, X. R., Gitler, A. D. ALS genetics: Gains, losses, and implications for future therapies. Neuron. 108 (5), 822-842 (2020).
- Nijssen, J., Comley, L. H., Hedlund, E. Motor neuron vulnerability and resistance in amyotrophic lateral sclerosis. Acta Neuropathologica. 133 (6), 863-885 (2017).
- Marttinen, M., Kurkinen, K. M., Soininen, H., Haapasalo, A., Hiltunen, M. Synaptic dysfunction and septin protein family members in neurodegenerative diseases. Molecular Neurodegeneration. 10, 16 (2015).
- Bae, J. S., Simon, N. G., Menon, P., Vucic, S., Kiernan, M. C. The puzzling case of hyperexcitability in amyotrophic lateral sclerosis. Journal of Clinical Neurology. 9 (2), 65-74 (2013).
- Kiernan, M. C. Hyperexcitability, persistent Na+ conductances and neurodegeneration in amyotrophic lateral sclerosis. Experimental Neurology. 218 (1), 1-4 (2009).
- Krarup, C. Lower motor neuron involvement examined by quantitative electromyography in amyotrophic lateral sclerosis. Clinical Neurophysiology. 122 (2), 414-422 (2011).
- Vucic, S., Nicholson, G. A., Kiernan, M. C. Cortical hyperexcitability may precede the onset of familial amyotrophic lateral sclerosis. Brain. 131, 1540-1550 (2008).
- Marchand-Pauvert, V., et al. Absence of hyperexcitability of spinal motoneurons in patients with amyotrophic lateral sclerosis. Journal of Physiology. 597 (22), 5445-5467 (2019).
- Fischer, L. R., et al. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Experimental Neurology. 185 (2), 232-240 (2004).
- Markert, S. M., et al. Overexpression of an ALS-associated FUS mutation in C. elegans disrupts NMJ morphology and leads to defective neuromuscular transmission. Biology Open. 9 (12), (2020).
- Shahidullah, M., et al. Defects in synapse structure and function precede motor neuron degeneration in Drosophila models of FUS-related ALS. Journal of Neuroscience. 33 (50), 19590-19598 (2013).
- Liu, Y., et al. C9orf72 BAC Mouse Model with Motor Deficits and Neurodegenerative Features of ALS/FTD. Neuron. 90 (3), 521-534 (2016).
- Freibaum, B. D., et al. GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature. 525 (7567), 129-133 (2015).
- Zhang, K., et al. The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature. 525 (7567), 56-61 (2015).
- Perry, S., Han, Y., Das, A., Dickman, D. Homeostatic plasticity can be induced and expressed to restore synaptic strength at neuromuscular junctions undergoing ALS-related degeneration. Human Molecular Genetics. 26 (21), 4153-4167 (2017).
- Romano, G., et al. Chronological requirements of TDP-43 function in synaptic organization and locomotive control. Neurobiology of Disease. 71, 95-109 (2014).
- Armstrong, G. A., Drapeau, P. Calcium channel agonists protect against neuromuscular dysfunction in a genetic model of TDP-43 mutation in ALS. Journal of Neuroscience. 33 (4), 1741-1752 (2013).
- Diaper, D. C., et al. Loss and gain of Drosophila TDP-43 impair synaptic efficacy and motor control leading to age-related neurodegeneration by loss-of-function phenotypes. Human Molecular Genetics. 22 (8), 1539-1557 (2013).
- Schwiening, C. J. A brief historical perspective: Hodgkin and Huxley. Journal of Physiology. 590 (11), 2571-2575 (2012).
- Dong, H., et al. Curcumin abolishes mutant TDP-43 induced excitability in a motoneuron-like cellular model of ALS. Neurosciences. 272, 141-153 (2014).
- Chand, K. K., et al. Defects in synaptic transmission at the neuromuscular junction precede motor deficits in a TDP-43(Q331K) transgenic mouse model of amyotrophic lateral sclerosis. Federation of American Societies for Experimental Biology Journal. 32 (5), 2676-2689 (2018).
- Perkins, E. M., et al. Altered network properties in C9ORF72 repeat expansion cortical neurons are due to synaptic dysfunction. Molecular Neurodegeneration. 16 (1), 13 (2021).
- Ceccarelli, B., Hurlbut, W. P. Vesicle hypothesis of the release of quanta of acetylcholine. Physiological Reviews. 60 (2), 396-441 (1980).
