Primed Mycobacterial Uveitis (PMU) as a Model for Post-Infectious Uveitis
Summary
This protocol outlines the steps for inducing Primed Mycobacterial Uveitis (PMU) in mice. This method outlines the steps to help produce reliable and robust ocular inflammation in the mouse model system. Using this protocol, we generated uveitic eyes and uninflamed fellow eyes from single animals for further evaluation with immunologic, transcriptomic, and proteomic assays.
Abstract
The term ‘uveitis’ describes a heterogeneous set of conditions that all feature intraocular inflammation. Broadly, uveitis is defined by etiology: infection or autoimmunity. Infectious uveitis requires treatment with the appropriate antimicrobial agents, while autoimmune uveitis requires treatment with corticosteroids or other immunosuppressive agents. Post-infectious uveitis is a form of chronic uveitis that requires corticosteroids to control immune sequela following the initial infection. Uveitis associated with Mycobacterium tuberculosis (Mtb) infection is a well-recognized form of post-infectious uveitis, but the mechanisms of disease are not fully understood. To understand the role mycobacterial antigens and innate ligands play in stimulating chronic ocular inflammation following mTB infection, the model Primed Mycobacterial Uveitis (PMU) was developed for use in mice. This manuscript outlines the methods for generating PMU and monitoring the clinical course of inflammation using color fundus and optical coherence tomography (OCT) imaging. PMU is induced by immunization with heat-killed mycobacterial extract followed by intravitreal injection of the same extract into one eye seven days later. Ocular inflammation is monitored longitudinally using in vivo imaging and followed by sample collection for a wide range of assays, including histology, flow cytometry, cytokine analysis, qPCR, or mRNA sequencing. The mouse model of PMU is a useful new tool for studying the ocular responses to mTB, the mechanism of chronic uveitis, and for preclinical effectiveness tests of new anti-inflammatory therapies.
Introduction
The term 'uveitis' describes a heterogeneous set of conditions that all feature intraocular inflammation1. Animal models of uveitis are important for understanding disease mechanisms and for preclinical testing of new therapies. A number of animal models of uveitis have been established2. The two that have been studied most extensively are experimental autoimmune uveitis (or uveoretinitis; EAU) and endotoxin-induced uveitis (EIU). EAU is typically generated by immunization with ocular antigens or can occur spontaneously when central tolerance is disrupted in the absence of the AIRE gene3,4. Other variants of the model have since been developed5,6,7 to include different uveitogenic peptides; these have been reviewed extensively8,9,10. EAU is the primary model for forms of T cell-dependent autoimmune uveitis such as Vogt-Koyanagi-Harada disease and birdshot chorioretinitis in humans. EIU is generated by systemic or local injection of bacterial lipopolysaccharide (LPS)10,11. EIU has been used as a model of acute uveitis generated by activation of innate immune signaling pathways12. Both models have been instrumental to the current understanding of ocular immunology, but neither are effective models for post-infectious chronic uveitis. Recently established in mice, the Primed Mycobacterial Uveitis (PMU) model now provides an approach to interrogate and evaluate clinical and cellular aspects of this form of uveitis13.
There is a high prevalence of mycobacterial infection worldwide, with over 10 million new cases and more than 1.4 million deaths reported by the World Health Organization in 201914. Extrapulmonary manifestation of active tuberculosis (TB) infection includes uveitis and is a well-recognized cause of infectious uveitis15,16. The manifestations of TB-associated uveitis are protean, which likely reflects multiple distinct mechanisms of disease to include direct ocular infection as well as less well-understood immune-mediated inflammation17,18,19. The proposed mechanisms for these post-infectious sequelae include a chronic inflammatory response stimulated by the persistence of a pauci-bacillary infection in the retinal pigment epithelium (RPE), a chronic inflammatory response stimulated by the presence of residual pathogen-associated molecular patterns (PAMPs) from a successfully cleared ocular infection, and inappropriate activation of the adaptive immune response against ocular antigens through a process of molecular mimicry or antigen spread caused by systemic TB infection20,21,22,23.
In order to gain a better mechanistic understanding of chronic post-infectious uveitis and study the role of mycobacterial antigens in the initiation of disease, the PMU model was developed for use in mice13,24. Accordingly, to elicit inflammation, the mouse first receives a subcutaneous injection of antigen from the heat-killed Mycobacterium tuberculosis H37Ra strain to mimic systemic infection, followed seven days later by intravitreal injection of the same antigen administered to the left or right eye to mimic local ocular infection. The intensity and duration of the ensuing uveitis are monitored by longitudinal in vivo Optical Coherence Tomography (OCT) and fundal imaging of the eye25. PMU is characterized by an acute, myeloid-dominant panuveitis that develops into a chronic T cell-dominant posterior uveitis with vitritis, perivascular retinal inflammation, and focal areas of outer retinal damage26. The presence of granulomatous inflammation in the posterior segment of the eye suggests that the PMU model can be used to study some forms of anterior (granulomatous and non-granulomatous) and intermediate uveitis, seen in patients with immunological evidence of past Mtb infection27. Additionally, the components of heat-killed Mtb used in the PMU model have been suggested to trigger immune responses underlying the aspects of recurrent uveitis in patients with ocular tuberculosis who respond to anti-tubercular therapy (ATT)28. Due to the differences in disease initiation and inflammatory course when compared to EAU and EIU, PMU represents a new animal model of uveitis that is not dependent on immunization with ocular antigens and may help elucidate mechanisms of disease in patients with chronic uveitis. This protocol outlines the methods for generating PMU, monitoring the clinical course of inflammation, and collecting ocular samples for post-mortem analysis with flow cytometry.
Protocol
All procedures performed were approved locally by the Animal Care and Use Committee at the University of Washington (animal study protocol # 4481-02) or in concordance with the United Kingdom Home Office license (PPL 30/3281) and the University of Bristol Ethical Review Group. Experiments conducted at both institutions were concordant with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Visual Research. PMU was generated in 6-10-week-old C57BL/6J mice; all mice weighed at least 18 g at the time of uveitis induction and were confirmed negative for the rd8 mutation of the Crb1 gene29. Mice were maintained with standard chow and medicated water (acetaminophen 200-300 mg/kg/day) ad libitum under specific pathogen-free conditions. Animal euthanasia was performed using a standard carbon dioxide inhalation method30.
1. Antigen preparation for subcutaneous injection
- Perform all procedures in this section inside a chemical fume hood to prevent inhalation or skin contact with the Mtb H37Ra powder. Handle according to your institutional policies for Complete Freund's Adjuvant (typically BSL-1). This includes using chemical-resistant gloves, safety glasses, and protective work clothing (lab coat).
- Use a good sterile technique to prevent contamination of reagents that will be introduced into experimental animals.
- Make the Mtb suspension in PBS by mixing 5 mg of lyophilized, heat-killed M. tuberculosis H37Ra powder with 2.5 mL of cold PBS in a 5 mL microcentrifuge tube. Vortex once for 30 s and then place on ice.
- To generate a fine suspension of the H37Ra in PBS, sonicate the suspension on ice for 5 min.
- Unclamp the body of the converter unit and clean the probe with a 70% (v/v) alcohol swab.
- Switch on the sonicator, adjust the power setting to 4 by turning the power control knob and immerse the probe's tip into the PBS-containing mycobacterial powder. Ensure that the probe tip is immersed to at least half the depth of the sample and that the probe tip is not touching the wall of the microcentrifuge tube.
- Sonicate the mixture on ice for 30 s, pause 30 s and repeat for a total of 5 min to fully disperse the powder into an even suspension without heating the liquid.
- Add 2.5 mL of Freund's Incomplete Adjuvant to the mixture and repeat the sonication process on ice until the emulsion forms a toothpaste-like consistency.
- Set the power to 0 using the control knob and turn off the unit to end the sonication. Remove the tip from the suspension and wipe the probe with an alcohol swab.
- Store the antigen emulsion at 4 °C. Making batches of the emulsion will help ensure consistency across experiments. The emulsion can be stored at 4 °C for up to 3 months.
2. Subcutaneous injection
- Perform subcutaneous injection a week prior to the intravitreal injection (designated as day -7).
- Load a 1 mL syringe (no needle attached) with the mycobacterial emulsion. Due to the viscosity and opacity of the emulsion, difficult-to-see air bubbles can fill the syringe.
- To prevent airbubbles in the syringe, after loading 0.2-0.3 mL of emulsion, invert the syringe (tip facing up) and gently tap the syringe repeatedly on the edge of a counter to bring the bubbles to the surface.
