In Ovo Feeding of Commercial Broiler Eggs: An Accurate and Reproducible Method to Affect Muscle Development and Growth
Summary
A robust methodology has been developed for conducting in ovo feeding research trials utilizing unincubated commercial broiler eggs to test the ability of natural and synthetic compounds, in this case, nicotinamide riboside, to influence muscle development and growth.
Abstract
Within the past three decades, red meat and poultry scientists focused on developing strategies and technologies to manipulate muscle development during embryonic and fetal development. This area continues to be an area of focus because muscle fiber number is established during this time and determines the basis for all future growth. In poultry, numerous studies demonstrated in ovo feeding of growth factors, vitamins, or other nutrients improved chick embryonic muscle and intestinal development. Improving in ovo muscle development could benefit the poultry industry by possibly influencing meat yield, growth rate, or myopathy conditions. During the past five years, the Gonzalez Laboratory at the University of Georgia developed a nicotinamide riboside in ovo feeding methodology for broiler-chicken embryos, which altered muscle development. When injected into a developing embryo's yolk sac, nicotinamide riboside increased pectoralis major muscle weight and muscle fiber density at hatch. This protocol will demonstrate a methodology to accurately and reproducibly conduct in ovo feeding studies utilizing commercial standard- and high-yielding broiler embryos. These data and methods will allow other research groups to perform in ovo feeding studies with much success and reproducibility.
Introduction
Since 1960, the United States' per capita consumption of meat from poultry has risen at an astounding rate, while other primary protein sources have remained stagnant, declined, or minimally increased. The poultry industry invested considerable time and research efforts optimizing nutrition and genetics to produce an efficient bird to keep up with demand. Because the poultry industry's main objective is to produce muscle for conversion to meat, their efforts have drastically changed the bird's ultimate muscle mass at harvest.
Like most species, poultry develops muscle in a biphasic manner. Primary myogenesis utilizes mesenchymal stem cells to produce primary muscle fibers, which serve as the scaffold for the second wave of muscle fiber development1. In poultry, primary myogenesis occurs during embryonic days 3 to 8, and secondary myogenesis happens from days 8 to 212. Once developed, primary and secondary muscle fibers serve as the basis for all future muscle growth through cellular hypertrophy. Therefore, scientists and industry spent considerable effort attempting to manipulate primary and secondary myogenesis in all meat-producing species to maximize meat yield.
One technology explored in poultry, called in ovo feeding, involves feeding compounds through injection. In ovo feeding, a technology employed by the poultry industry for almost 40 years, was initially developed for vaccine administration3. The literature documents that in ovo feeding of various compounds and nutrients at different developmental periods and locations within the egg positively affected in ovo muscle development and growth4,5,6. To date, the Gonzalez Laboratory at the University of Georgia is the pioneer in utilizing nicotinamide riboside in ovo feeding to manipulate poultry muscle development.
Nicotinamide riboside, a pyridine-nucleoside analog of vitamin B3, produces NAD+ through the salvage pathway7. Since this pathway utilizes fewer enzymatic steps to produce NAD+, production is the most efficient8. Gonzalez and Jackson9 demonstrated that supplementation of developing broiler embryo yolk sac with nicotinamide riboside increased hatched chick pectoralis major muscle weight and muscle fiber density. This was later confirmed by Xu et al.10, who found that increasing nicotinamide riboside dose increased muscle weight and increased muscle fiber density. These first two studies were conducted in a commercial yield broiler. Because high yield broilers possess a more significant genetic potential for ultimate muscle mass size, the study's objective was to determine the effects of nicotinamide riboside dose on high yield broiler hatched chick pectoralis major muscle development and growth at hatch.
Protocol
All methodologies were approved by the University of Georgia Institutional Animal Care and Use Committee.
1. Egg incubation and treatment administration
- Egg procurement and treatment assignment
- Obtain unincubated, fertilized high-yield broiler eggs and transport them to the laboratory.