- Ettinger, A., Wittmann, T. Fluorescence live cell imaging. Methods in Cell Biology. 123, 77-94 (2014).
- Ryan, J., Gerhold, A. R., Boudreau, V., Smith, L., Maddox, P. S. Introduction to Modern Methods in Light Microscopy. Methods in Molecular Biology. 1563, 1-15 (2017).
- Wang, L., Frei, M. S., Salim, A., Johnsson, K. Small-molecule fluorescent probes for live-cell super-resolution microscopy. Journal of the American Chemical Society. 141 (7), 2770-2781 (2019).
- Neher, E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 20 (3), 389-399 (1998).
- Dolphin, A. C., Lee, A. Presynaptic calcium channels: specialized control of synaptic neurotransmitter release. Nature Reviews Neuroscience. 21 (4), 213-229 (2020).
- Tsien, R. Y., Rink, T. J., Poenie, M. Measurement of cytosolic free Ca2+ in individual small cells using fluorescence microscopy with dual excitation wavelengths. Cell Calcium. 6 (1-2), 145-157 (1985).
- Takahashi, N., et al. Cytosolic Ca2+ dynamics in hamster ascending thin limb of Henle’s loop. American Journal of Physiology. 268 (6), 1148-1153 (1995).
- Cleemann, L., DiMassa, G., Morad, M. Ca2+ sparks within 200 nm of the sarcolemma of rat ventricular cells: evidence from total internal reflection fluorescence microscopy. Advances in Experimental Medicine and Biology. 430, 57-65 (1997).
- Roe, M. W., Lemasters, J. J., Herman, B. Assessment of Fura-2 for measurements of cytosolic free calcium. Cell Calcium. 11 (2-3), 63-73 (1990).
- Lin, M. Z., Schnitzer, M. J. Genetically encoded indicators of neuronal activity. Nature Neuroscience. 19 (9), 1142-1153 (2016).
- Tian, L., et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nature Methods. 6 (12), 875-881 (2009).
- Nakai, J., Ohkura, M., Imoto, K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nature Biotechnology. 19 (2), 137-141 (2001).
- Chen, T. W., et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 499 (7458), 295-300 (2013).
- Horikawa, K. Recent progress in the development of genetically encoded Ca2+ indicators. Journal of Medical Investigation. 62 (1-2), 24-28 (2015).
- Bohme, M. A., Grasskamp, A. T., Walter, A. M. Regulation of synaptic release-site Ca(2+) channel coupling as a mechanism to control release probability and short-term plasticity. Federation of European Biochemical Society Letters. 592 (21), 3516-3531 (2018).
- Li, Y. C., Kavalali, E. T. Synaptic vesicle-recycling machinery components as potential therapeutic targets. Pharmacological Reviews. 69 (2), 141-160 (2017).
- Gaffield, M. A., Betz, W. J. Imaging synaptic vesicle exocytosis and endocytosis with FM dyes. Nature Protocols. 1 (6), 2916-2921 (2006).
- Verstreken, P., Ohyama, T., Bellen, H. J. FM 1-43 labeling of synaptic vesicle pools at the Drosophila neuromuscular junction. Methods in Molecular Biology. 440, 349-369 (2008).
- Jensen, B. K., et al. Synaptic dysfunction induced by glycine-alanine dipeptides in C9orf72-ALS/FTD is rescued by SV2 replenishment. European Molecular Biology Organization Molecular Medicine. 12 (5), 10722 (2020).
- Kia, A., McAvoy, K., Krishnamurthy, K., Trotti, D., Pasinelli, P. Astrocytes expressing ALS-linked mutant FUS induce motor neuron death through release of tumor necrosis factor-alpha. Glia. 66 (5), 1016-1033 (2018).
- Fernandopulle, M. S., et al. Transcription Factor-Mediated Differentiation of Human iPSCs into Neurons. Current Protocols in Cell Biology. 79 (1), 51 (2018).
- Ye, L., Haroon, M. A., Salinas, A., Paukert, M. Comparison of GCaMP3 and GCaMP6f for studying astrocyte Ca2+ dynamics in the awake mouse brain. Public Library of Science One. 12 (7), 0181113 (2017).
- Angleson, J. K., Betz, W. J. Monitoring secretion in real time: capacitance, amperometry and fluorescence compared. Trends in Neuroscience. 20 (7), 281-287 (1997).