- Expel the air from the syringe and continue filling the syringe. Invert and intermittently tap until filled.
- Place a 25 G needle on the syringe and advance the emulsion to fill the needle. Store the syringe on ice until used.
- To perform the subcutaneous injection safely, either anesthetize the mouse or utilize humane restraint methods that allow easy access to the animal hindquarters31.
- To anesthetize for subcutaneous injection, place the animal in an isoflurane induction chamber (3%-4% for induction and 1%-3% for maintenance). Once anesthetized, ensure that the mouse has a slow respiratory rate and exhibits no signs of respiratory distress.
- Place the subcutaneous injections on either the dorsal surface of the hips, or on the ventral surface of the legs proximal to the region of the inguinal lymph nodes.
- Carefully insert the needle to prevent injecting into the muscle. Inject 0.05 mL of the Mtb emulsion into the subcutaneous space. Do not remove the needle immediately in order to allow the thick emulsion to be fully injected.
- Repeat the injection on both left and right sides for a total of 0.1 mL per animal.
- If anesthetized, place the mouse on a warm heating pad until complete recovery. Do not leave the mouse unattended until it has regained sufficient consciousness to maintain sternal recumbency.
- Return the mouse to its cage upon complete recovery and label the cage card with the date of subcutaneous injection.
- Provide analgesia with oral acetaminophen (200 mg/kg/day), but not NSAIDs as anti-inflammatory agents can impact the induction of uveitis.
3. Antigen stock preparation for intravitreal injection
- Perform all procedures in this section under appropriate sterile conditions to prevent contamination of the intravitreal Mtb suspension.
- Make the intravitreal suspensions.
- For induction of mild to moderate panuveitis, make the intravitreal suspension at a 5 mg/mL concentration by adding 5 mg of the mycobacteria extract to 1 mL of 1x PBS.
- For induction of moderate to severe panuveitis, make the intravitreal suspension at a 10 mg/mL concentration by adding 10 mg of the mycobacteria extract to 1 mL of 1x PBS.
- Vortex once for 30 s and then place on ice.
- To generate a fine suspension of the H37Ra in PBS, sonicate the suspension on ice for 10 min as described in step 1.4. Aliquot this stock solution in 100 µL volumes and store at -20 °C.
- Prior to use, thaw at room temperature and vortex on high for 1 min. Keep the aliquots on ice while transport to the animal facility.
4. Intravitreal injection procedure on day 0
- Animal preparation
- Wear fitted examination gloves, place the mouse on a weighing balance to obtain its weight in grams.
- Give an intraperitoneal injection of 0.02 mL/g bodyweight of a solution containing 100 mg/mL Ketamine and 20 mg/mL Xylazine mixed with sterile water to anesthetize the animal. An alternative approach includes induction using ~1.5% isoflurane (inhaled).
- Wait approximately 2 min for the mouse to fall asleep, and then place the mouse in a warming box and cover the lid. Perform pain reflex tests like ear, toe, and tail pinch to assess the depth of anesthesia for the procedure32.
- Once asleep, anesthetize the cornea with 1 drop of 0.5% (v/v) tetracaine. Avoid getting tetracaine near the nose or mouth of the mouse. After 10 s, dab off the excess liquid.
NOTE: It has been observed that iris dilation and anterior chamber (AC) visualization are improved when topical anesthesia is administered, possibly due to improved corneal reflex suppression with the combined systemic and topical anesthesia. However, this step could be omitted if desired. - Dilate the pupil with 1 drop of 2.5% (v/v) phenylephrine. Use caution to avoid any excess droplets that might enter the nose or mouth. After 2-3 min, dab off the excess liquid.
- To decrease the risk of endophthalmitis, add 1 drop of 5% betadine to the eye surface and surrounding hair. Leave on the eye for 2-3 min.
NOTE: Perform all procedures in this section under appropriate sterile conditions to prevent endophthalmitis. - Remove betadine and cover the eye with hypromellose (0.3%) or carbomer eye gel 0.2% w/w) to prevent dryness under anesthesia. This will also help prevent cataract formation.
- Setting up the microinjection system
- Perform the intravitreal injection using a micropump connected to a microsyringe pump controller and an injection syringe (Figure 1A-C). Alternatively, inject with a 33 G needle attached to a Hamilton syringe as described in subsection 4.4.
- Connect a 34 G needle to the injection holder to assemble the injector. Loosen the silver screw cap at the front end of the injection holder and slide the needle into the body of the holder about halfway. Tighten the silver screw cap finger tight.
- Connect the tubing to the injection holder as mentioned in steps 4.2.4-4.2.5.
- To insert the tubing on the injection holder, loosen the plastic screw on the back end of the holder, slide the tubing through the gasket inside and tighten the screw.
- Maintain a slight gap with the end of the tubing to prevent tubing damage during the injection. Refer to Figure 1C.
- Thaw a 100 µL aliquot of the mycobacterium stock suspension.
- Add 3 µL of a 1% fluorescein sodium (AK-Fluor) solution and vortex well.
- Load a 10 µL syringe with the antigen and fluorescein mix without including any air bubbles.
- Remove the loading needle from the syringe, and slide the tubing through the silver screw cap gasket until the tip reaches the zero mark in the syringe body.
- Once the tip of the tubing is correctly aligned to the desired position, tighten the screw cap finger tight.
- Flush the solution in the syringe through the injection tubing to fully load the system. Then repeat steps 4.2.8-4.2.11 to reload the syringe for injection.
- To install the loaded syringe on the micropump, press the clamp release button at the end of the micro-pump to open the syringe clamps.
- Position the cap of the plunger into the plunger cap holder at the rear end of the micropump.
- Then slide the syringe collar onto the collar stop and the syringe body into the syringe clamp.
- Release the clamp button and tighten the plunger retaining screw. Refer to Figure 1D.
- Slide the injection holder and the needle through the o-clamp on the stereotactic injection apparatus. This is a custom platform; alternatively, the syringe can be held and positioned manually.
- Set the infusion volume and the rate of infusion volume on the microsyringe pump controller to inject 500 nL per cycle at a rate of 40 nL/s, respectively.
NOTE: Faster injection rates can be used, however, more reflux may be experienced before needle repositioning can be achieved. - Test the system to ensure correct functioning prior to performing an intravitreal injection.
NOTE: When the injection system is functioning correctly, activation of an injection cycle using the foot pedal or the control pad will produce visible movement of the plunger cap holder and a small droplet of greenish liquid will be seen at the tip of the needle. In the case that no liquid is produced, activate additional cycles or flush and reload the syringe. - Prior to injecting the eye, gently wipe the needle with a 95% ethanol pad.
- Intravitreal injection procedure
- The mouse is placed on a stereotaxic apparatus to perform the injection procedure.
- Keep the stage/platform on which the mouse rests warm by attaching 2-3 paper towels on its surface.
- Place the mouse in a prone position on the platform. Use the right and left ear bars to gently fix the animal's head. Refer to Figure 1E.
- Position the mouse and orient under the scope so that the superior nasal aspect of the right eye is visible.
- Use a 30 G needle to displace the eyelashes and expose the sclera. Visualize the limbus and the radial blood vessel.
- Use a sterile 30 G needle to make a guide hole in the sclera 1-2 mm posterior to the limbus.
- Insert the 34 G needle attached to the injection holder into the eye through the guide hole at an angle that will avoid the lens, but place the needle tip into the vitreous cavity.
- Using the microsyringe pump controller, carefully inject 1 µL of the Mtb extract into the vitreous cavity. In case of consistent reflux, increase the injection volume to 1.5 µL to ensure adequate dose delivery.
NOTE: For sham controls, inject 1 µL of PBS into the eye of the animal. - Verify intravitreal placement by visualization of a greenish reflex in the eye. Refer to Figure 1F.
- After 10 s, withdraw the needle from the eye. Note any reflux.
- Remove the mouse from the platform, place 0.3% hypromellose or 0.2% w/w carbomer eye ointment on both eyes for corneal protection, and move to the recovery warming box.
- Do not leave the mouse unattended until it has regained sufficient consciousness to maintain sternal recumbency. Do not return to the company of other animals until fully recovered.
- When the mouse is fully awake, return to the cage and add acetaminophen (200-300 mg/kg/day) medicated water bottle. Label the cage card with the date of IVT injection.
- Intravitreal injections are generally well tolerated. Clinical signs that may indicate pain and the need for removal from the study include periocular alopecia (indicative of self-trauma), corneal ulceration, weight loss, and hunched posture.