- Inspect and discard eggs deemed poor quality.
NOTE: Eliminate misshapen eggs (round, elongated, slab-sided), cracked, dirty/stained, thin-shelled, and wrinkled. This is important to minimize the risk of rotten eggs. - Assign individual egg numbers, weigh, and record egg numbers and weights in a spreadsheet software program.
- Utilize the spreadsheet software program to sort eggs by weight.
- Highlight the egg number and egg weight columns.
- Select the Data tab and then Sort – sort data by egg weight from smallest to largest.
NOTE: For the best hatching rate, use eggs weighing between 40 and 70 g. - Based on the design of the experiment, assign eggs (numerically or alphabetically) an injection treatment and day of euthanasia. Enter treatment number and day of euthanasia in separate columns and randomly assign these factors within each stratum.
NOTE: For this publication, treatments were randomly assigned within each 8-egg strata.
- Generate a PivotTable within the spreadsheet software program to ensure each treatment possesses similar starting egg weights.
- Highlight all data within the spreadsheet to be analyzed.
- Select PivotTable option under Insert tab.
- Select the independent variable (Day of Euthanasia column) within the PivotTable Fields sub-window and drag to Rows field.
- Select the independent variable (Treatment column) within the B sub-window and drag to Rows field, under Day of Euthanasia.
- Select the dependent variable of interest (Egg Weight) and drag it to the Values field.
- Change Value field settings by clicking on the dependent variable and selecting Value Field Settings.
- Change the setting to Average.
- Tray assignment
- In the spreadsheet software program, assign eggs to a tray (numerically or alphabetically), so treatments are equally represented within a tray.
- Assign the first four eggs with assigned treatments to tray 1. Assign the next four eggs to tray 2 and continue until all eggs are assigned to a tray.
NOTE: This step will vary depending on the number of incubators and trays used in the experiment.
- Assign the first four eggs with assigned treatments to tray 1. Assign the next four eggs to tray 2 and continue until all eggs are assigned to a tray.
- In the spreadsheet software program, assign eggs to a tray (numerically or alphabetically), so treatments are equally represented within a tray.
- Ensure all treatments are equally represented on a tray using the PivotTable function.
- Highlight all data within the spreadsheet to be analyzed.
- Select the PivotTable option under the Insert tab.
- Select the independent variable (Tray column) within the PivotTable Fields sub-window and drag to the Rows field.
- Select the dependent variable of interest (Egg Weight) and drag it to the Values field.
- Change Value field settings by clicking on the dependent variable and selecting Value Field Settings.
- Change the setting to Count.
- Incubation
- Place eggs in their appropriate incubation tray and pre-incubate them at 26.6 °C with 40% ± 4% relative humidity for 6 h.
NOTE: Some incubators have self-monitoring systems that may not be entirely accurate. Use other temperature and humidity monitoring devices to control conditions. - Increase the incubator temperature to 37 °C with 40% ± 4% relative humidity and maintain these conditions until incubation day 18.
- To ensure proper incubator temperature, measure several eggs' surface temperatures throughout the incubator twice daily with a thermal surface thermometer to ensure surface temperatures are 37 °C.
- Rotate eggs hourly to reposition.
- Record egg weights daily to ensure 10%-12.5% egg weight loss during the first 18.5 days of incubation.
NOTE: If weight loss is not within the desired range, adjust (increase or decrease) humidity.
- Place eggs in their appropriate incubation tray and pre-incubate them at 26.6 °C with 40% ± 4% relative humidity for 6 h.
- Incubation day-10 in ovo injections
- Calculate the amount of nicotinamide riboside needed for each treatment using the formula weight of 290.07 g/mol, with 100 µL of solution injected into each eggs' yolk sac.
NOTE: Sterile saline (0.9%) solution will be used as the diluent for all solutions.