- Ryan, T. A., et al. The kinetics of synaptic vesicle recycling measured at single presynaptic boutons. Neuron. 11 (4), 713-724 (1993).
- Kraszewski, K., et al. Synaptic vesicle dynamics in living cultured hippocampal neurons visualized with CY3-conjugated antibodies directed against the lumenal domain of synaptotagmin. Journal of Neuroscience. 15 (6), 4328-4342 (1995).
- Betz, W. J., Mao, F., Bewick, G. S. Activity-dependent fluorescent staining and destaining of living vertebrate motor nerve terminals. Journal of Neuroscience. 12 (2), 363-375 (1992).
- Betz, W. J., Bewick, G. S. Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science. 255 (5041), 200-203 (1992).
- Ryan, T. A., Smith, S. J. Vesicle pool mobilization during action potential firing at hippocampal synapses. Neuron. 14 (5), 983-989 (1995).
- Betz, W. J., Ridge, R. M., Bewick, G. S. Comparison of FM1-43 staining patterns and electrophysiological measures of transmitter release at the frog neuromuscular junction. Journal of Physiology-Paris. 87 (3), 193-202 (1993).
- Wu, L. G., Betz, W. J. Nerve activity but not intracellular calcium determines the time course of endocytosis at the frog neuromuscular junction. Neuron. 17 (4), 769-779 (1996).
- Ryan, T. A., Smith, S. J., Reuter, H. The timing of synaptic vesicle endocytosis. Proceedings of the National Academy of Sciences of the United States of America. 93 (11), 5567-5571 (1996).
- Ramaswami, M., Krishnan, K. S., Kelly, R. B. Intermediates in synaptic vesicle recycling revealed by optical imaging of Drosophila neuromuscular junctions. Neuron. 13 (2), 363-375 (1994).
- Kayser, M. S., McClelland, A. C., Hughes, E. G., Dalva, M. B. Intracellular and trans-synaptic regulation of glutamatergic synaptogenesis by EphB receptors. Journal of Neuroscience. 26 (47), 12152-12164 (2006).
- Washburn, H. R., Xia, N. L., Zhou, W., Mao, Y. T., Dalva, M. B. Positive surface charge of GluN1 N-terminus mediates the direct interaction with EphB2 and NMDAR mobility. Nature Communications. 11 (1), 570 (2020).
- Magrane, J., Sahawneh, M. A., Przedborski, S., Estevez, A. G., Manfredi, G. Mitochondrial dynamics and bioenergetic dysfunction is associated with synaptic alterations in mutant SOD1 motor neurons. Journal of Neuroscience. 32 (1), 229-242 (2012).
- Casci, I., et al. Muscleblind acts as a modifier of FUS toxicity by modulating stress granule dynamics and SMN localization. Nature Communications. 10 (1), 5583 (2019).
- Hruska, M., Henderson, N., Le Marchand, S. J., Jafri, H., Dalva, M. B. Synaptic nanomodules underlie the organization and plasticity of spine synapses. Nature Neuroscience. 21 (5), 671-682 (2018).
- Rein, M. L., Deussing, J. M. The optogenetic (r)evolution. Molecular Genetics and Genomics. 287 (2), 95-109 (2012).
- Bertucci, C., Koppes, R., Dumont, C., Koppes, A. Neural responses to electrical stimulation in 2D and 3D in vitro environments. Brain Research Bulletin. 152, 265-284 (2019).
- Zhang, Y., et al. jGCaMP8 Fast genetically encoded calcium indicators. Janelia Research Campus. , (2020).
- Guerra-Gomes, S., Sousa, N., Pinto, L., Oliveira, J. F. Functional roles of astrocyte calcium elevations: From synapses to behavior. Frontiers in Cellular Neuroscience. 11, 427 (2017).
- Westergard, T., et al. Cell-to-cell transmission of dipeptide repeat proteins linked to C9orf72-ALS/FTD. Cell Reports. 17 (3), 645-652 (2016).
- Wen, X., et al. Antisense proline-arginine RAN dipeptides linked to C9ORF72-ALS/FTD form toxic nuclear aggregates that initiate in vitro and in vivo neuronal death. Neuron. 84 (6), 1213-1225 (2014).
- Daigle, J. G., et al. Pur-alpha regulates cytoplasmic stress granule dynamics and ameliorates FUS toxicity. Acta Neuropathologica. 131 (4), 605-620 (2016).