- Alternative method for intravitreal injection
NOTE: This procedure is performed using an operating microscope and a 33 G needle on a microsyringe.- Proptose the eye and hold it in position with a pair of forceps.
- Then apply carbomer eye gel 0.2 % w/w or 0.3% hypromellose eye gel and place a circular coverslip (7 mm diameter) over the eye.
- Mount a 33 G hypodermic needle on a 5 µL Hamilton syringe and insert it approximately 2 mm circumferential to the corneal limbus with a ~45° injection angle.
- Guide the needle bevel into the vitreous, stopping between the lens and the optic disc (from the relative viewpoint of the surgeon, this is above/covering the optic disc – approximately 1.5 mm from the site of insertion), and inject 2 µL of Mtb (at 2.5 ug/µL in PBS) slowly.
- Hold the needle in place briefly (to reduce the amount of reflux of injectate) and then remove it.
- Post-injection, reposit the globe by releasing the forceps. A drop of 1% w/w chloramphenicol ointment can be administered to the eye at this time to provide additional protection from post-injection endophthalmitis.
- Post-injection, move to the recovery warming box as mentioned in steps 4.3.11-4.3.13.
5. OCT imaging to detect and quantify uveitis
- Animal preparation
- Anesthetize the mouse as described in steps 4.1.2-4.1.4
- Dilate the pupil with 1 drop of 2.5% phenylephrine. Use caution to avoid any excess droplets that might enter the nose or mouth. After 2-3 mins, dab off the excess liquid.
- Place 0.3% hypromellose or 0.2% w/w carbomer gel on the eye to prevent dryness while under anesthesia. This will also help to prevent cataract formation.
- Wrap the mouse in a layer of surgical gauze to maintain body warmth and place it on the animal cassette. Position the head with the bite bar.
- Acquire the OCT images of the anterior and posterior chambers.
NOTE: If obtaining anterior and posterior chamber images, obtain the posterior chamber (PC) images first to prevent image degradation following cataract formation. Cataract formation can be prevented with frequent lubrication and applying 0.3% hypromellose or 0.2% w/w carbomer eye gel. For extended imaging (>10 min), keeping the mouse warm (through the use of a heat pad) also helps.- After turning on the OCT imaging system, secure the correct imaging lens and adjust the reference arm position as needed.
- Open the imaging software, create the unique mouse ID and begin imaging per OCT manufacturer's protocol.
- Using the Free Run option with the fast scan protocol, position the eye with the optic nerve centered on posterior chamber images or the apex of the cornea on anterior chamber images.
NOTE: Table 1 contains imaging protocol parameters for two commercially available small animal imaging systems. Refer to the Table of Materials for product specifications. - For posterior chamber imaging, bring the OCT close to the surface of the eye. Use caution to avoid bringing the surface of the lens in contact with the eye.
- Once the eye is correctly positioned, stop the fast scan and select the volume scan protocol, and activate the scan with the Aim option.
- For posterior segment images, adjust untilthe optic nerve is centered in the Horizontal B-Scan Alignment image and that the retina is aligned with the Vertical Alignment axis
- For anterior segment images, adjust the position to center the apex of the cornea in both the Horizontal B-Scan Alignment image and Vertical Alignment B-Scan Alignment image. The presence of a reflection artifact in both images will confirm proper alignment. Then shift the horizontal image enough to remove the reflection artifact. Refer to Figure 2.
- Click on Snapshot to capture the volume scan image and then click on Save.
- Next, obtain the averaged central line scan. Open the scan protocol and click on Aim followed by Snapshot. Right-click on the same panel and then click on Average.
- Repeat steps 5.2.1-5.2.9 for each eye with both lenses.
- After all images are collected, remove the mouse from the cassette and provide corneal protection during recovery as listed in steps 4.3.11-4.3.13.
6. Scoring inflammation by OCT
- Score the OCT images with the help of graders who are masked to the treatment condition.
NOTE: For PMU in the mouse model, the scoring system provided in Table 2 is recommended. - If both anterior chamber (AC) and posterior chamber (PC) images were obtained, combine these scores to obtain the final score for each eye.
NOTE: Anterior chamber inflammation resolves before posterior chamber inflammation.
7. Scoring inflammation by post-mortem histology
- At the end of the experiment, collect individual eyes by enucleation, fix in 4% formaldehyde overnight, and proceed for paraffin embedding, sectioning, and H&E staining33.
NOTE: Multiple 4-8 µm sections along the pupillary-optic nerve axis are recommended.
NOTE: Three sections per eye are scored by a masked grader using the scoring system provided in Table 3, and the average score of the three sections is reported as the final histology inflammation score.
Representative Results
This protocol demonstrates the induction of uveitis in mice using the primed mycobacterial uveitis model (PMU). Ensuring consistency in the subcutaneous injection and accuracy of the intravitreal injection are key steps in developing the primed mycobacterial uveitis model (PMU). Figure 1 demonstrates the mouse intravitreal injection procedure using a stereotaxic apparatus. Ear bars help to gently position the head in the same location under the microscope (Figure 1E). They also keep the head stable during the intravitreal injection procedure, which decreases the risk of injection trauma. Following a successful injection, fluorescein in the injection solution produces a greenish reflection from within the eye that can be seen under the microscope or from a side view as pictured in Figure 1F.
When performed as outlined, the protocol generates robust acute uveitis that can be detected using OCT and fundus imaging as early as 10 h after intravitreal injection. Figure 2 demonstrates the correct alignment of the eye for OCT imaging. Table 1A lists the parameters used in the OCT protocol. A systematic approach to obtaining images will provide high-quality images that can be compared over time. Anterior chamber images are centered on the apex of the cornea using the en face SLO image (Figure 2A) with the iris aligned in parallel to both the horizontal and vertical planes (Figure 2B,C). Volume and line scans are captured with a vertical alignment such that inferior and superior regions can be viewed simultaneously. Posterior segment images are centered on the optic nerve using the en face SLO image (Figure 2D), and the bright band of the RPE is used to align the retina in parallel to both the horizontal and vertical planes (Figure 2E,F).
Figure 3 shows typical findings of PMU ocular inflammation using OCT imaging. Twenty four hours after intravitreal injection, inflammatory cells are seen in the aqueous and vitreous (Figure 3B). In the presence of moderate or severe inflammation, a hypopyon will be seen in the inferior angle in the AC. The degree of ocular inflammation can be scored on these OCT images using the criteria listed in Table 2. Representative examples of images demonstrating the inflammatory features typical for each score are shown in Figure 4. AC and PC chamber scores can be added together to generate the combined OCT score. Combined scores >0 but ≤2.5 represent mild inflammation. Moderate inflammation is determined by scores >2.5 but ≤4.5. Scores >4.5 identify severe inflammation. Inflammation typically peaks 48 h after intravitreal injection with OCT scores in the AC and PC between 1 and 3 (combined scores between 2 and 6). AC and PC scores of 0.5 or 4 are less common. In the case scores outside the typical range are encountered frequently, troubleshooting may be required to identify the factors contributing to outlier scores (see discussion section). Inflammation scores in the anterior chamber tend to return to zero within one week following intravitreal injection. In contrast, posterior scores do not return to zero; instead, low-level chronic inflammation persists in the form of vitritis, and perivascular lymphocytes infiltrate in the retina for 1-2 months following intravitreal injection.
Inflammation score in PMU can also be determined using histology. Figure 5 shows representative H&E sections for use in scoring the severity of PMU by histology. The number of inflammatory cells in the aqueous and vitreous are counted and used to determine the severity using the score criteria listed in Table 3. Inflammatory cell infiltration of the ciliary body is commonly seen on one side of the histology section (unilateral involvement) in mild or moderate inflammation. When inflammation is severe, this is reflected by the presence of an inflammatory cell infiltrate in the ciliary body on both sides of the lens (termed bilateral involvement). During later time points after intravitreal injection, chronic inflammation manifestations, including the presence of perivascular and intraretinal leukocytes and outer retinal folds, can also be identified. Histology can also be helpful in identifying eyes impacted by poor injection techniques. Trauma to the lens during the intravitreal injection can be identified by the presence of amorphous eosin-stained (pink) lens proteins outside the lens capsule adjacent to the area of trauma. Reflux of the intravitreal mTB into the subconjunctival space will generate inflammation outside the eye that can be identified by a careful review of periocular structures present on the sections. Due to the failure to retain mTB extract within the eyes, these eyes will typically have low OCT scores of inflammation.