Calculation: 50 eggs × 100 µL = 5,000 µL (5 mL) of solution needed. Round up to 6 mL to ensure enough solution is available for injection (Figure 1).- Once solutions are made, place them in a 37 °C water bath to keep them at the temperature of the eggs.
- Remove eggs from the incubator one tray at a time and cover with a warm towel.
- Candle egg to locate the yolk sac and clean the area of injection with 70% ethanol.
- Insert a sterile 20 G, 2.54 cm hypodermic needle ~1 cm into the eggshell and inject the assigned dose into the yolk sac. Inject the eggs from the 0 mM nicotinamide riboside treatment with 100 µL of sterile saline (0.9%).
- Immediately, cover the injection site with a small piece of absolute waterproof tape to avoid excessive moisture loss.
- Once all eggs have received their treatment, place the tray back into the incubator.
- On incubation day 18, remove eggs from trays and place them in hatching boxes according to their treatments.
- Place hatching boxes into the incubator and increase humidity to 60 ± 2% until all eggs hatch or until day 23 of incubation.
NOTE: If eggs do not contain an embryo at candling, discard the egg. This will prevent the occurence of rotten eggs.
- Calculate the amount of nicotinamide riboside needed for each treatment using the formula weight of 290.07 g/mol, with 100 µL of solution injected into each eggs' yolk sac.
2. Euthanasia and pectoralis major muscle sample collection
- Chick euthanasia
- On incubation day 18, remove the embryonic eggs from the incubator and place them at room temperature for 1 h to cease metabolism. Remove the embryos from the eggs, weigh without the yolk sac, and then decapitate. 12 h post-hatching, euthanize the chicks by exposure to CO2 for 10 min, weigh, quickly collect crown-to-rump length measurement, and then decapitate.
NOTE: The fact that the bird no longer has their head ensures euthanasia. - Consider the following measurements (steps 2.1.2.1-2.1.2.4) using digital calipers for embryos and chicks.
- For determining crown-to-rump length, lay the chick on its side with its head tucked down and legs under its body. Measure from the top of the head to the tail.
- To measure the head width, measure from one ear hole to the other ear hole.
- For determining head length, measure from the rear of the beak to the back of the cranium.
- Take a non-elastic string and wrap it around the skull from one ear hole to the other to measure the head circumference. Place string on a metric ruler to obtain a measurement.
- Collect chest circumference by wrapping a string around the chest, under where the wings contact the body and placing the string on a metric ruler to obtain the measurement.
- Spray breasts with 70% ethanol, and using fingers, pull the feathers and skin to reveal the pectoralis major muscles and take the measurements (steps 2.1.4.1-2.1.4.2) with digital calipers.
- For determining the chest width, measure across the chest where the wings contact the body.
- For determining the chest length, measure from the bottom of the clavicle to the top of the fat pad.
- On incubation day 18, remove the embryonic eggs from the incubator and place them at room temperature for 1 h to cease metabolism. Remove the embryos from the eggs, weigh without the yolk sac, and then decapitate. 12 h post-hatching, euthanize the chicks by exposure to CO2 for 10 min, weigh, quickly collect crown-to-rump length measurement, and then decapitate.
- Extraction of pectoralis major muscle, measurement, and collection
- Using surgical scissors or scalpel and forceps, remove the right pectoralis major muscle by cutting along the keel bone and releasing the muscle from the body wall.
NOTE: Be sure not to collect the pectoralis minor muscle by visually identifying that the muscle remains on the rib cage. - After removing the pectoralis major muscle, lay the muscle flat on a popsicle stick, and collect the following measurements (steps 2.2.2.1-2.2.2.3) using digital calipers.
- For determining the muscle length, measure from the cranial to the caudal portion of the muscle.
- For determining the muscle width, measure at the widest portion of the cranial part of the muscle.
- For determining the muscle thickness, pick up the breast with forceps and measure at the thickest portion of the cranial part of the muscle.
- If desired, store this muscle and the left pectoralis major muscle for further analyses (such as histology, protein and gene expression, etc.) at -80 °C for up to a year.