Brightfield fundus imaging can also be used to identify clinically relevant aspects of PMU, including the development of a hypopyon, vitritis, and retinal or perivascular inflammatory cell infiltration. Figure 6 shows two examples where retinal and perivascular inflammation can be seen on fundus images. These two eyes also show the range of inflammation that is common in the PMU model. Table 1B lists the parameters used in the fundus/retinal OCT imaging system. Note the impact that severe inflammation has on the image quality (Figure 6, day 2, OCT and fundus images in the top row) and the extent of disease present on day 21. Corneal edema can also decrease image quality during acute inflammation; however, it is uncommon for corneal edema to be severe from inflammation alone. More commonly, the image quality will be degraded by epithelial damage resulting from incomplete surface protection during imaging and anesthesia events.
The PMU model can be used to induce uveitis in any mouse breed or genotype. In albino eyes, OCT can still be used to score inflammation, but the absence of fundus pigment makes visualization of inflammation challenging by brightfield imaging13,34. Post mortem studies can be performed on ocular tissues, regional lymph nodes, or the spleen. Some examples include assays for the presence of immune cells such as flow cytometry and immunohistochemistry and measurement of inflammatory cytokines. At all time points tested after initiation of inflammation with PMU (day 1 to day 56), there are sufficient CD45+ inflammatory cells present in individual eyes to detect many major leukocyte populations in the eye by multi-parameter flow analysis12,35. Aqueous (2-5 µL) and vitreous (5-10 µL) humors can be collected from inflamed eyes for protein concentration determination, proteomic studies, or cytokine concentration determination36.
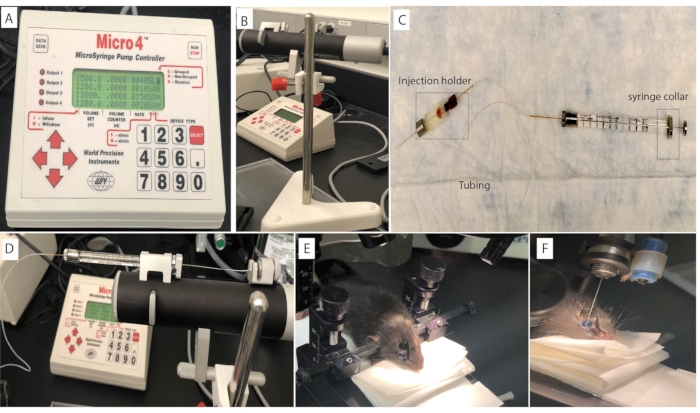
Figure 1: Mouse intravitreal injection set up. The intravitreal injection is performed on the mouse eye using (A) a microsyringe pump controller connected to the (B) Micropump and (C) an injection syringe. The syringe is loaded and mounted on the (D) Micropump. The mouse head is positioned using (E) ear bars to ensure stability and consistency during the intravitreal injection procedure. (F) Fluorescein in the injection solution produces a greenish reflection from within the eye after a successful procedure. Please click here to view a larger version of this figure.
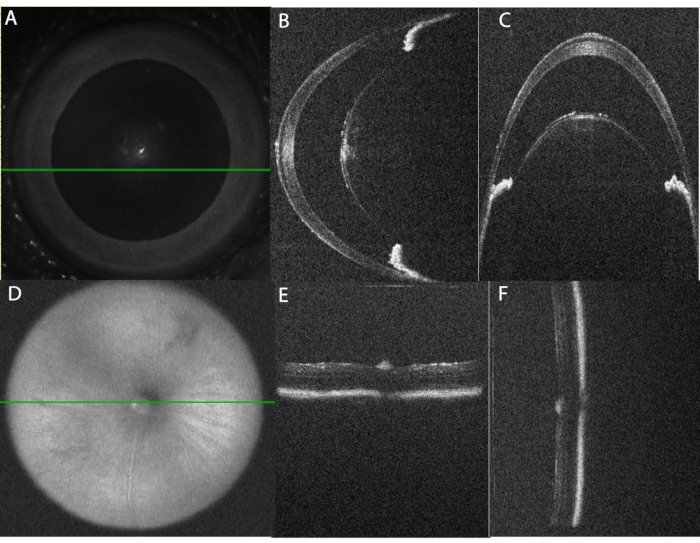
Figure 2: Proper alignment of the eye for OCT imaging. (A) Using the en-face scanning laser ophthalmoscope (SLO) image, the eye is centered for anterior chamber imaging. The green line indicates the position of the horizontal line scan shown in panel (B). Note that the central cornea is avoided to decrease reflection artifact. (C) Vertical B-Scan through the paracentral anterior chamber. This scan is obtained at 90° from the horizontal scan. Note that the alignment of the iris sections on each side of the lens is level in the horizontal scan (panel B) and arranged one above the other in the vertical scan (panel C). (D) Using the SLO image, the posterior chamber image is centered on the optic nerve. (E) Horizontal B-Scan Alignment, (F) Vertical B-Scan Alignment. Please click here to view a larger version of this figure.
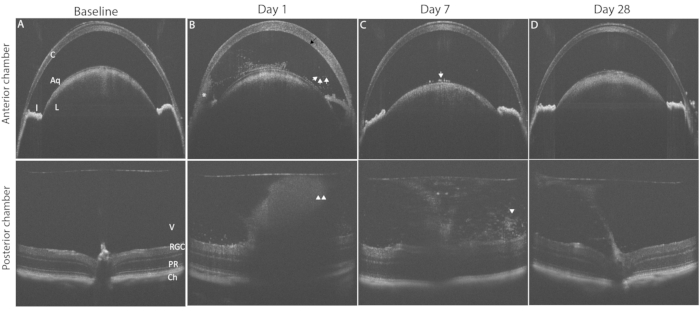
Figure 3: Induction of PMU generates panuveitis that can be monitored by longitudinal OCT imaging. The top row shows anterior chamber (AC); the bottom row shows posterior chamber (PC) OCT images to highlight pathological changes in the disease course. (A) Baseline OCT image of the AC (top) and PC (bottom) prior to induction of uveitis, both score 0. (B) Day 1 after intravitreal injection showing the presence of corneal edema (black arrow), a hypopyon (*) multiple free-floating inflammatory cells in the AC (white arrows), and vitritis (white arrowheads) in the PC. (C) Day 7 after intravitreal injection with few AC cells on the anterior lens capsule (white arrow) and decreased vitritis (white arrowhead). (D) Day 28 after intravitreal injection anterior chamber with resolved AC inflammation and mild vitritis. Abbreviations: C- cornea, L – lens, I – iris, Aq – aqueous, V vitreous, RGC – retinal ganglion cells, PR – photoreceptors, Ch- choroid. Please click here to view a larger version of this figure.
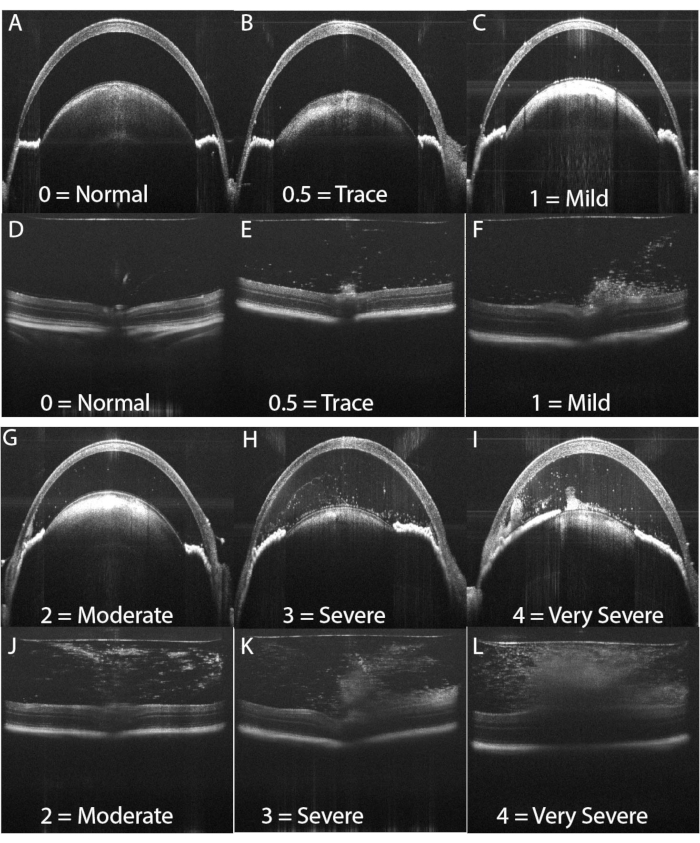
Figure 4: OCT score examples. An OCT score between 0 and 4 is assigned to each AC image and PC image using the categorical system shown in Table 2. The AC and PC scores are combined for the final OCT score for the eye. (A,D) Examples of a score of zero. (B,E) Examples of a score of 0.5. (C,F) Examples of a score of 1. (G,J) Examples of a score of 2. (H,K) Examples of a score of 3. (I,L) Examples of a score of 4 are shown in panels I and L. Please click here to view a larger version of this figure.
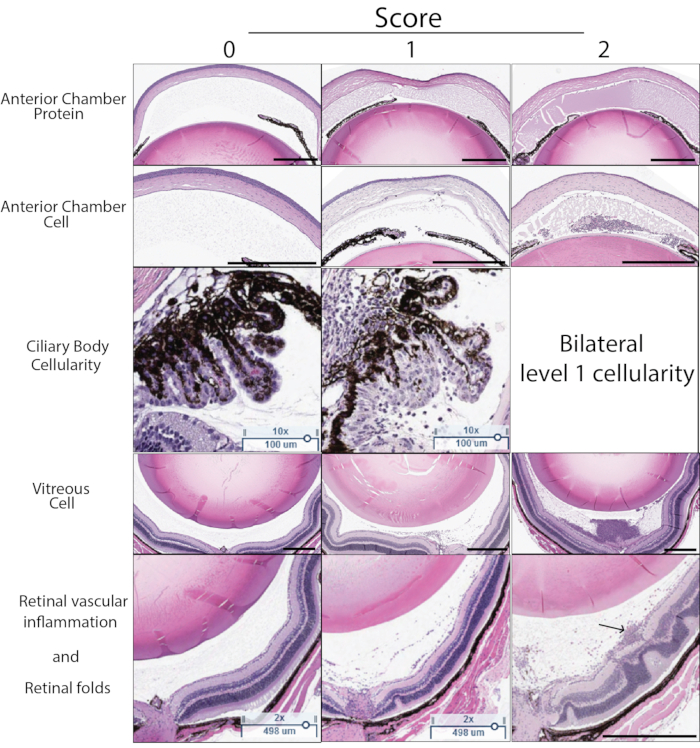
Figure 5: Histology score examples. Histology score is determined based on five characteristics visible in H&E sections: Anterior chamber protein density, anterior chamber cell number, immune cell infiltration of the ciliary body, vitreous cell density, retinal vascular inflammation, and structural retinal changes. A score of 0-2 is assigned for each characteristic. The description of each score is found in Table 3. A representative example score of 0-2 for each characteristic is shown in this figure. The left column demonstrates the score of zero. The center column shows examples of score 1. The right column shows examples of score 2. A score of 2 for ciliary body score is assigned if the ciliary body on either side of the lens in the same section demonstrates cellular inflammation. The final histology score is the sum of the score for each of the five criteria (max score 10). The arrow in the bottom right panel indicates perivascular leukocytes associated with a superficial retinal vessel. Black scale bar indicates 500 µm. Ciliary scale bars indicate 100 µm. Please click here to view a larger version of this figure.
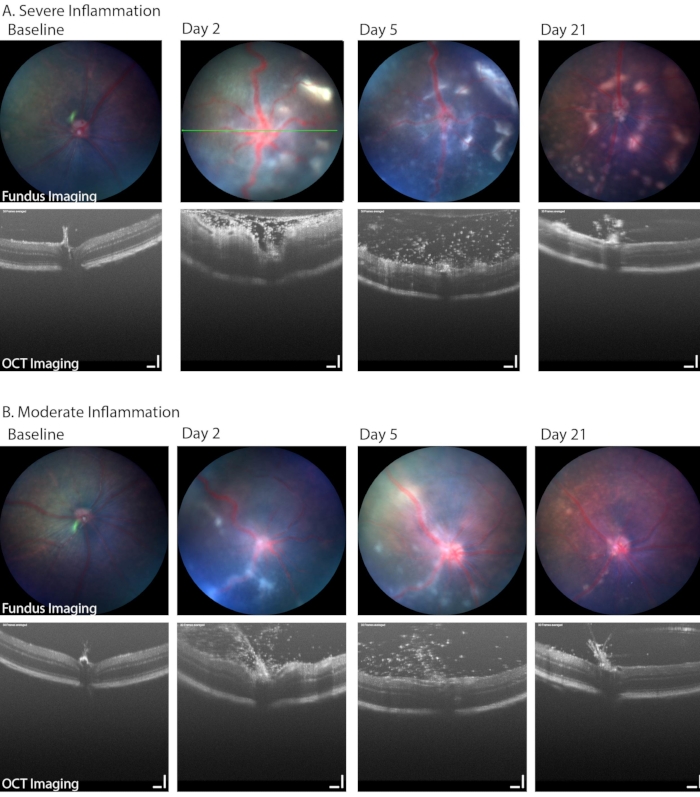
Figure 6: Longitudinal fundus imaging in PMU identified a range of disease severity. (A) Severely inflamed eyes demonstrate multiple white infiltrates in the retina and vascular tortuosity on color fundus imaging (top row) as well as dense vitritis and retinal edema on OCT (bottom row) on day 2. Progression in the number of retinal lesions can be seen over time while the vitritis improves. Green line indicates the position of the OCT image. (B) Mildly inflamed eyes demonstrate fewer and more discrete linear lesions in the fundus and a number of infiltrating cells in the vitreous space. Scale bar = 100 µm. Please click here to view a larger version of this figure.
| A | |||
| Mouse Anterior Chamber | Fast Scan | Volume Scan | Linear Scan |
| Length X Width | 4.0 mm x 4.0 mm | 3.6 mm x 3.6 mm | 3.6 mm |
| Angle | 0 | 90 | 90 |
| A-scan/B-Scan | 800 | 1000 | 1000 |
| # B-scans | 50 | 400 | 1 |
| Frames/ B-scan | 1 | 3 | 20 |
| Mouse Posterior Chamber | Fast Scan | Volume Scan | Linear Scan |
| Length X Width | 1.6 mm x 1.6 mm | 1.6 mm x 1.6 mm | 1.6 mm |
| Angle | 0 | 0 | 0 |
| A-scan/B-Scan | 800 | 1000 | 1000 |
| # B-scans | 50 | 200 | 1 |
| Frames/ B-scan | 1 | 3 | 20 |
| B | |||
| Mouse Posterior Chamber | Volume Scan | Linear Scan | |
| Length X Width | 0.9 mm x 0.9 mm | 1.8 mm | |
| Angle | 0 | Any (typically 0 or 90) | |
| A-scan/B-Scan | 1024 | 1024 | |
| # B-scans | 512 | 1 | |
| Frames/ B-scan | 1 | 30 |
Table 1: Scan parameters. (A) OCT scan parameters. (B) Fundus/retinal OCT scan parameters
| OCT Score descriptions | ||||||
| Score | Anterior Chamber | Posterior Chamber | ||||
| NA | No view beyond anterior cornea | No view of posterior segment | ||||
| 0 | No inflammation | No inflammation | ||||
| 0.5 | 1–5 cells in the aqueous | Few cells occupying less than 10% of the vitreous area | ||||
| OR corneal edema | No subretinal or intraretinal infiltrates or retinal architecture disruption | |||||
| 1 | 6–20 cells in the aqueous | Diffuse cells (no dense clumps) occupying between 10 and 50% of the vitreous area. | ||||
| OR a single layer of cells on the anterior lens capsule | No subretinal or intraretinal infiltrates or retinal architecture disruption | |||||
| 2 | 20–100 cells in the aqueous | Diffuse cells (no dense clumps) occupying > 50% of the vitreous area | ||||
| OR fewer than 20 cells and a hypopyon present | No subretinal or intraretinal infiltrates or retinal architecture disruption | |||||
| 3 | 20–100 cells in the aqueous | Diffuse cells equal to grade 2 and 1 | ||||
| AND a hypopyon OR a pupillary membrane | AND at least one dense vitreous opacity occupying 10%–20% of the vitreous area OR the presence of vitreous cells equal to grade 2 and rare (≤ 2) subretinal or ntraretinal opacities | |||||
| 4 | Any number of aqueous cells | Dense vitreous opacity occupying > 20% of the vitreous area. | ||||
| AND a large hypopyon and pupillary membrane OR anterior structure loss due to severe inflammation | OR diffuse vitreous cells with large subretinal or intraretinal opacities | |||||
Table 2: PMU OCT score criteria: OCT images are scored according to the criteria listed in the table. AC and PC scores are added to obtain the final score of the eye. In cases where a clear view of the eye was not acquired, a score of NA was assigned to the images, and these were excluded from the study.