- Using surgical scissors or scalpel and forceps, remove the right pectoralis major muscle by cutting along the keel bone and releasing the muscle from the body wall.
3. Statistics
- Analyze the data as a completely randomized design with egg/chick as the experimental unit.
NOTE: Nicotinamide riboside dose (DOS) served as the fixed effect. All data were analyzed with a statistical analysis software program (see Table of Materials), and pairwise comparisons between least-square means of treatments were computed. Differences were considered significant at P < 0.05.
Representative Results
There were no DOS effects for the bodyweight of day-18 embryos and hatched chicks (P > 0.52; Figure 2). There were no DOS effects for all day-18 embryo pectoralis major muscle measurements (P > 0.24; Figure 3). There were no DOS effects for hatched chick pectoralis major muscle length and width measurements (P > 0.26); however, DOS did affect muscle weight and depth (P < 0.03; Figure 4). Chicks from embryos not injected with nicotinamide riboside had pectoralis major muscles that weighed less than chick from embryos injected with 500 and 1,000 mM nicotinamide riboside (P < 0.03), but these treatments did not differ (P = 0.41) from each other. Chicks from embryos injected with 250 mM nicotinamide riboside did not vary in pectoralis major weight compared to the other treatments (P > 0.06). Chicks from embryos injected with 0 and 250 mM nicotinamide riboside had less pectoralis major depth than chicks from embryos injected with 500 and 1,000 mM nicotinamide riboside (P < 0.05), but these treatments did not differ (P = 0.95). Chick from embryos injected with 500 and 1,000 mM nicotinamide riboside did not vary (P = 0.73) in pectoralis major depth.

Figure 1: Nicotinamide riboside dose general calculation and examples of the three doses utilized in the current experiment. Please click here to view a larger version of this figure.
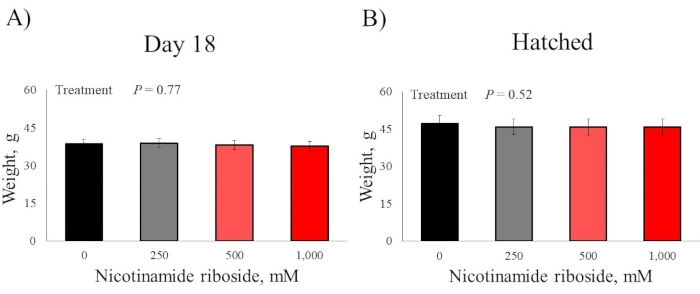
Figure 2: Effect of in ovo feeding of four doses of nicotinamide riboside on (A) day-18 embryo and (B) hatch chick body weights. Embryos were injected into the yolk sac with four nicotinamide riboside doses at day-10 of incubation. Please click here to view a larger version of this figure.
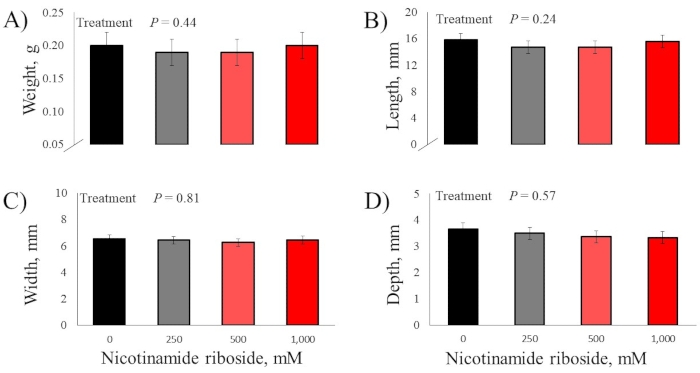
Figure 3: Effect of in ovo feeding of four doses of nicotinamide riboside on day-18 embryo pectoralis major muscle. (A) Weight. (B) Length. (C) Width. (D) Depth. Embryos were injected into the yolk sac with one of four nicotinamide riboside doses at day 10 of incubation. Please click here to view a larger version of this figure.