| Histology Score Description | |||
| Characteristic | 0 | 1 | 2 |
| Anterior Chamber (AC) Protein | Scant acellular particles staining with eosin in the AC | Moderate, but not confluent, extracellular eosin staining anywhere in the AC | Confluent or near confluent extracellular eosin staining throughout the AC |
| Anterior Chamber (AC) Cell | No cells | 1–100 cells, but no dense aggregations of cells | >100 cells, or dense aggregations of cells |
| Ciliary Body Inflammation | No leukocyte infiltration of the ciliary body or surrounding vitreous | Unilateral presence of leukocytes infiltrating the ciliary body and/or the surrounding vitreous. | Bilateral presence of leukocytes infiltrating the ciliary body and/or the surrounding vitreous. |
| Retinal Vascular Inflammation | No retinal vessels with perivascular leukocytes | One vessel per section with perivascular leukocytes | >1 vessel per section with perivascular leukocytes |
| Retinal fold or damage | No retinal damage | 1–3 retinal folds per section | >3 retinal folds per section, or any other retinal layer destruction or intraretinal hemorrhage |
Table 3: PMU histology score criteria: H&E sections of the eye were scored based on the criteria listed in the table. Three sections from the same eye were scored and averaged to obtain the final histology score of the eye.
Discussion
Animal models of uveitis have been instrumental in understanding the mechanisms of ocular inflammation and homeostasis as well as enabling preclinical evaluation of medical and surgical therapies for patients with uveitis37. Both rabbit and rat variants of the PMU model have demonstrated their value in preclinical therapy via proof of concept studies38,39,40. Due to the availability of a diverse range of transgenic strains in mice, establishing the mouse PMU model system now permits more detailed mechanistic studies to identify specific cell types, pathways, and genes that contribute to the pathology of this disease.
Animal models of uveitis can demonstrate animal to animal variability in the incidence and intensity of inflammation41. In the C57BL/6 mouse strain, PMU is reliably generated using the protocol outlined here. Strain-specific variations in uveitis course and intensity have been reported for both EAU and EIU42,43. While strain-specific impacts on severity and course of PMU have not been measured experimentally, this model has been used in wild-type C57BL/6J as well as in albino mice (B6(Cg)-Tyrc-2J/J) and produced similar inflammatory responses. In generating the PMU model, controlling the considerations listed below can help new researchers limit variability and produce the most consistent and reproducible uveitis.
Ensure consistency in the subcutaneous injections:
To provide a consistent subcutaneous injection, ensure that all air bubbles are removed from the emulsion. Considerations include a short centrifuge (30 s at 400 x g) of the premade emulsion prior to loading the syringe. This will remove air trapped in the emulsion. Also, when loading the syringe, periodically invert (tip-up) and tap the syringe to remove any air bubbles. While injecting, do not place the syringe too deep in order to avoid intramuscular injection. Conversely, a shallow (intradermal) injection can result in erosion of the emulsion through the skin. Remember to pause briefly before removing the syringe from the injection site to ensure complete injection of the thick viscous emulsion and to prevent reflux from the skin.
Seven days after placing the subcutaneous injection, confirm the presence of palpable nodules on either side of the hind legs. If no nodules can be identified, it is possible that air was injected rather than emulsion. In this case, acute inflammation may not be as robust, and chronic inflammation may not develop.
Prevent the development of infectious endophthalmitis:
Bacterial or fungal endophthalmitis will generate a confounding variable if not prevented44. In order to prevent bacterial endophthalmitis, always practice good aseptic technique when making the intravitreal suspension, handling, and cleaning all reusable tools that will come in contact with the eye. Using sterile single-use items, autoclaving, or cleaning with 95% alcohol washes or wipes is important. Appropriate use of betadine applied to the ocular surface, lids, and periocular fur will also help prevent endophthalmitis45. It is straightforward to recognize an eye with infection as the ocular structures will be obliterated by extreme inflammation during the post-injection course. This is not typical for PMU. The presence of intraocular bleeding can also suggest endophthalmitis or trauma from the injection. In such cases, exclude these animals from the study.
Ensure consistency in the intravitreal injection:
The intravitreal injection is a critical step in the induction of reliable and reproducible inflammation in PMU. Providing a consistent amount of Mtb suspension with each injection, avoiding trauma, and preventing reflux of the suspension are all factors that should be considered when performing the injections. To ensure a consistent suspension, vortex the stock suspension thoroughly upon thawing and before loading it in the syringe. Since this Mtb extract used does not form a solution, the suspension can undergo sedimentation over time. To ensure uniform concentration of the Mtb extract in each injection, use or expel and reload the syringe within 15 minutes of loading. Phenylephrine is used for dilation to provide a larger field of view to the posterior eye and reduce the risk of trauma to the eye during the injection. This drop generates natural lid retraction and slight proptosis of the globe, allowing good visualization of area 1-2 mm posterior to the limbus without the need to grasp the eye with forceps. Using forceps to restrain the eye could cause potential trauma and transiently increase intraocular pressure and the risk of reflux of the Mtb suspension. Trauma can also be caused by attempting to inject too much volume into the eye. The injection volume is limited to 2 µL to prevent significant and prolonged elevation of intraocular pressure and trauma to the eye. Additionally, younger animals will have eyes that are smaller than adult mice. Typically 6-8 week mice (20-25 g) provide a uniform eye size and ensure greater consistency in the inflammation following injection of Mtb. A higher frequency of post-injection reflux of the mycobacterial suspension was observed in smaller mice. This, in turn, leads to a less-than-expected acute inflammation. A dilute fluorescein solution is used to provide the novice injector visual feedback on the success of their injection technique. Dilation at the time of injection will allow for direct visualization of the injected material in the vitreous cavity and the opportunity to note any evidence of lens trauma. In the case of lens trauma, it can cause a change in lens clarity that will cause a cataract which can be visualized on OCT. In the case of ocular trauma, the eyes need to be excluded from the study due to the possibility of lens-induced uveitis46. We recommend pausing for 10 s before removing the syringe from the eye to allow dispersion of the Mtb suspension within the eye and decrease reflux.
The PMU model can be modified to change the intensity of acute inflammation by varying the concentration of the Mtb in the intravitreal injection. Different dosages ranging from 2.5 µg/µL to 15 µg/µL have been tested previously in our lab. However, doses higher than 10 µg/µL were found to cause severe eye damage, including spontaneous lens rupture, severe corneal edema and scarring, and hyphema. This degree of severity is not typical in human patients with post-infectious uveitis, and therefore, these concentrations are not recommended. A 5 µg/µL dose was found to reliably produce mild to moderate acute inflammation and mild chronic uveitis; the 10 µg/µL dose produces a reliably moderate to severe acute disease and more notable chronic disease. Thus, varying the intravitreal concentration can provide alternative disease severities for use as needed based on the experimental question. Controls should be selected to ensure results are due to the response to mTB and not trauma associated with the subcutaneous or intravitreal injections. In the sham injection controls, PBS can be used in place of the mTB extract. For comparisons to unexposed animals, true naive samples should be considered as fellow eyes are not always equivalent.
Due to the small size of the mouse eye, OCT can be a more sensitive assay to detect inflammation in the anterior chamber than direct visualization or microscopic bright field photography. Prior work with PMU in rats25 determined that more cells can be detected by histology than by OCT but that there is a good correlation between the two modalities. OCT has the added advantage that it can be used to monitor the inflammation longitudinally in the same animal. Other major mouse models of uveitis, such as EAU and EIU, have also employed OCT for quantitative analysis12,47,48. In the PMU model of mice, anterior chamber cells are only visible on OCT and cannot be seen on clinical exams unless a large hypopyon is present. Vitreous inflammation (vitritis) can be observed with color fundus imaging, but detecting quantitative change is possible only with OCT imaging. Other aspects of the model, such as retinal vascular inflammation and retinal damage, can be easily identified with either OCT and microscopic brightfield fundus photography.
When using OCT, it is important to consider how localized imaging can be impacted by regional differences in the degree of inflammation. Prior reports have identified an uneven distribution of cells in the anterior chamber of humans, with more cells located inferiorly49. In mice, a similar predisposition is common. Thus, vertical or radial scans through the AC will help ensure images that capture the range of inflammation. Additionally, performing imaging in the same place will also provide consistency to images collected in the same eye longitudinally. To obtain images in the same part of the eye, use stable landmarks and a systematic approach. For anterior chamber images, the image is centered immediately adjacent to the apex of the cornea and oriented vertically so that the presence of a hypopyon can be detected in the inferior angle. For posterior segment images, the image is centered on the optic nerve. It is recommended to consider using at least 3 line scans for scoring to ensure regional variability is captured. In cases where inflammation is restricted to peripheral locations, acquiring volume scans can be helpful. The collection of volume scans can also help capture regional variations but will increase data storage requirements.
Other in vivo assays that can be used to characterize inflammation in the PMU mouse model include bioluminescence imaging13,35. Post-mortem assays like multi-parameter flow cytometric analysis can be performed to identify and quantify infiltrating immune cell type populations in the aqueous and posterior chamber of the eye12,26. In the PMU model, acute inflammation is characterized by an innate response with a predominant neutrophil infiltrate, followed by a chronic and persistent adaptive T cell dominant response that persists for over a month35. Other assays of immune function that can be performed on post-mortem tissues include ocular fluid cytokine analysis. Additionally, other downstream assays like mRNA sequencing and immunofluorescence imaging can be used to assess gene and protein expression patterns of retinal immune cell populations in uveitis50,51.
The PMU model can be replicated in other rodent systems using adaptations appropriate for the different species. PMU model has been previously used in rats and rabbits38,39,40. In rats, acute panuveitis develops following intravitreal injection that resolves spontaneously over 14 days without developing signs of chronic inflammation by histology24. In rabbits, induction of uveitis utilizes two rounds of subcutaneous injection prior to intravitreal injection but also generates a robust panuveitis. One of the advantages of using the mouse model is the ready availability of numerous transgenic and knockout strains that can help understand the basic mechanism of uveitis52. All rodent models can be used for preclinical therapy testing if the agent is administered systemically or as a topical drop. However, due to their larger size, rat and rabbit eyes are better models for use in preclinical studies of implantable or local injection treatment options for uveitis.
In summary, this protocol provides researchers interested in studying the mechanisms of chronic ocular inflammation with a new tool that is not dependent on prior immunization with ocular antigens.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work is supported by funding from the National Institutes of Health, Bethesda, Maryland, United States (KP) K08EY0123998, (KP) R01EY030431, (KP) R21 EY02939, UW vision research core grant (NEI P30EY01730), gifts from the Mark Daily, MD Research Fund and the Christopher and Alida Latham Research fund, an unrestricted departmental grant from Research to Prevent Blindness, and career development award from Research to Prevent Blindness (KP). The work conducted in Bristol was supported by additional funding from Sight Research UK and The Underwood Trust.
Materials
| AK-FLUOR | Akorn Pharmaceuticals, IL, USA | 10% Fluorescein sodium 100 mg/mL in 5 mL vial | |
| AnaSed | Akorn Animal Health, IL, USA | NDC 59399-110-20 | Xylazine 20 mg/mL |
| Betadine 5% Sterile Ophthalmic Prep Solution | Alcon, TX, USA | 8007-1 | |
| B-D Precision Glide Needles -25 G | Becton, Dickinson and Company, NJ, USA | 305122 | |
| B-D Precision Glide needle -30-G | Becton, Dickinson and Company, NJ, USA | 305106 | |
| Bond MAX, Bond Rx | Leica Biosystems, IL,USA | Automated IHC staining system | |
| Chloramphenicol ointment | Martindale Pharma, Romford, UK | 1% w/w Chloramphenicol | |
| EG1150H | Leica Biosystems, IL,USA | Tissue Embedding | |
| Envisu R2300 | Bioptigen/Leica | OCT Machine | |
| Freund's Incomplete Adjuvant | BD Difco, NJ, USA | 263910 | |
| GenTeal lubricant eye ointment | Alcon, TX, USA | — | |
| GenTeal lubricant eye gel | Alcon, TX, USA | — | |
| H37Ra lyophilized Mycobacteria extract | BD Difco, NJ, USA | 231141 | |
| Hamilton RN Needle (33/12/2)S | Hamilton, Reno, NV | 7803-05(33/12/2) | 33 G |
| Hamilton syringe | Hamilton, Reno, NV | CAL7633-01 | 5 µL |
| Insulin needle | Exel International, USA | 26029 | 1 mL |
| Isoflurane | |||
| Ketaset | Zoetis, USA | 377341 | Ketamine HCL 100 mg/mL |
| Microinjection Syringe Pump and Micro4Controller | World Precision Instruments, FL, USA | UMP3 | |
| Micron IV | Phoenix Research Laboratories, Pleasanton, CA | Alternative Imaging/OCT Machine | |
| Nanofil 10 µL syringe | World Precision Instruments, FL, USA | NANOFIL | |
| Nanofil Intraocular Injection Kit | World Precision Instruments, FL, USA | IO-KIT | |
| Olympus SZX10 | Olympus | Dissection scope | |
| PBS | Gibco | 14190 | |
| Phenylephrine Hydrochloride Ophthalmic Solution USP 2.5% Sterile 15 mL | Akorn Pharmaceuticals, IL, USA | 17478020115 | |
| RM2255 | Leica Biosystems, IL,USA | Tissue Sectioning | |
| TB Syringe | Becton, Dickinson and Company, NJ, USA | 309602 | 1 mL |
| Tetracaine 0.5% | Alcon, TX, USA | 1041544 | |
| Tissue Tek VIP series | Sakura Finetek USA, Inc.,CA. | Histology Tissue Processing | |
| Tropicamide 1% | Chauvin Pharmaceuticals, Romford, UK | Minims | |
| Tylenol | Johnson & Johnson Consumer Inc, PA, USA | NDC 50580-614-01 | Acetaminophen |
| Viscotears | Novartis Pharmaceuticals, Camberley, UK | Carbomer eye gel 0.2% w/w |
References
- American Academy of Ophthalmology. Aao 2019-2020 Basic and Clinical Science Course, Section 09: Uveitis and Ocular Inflammation. American Academy of Ophthalmology. , (2019).
- Caspi, R. R. Animal models of autoimmune and immune-mediated uveitis. Drug Discovery today. Disease Models. 3 (1), 3-9 (2006).
- DeVoss, J., et al. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. The Journal of Experimental Medicine. 203 (12), 2727-2735 (2006).
- Caspi, R. R., et al. A new model of autoimmune disease. Experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. Journal of Immunology. 140 (5), 1490-1495 (1988).
- Tang, J., Zhu, W., Silver, P. B., Su, S. -. B., Chan, C. -. C., Caspi, R. R. Autoimmune uveitis elicited with antigen-pulsed dendritic cells has a distinct clinical signature and is driven by unique effector mechanisms: Initial encounter with autoantigen defines disease phenotype. The Journal of Immunology. 178 (9), 5578-5587 (2007).
- Broekhuyse, R. M., Kuhlmann, E. D., Winkens, H. J. Experimental melanin-protein induced uveitis (EMIU) is the sole type of uveitis evoked by a diversity of ocular melanin preparations and melanin-derived soluble polypeptides. Japanese Journal of Ophthalmology. 40 (4), 459-468 (1996).
- Pennesi, G., et al. A humanized model of experimental autoimmune uveitis in HLA class II transgenic mice. The Journal of Clinical Investigation. 111 (8), 1171-1180 (2003).
- Caspi, R. R. Understanding autoimmune uveitis through animal models. The Friedenwald lecture. Investigative Ophthalmology & Visual Science. 52 (3), 1872-1879 (2011).
- Bansal, S., Barathi, V. A., Iwata, D., Agrawal, R. Experimental autoimmune uveitis and other animal models of uveitis: An update. Indian Journal of Ophthalmology. 63 (3), 211-218 (2015).
- Smith, J. R., Hart, P. H., Williams, K. A. Basic pathogenic mechanisms operating in experimental models of acute anterior uveitis. Immunology and Cell Biology. 76 (6), 497-512 (1998).
- Rosenbaum, J. T., McDevitt, H. O., Guss, R. B., Egbert, P. R. Endotoxin-induced uveitis in rats as a model for human disease. Nature. 286 (5773), 611-613 (1980).
- Chu, C. J., et al. Multimodal analysis of ocular inflammation using the endotoxin-induced uveitis mouse model. Disease Models & Mechanisms. 9 (4), 473-481 (2016).
- Gutowski, M. B., Wilson, L., Van Gelder, R. N., Pepple, K. L. In vivo bioluminescence imaging for longitudinal monitoring of inflammation in animal models of uveitis. Investigative Ophthalmology & Visual Science. 58 (3), 1521-1528 (2017).
- World Health Organization. Global tuberculosis report 2019. World Health Organization. , (2019).
- Biswas, J., Badrinath, S. S. Ocular morbidity in patients with active systemic tuberculosis. International Ophthalmology. 19 (5), 293-298 (1995).
- Donahue, H. C. Ophthalmologic experience in a tuberculosis sanatorium. American Journal of Ophthalmology. 64 (4), 742-748 (1967).
- El-Asrar, M. A., Abouammoh, M., Al-Mezaine, H. S. Tuberculous uveitis. Middle East African Journal of Ophthalmology. 16 (4), 188-201 (2009).
- Bodaghi, B., et al. Chronic severe uveitis: etiology and visual outcome in 927 patients from a single center. Médecine. 80 (4), 263-270 (2001).
- Cunningham, E. T., Forrester, J. V., Rao, N. A., Zierhut, M. Post-infectious uveitis. Ocular Immunology and Inflammation. 24 (6), 603-606 (2016).
- Wroblewski, K. J., Hidayat, A. A., Neafie, R. C., Rao, N. A., Zapor, M. Ocular tuberculosis: a clinicopathologic and molecular study. Ophthalmology. 118 (4), 772-777 (2011).
- Yeh, S., Sen, H. N., Colyer, M., Zapor, M., Wroblewski, K. Update on ocular tuberculosis. Current Opinion in Ophthalmology. 23 (6), 551-556 (2012).
- Tagirasa, R., Parmar, S., Barik, M. R., Devadas, S., Basu, S. Autoreactive T cells in immunopathogenesis of TB-associated uveitis. Investigative Ophthalmology & Visual Science. 58 (13), 5682-5691 (2017).
- Agrawal, R., et al. Insights into the molecular pathogenesis of ocular tuberculosis. Tuberculosis. 126, 102018 (2021).
- Pepple, K. L., et al. Primed mycobacterial uveitis (PMU): Histologic and cytokine characterization of a model of uveitis in rats. Investigative Ophthalmology & Visual Science. 56 (13), 8438-8448 (2015).
- Pepple, K. L., Choi, W. J., Wilson, L., Van Gelder, R. N., Wang, R. K. Quantitative assessment of anterior segment inflammation in a rat model of uveitis using spectral-domain optical coherence tomography. Investigative Ophthalmology & Visual Science. 57 (8), 3567-3575 (2016).
- Pepple, K. L., Wilson, L., Van Gelder, R. N. Comparison of aqueous and vitreous Lymphocyte populations from two rat models of experimental uveitis. Investigative Ophthalmology & Visual Science. 59 (6), 2504-2511 (2018).
- Basu, S., Elkington, P., Rao, N. A. Pathogenesis of ocular tuberculosis: New observations and future directions. Tuberculosis. 124, 101961 (2020).
- Basu, S., Rao, N., Elkington, P. Animal models of ocular tuberculosis: Implications for diagnosis and treatment. Ocular Immunology and Inflammation. , 1-7 (2020).
- Mattapallil, M. J., et al. The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Investigative Ophthalmology & Visual Science. 53 (6), 2921-2927 (2012).
- Underwood, W., Anthony, R. . AVMA guidelines for the euthanasia of animals: 2020 edition. 2013 (30), 2020-2021 (2020).
- Donovan, J., Brown, P., Coligan, J. E. Handling and restraint. Current protocols in immunology. , (2006).
- Tremoleda, J. L., Kerton, A., Gsell, W. Anaesthesia and physiological monitoring during in vivo imaging of laboratory rodents: considerations on experimental outcomes and animal welfare. EJNMMI Research. 2 (1), 44 (2012).
- Cardiff, R. D., Miller, C. H., Munn, R. J. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harbor protocols. 2014 (6), 655-658 (2014).
- Paques, M., et al. Panretinal, high-resolution color photography of the mouse fundus. Investigative Ophthalmology & Visual Science. 48 (6), 2769-2774 (2007).
- John, S., Rolnick, K., Wilson, L., Wong, S., Van Gelder, R. N., Pepple, K. L. Bioluminescence for in vivo detection of cell-type-specific inflammation in a mouse model of uveitis. Scientific Reports. 10 (1), 11377 (2020).
- Fortmann, S. D., Lorenc, V. E., Hackett, S., Campochiaro, P. A. Murine Vitreous Tap (MurViTap): a novel technique to extract uncontaminated mouse vitreous humor, quantify retinal vascular permeability, and compare proteins secreted by diseased and normal retina. Investigative ophthalmology & visual science. 58 (8), 5978 (2017).
- Caspi, R. R. Understanding autoimmunity in the eye: from animal models to novel therapies. Discovery Medicine. 17 (93), 155-162 (2014).
- Mruthyunjaya, P., et al. Efficacy of low-release-rate fluocinolone acetonide intravitreal implants to treat experimental uveitis. Archives of Ophthalmology. 124 (7), 1012-1018 (2006).
- Jaffe, G. J., Yang, C. S., Wang, X. C., Cousins, S. W., Gallemore, R. P., Ashton, P. Intravitreal sustained-release cyclosporine in the treatment of experimental uveitis. Ophthalmology. 105 (1), 46-56 (1998).
- Pepple, K. L., et al. Uveitis therapy with shark variable novel antigen receptor domains targeting tumor necrosis factor alpha or inducible t-cell costimulatory ligand. Translational Vision Science & Technology. 8 (5), 11 (2019).
- Mattapallil, M. J., et al. Characterization of a New epitope of IRBP that induces moderate to severe uveoretinitis in mice with H-2b haplotype. Investigative Ophthalmology & Visual Science. 56 (9), 5439-5449 (2015).
- Silver, P. B., Chan, C. C., Wiggert, B., Caspi, R. R. The requirement for pertussis to induce EAU is strain-dependent: B10.RIII, but not B10.A mice, develop EAU and Th1 responses to IRBP without pertussis treatment. Investigative Ophthalmology & Visual Science. 40 (12), 2898-2905 (1999).
- Li, Q., Peng, B., Whitcup, S. M., Jang, S. U., Chan, C. C. Endotoxin induced uveitis in the mouse: susceptibility and genetic control. Experimental Eye Research. 61 (5), 629-632 (1995).
- Astley, R. A., Coburn, P. S., Parkunan, S. M., Callegan, M. C. Modeling intraocular bacterial infections. Progress in Retinal and Eye Research. 54, 30-48 (2016).
- Lau, P. E., Jenkins, K. S., Layton, C. J. Current evidence for the prevention of endophthalmitis in anti-VEGF intravitreal injections. Journal of Ophthalmology. 2018, 8567912 (2018).
- Nche, E. N., Amer, R. Lens-induced uveitis: an update. Graefe’s Archive for Clinical and Experimental Ophthalmology. 258 (7), 1359-1365 (2020).
- Chu, C. J., et al. Assessment and in vivo scoring of murine experimental autoimmune uveoretinitis using optical coherence tomography. PLoS ONE. 8 (5), 63002 (2013).
- Harimoto, K., Ito, M., Karasawa, Y., Sakurai, Y., Takeuchi, M. Evaluation of mouse experimental autoimmune uveoretinitis by spectral domain optical coherence tomography. The British Journal of Ophthalmology. 98 (6), 808-812 (2014).
- Li, Y., Lowder, C., Zhang, X., Huang, D. Anterior chamber cell grading by optical coherence tomography. Investigative Ophthalmology & Visual Science. 54 (1), 258-265 (2013).
- Bell, O. H., et al. Single eye mRNA-seq reveals normalisation of the retinal microglial transcriptome following acute inflammation. Frontiers in Immunology. 10, 3033 (2019).
- Lipski, D. A., et al. Retinal endothelial cell phenotypic modifications during experimental autoimmune uveitis: a transcriptomic approach. BMC Ophthalmology. 20 (1), 106 (2020).
- Agarwal, R. K., Silver, P. B., Caspi, R. R., Perl, A. Rodent Models of Experimental Autoimmune Uveitis. Autoimmunity. Methods in Molecular Biology (Methods and Protocols). , 443-469 (2012).