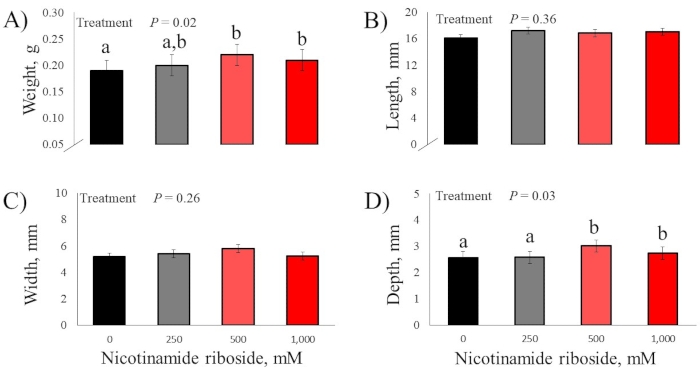
Figure 4: Effect of in ovo feeding of four doses of nicotinamide riboside on hatch chick body pectoralis major muscle. (A) Weight. (B) Length. (C) Width. (D) Depth. Embryos were injected into the yolk sac with one of four nicotinamide riboside doses at day 10 of incubation. a,b indicates the statistical difference from each other within a sub-figure (P < 0.05). Please click here to view a larger version of this figure.
Discussion
To date, the Gonzalez Laboratory at the University of Georgia is the only group to demonstrate positive effects of nicotinamide riboside in ovo feeding on pectoralis major muscle development and growth. The first study found that in ovo feeding of 250 mM nicotinamide riboside increased muscle weight and dimensions when injected in the yolk sac9. In the follow-up study, injecting increasing nicotinamide riboside dose into the yolk, similar to the doses tested in the current study, did not increase pectoralis major muscle morphometrics beyond the 250 mM dose10. These two studies utilized a commercial yield broiler line; therefore, this study was conducted to demonstrate the effects of in ovo feeding of high yield broiler embryos with nicotinamide riboside.
Through these studies, several critical steps have been identified located in this protocol that determines success. This process is critically important for those unfamiliar with selecting eggs for incubation to reduce bacteria spread and not biasing hatched-chick results. First, it is critically important not to choose dirty or misshapen eggs because they possess bacteria that could hamper the other eggs. These bacteria will spread rapidly through the incubator and cause rotten egg incidence to increase drastically; thus, affecting the number of embryos and chicks available for sampling.
As for assigning eggs to experimental treatments, researchers must utilize spreadsheet software methods described above to ensure all treatment starting egg weights are equal. Completing this step will be demonstrated in the embryo and hatched-chick whole-body morphometric data. This will ensure that all experimental treatment muscle differences are due to treatment application. There were no nicotinamide riboside effects on all body morphometric measures in the Gonzalez and Jackson9 and Xu et al.10 studies. Because of these consistent findings, only embryo and hatched-chick body weights were measured in the current research to establish a lack of a nicotinamide riboside effect on whole-body morphometrics; however, methodologies for collecting whole body morphometrics are presented in this publication for those wishing to collect those data. There were no nicotinamide riboside dose effects on embryo or chick body weights in the current study, continuing the trend reported previously.
Because this methodology strictly affects secondary myogenesis, future research teams may be tempted to inject embryos at an earlier time point. In the authors' experience, early injection, from incubation days 0 to 5, drastically reduces the hatchability of eggs by up to 70 to 80%. An early injection is a significant limitation of the technique. It could serve as a future area of research, but in the authors' experience, early injection is detrimental to hatchability which severely reduces the value of this technology.
When measuring morphometrics of the pectoralis major muscle, researchers must ponder two crucial considerations. First, the authors advise a single, well-trained researcher to remove all muscles utilized for morphometric analysis. Because the pectoralis major muscle is so small, much-unwanted variation or bias could be introduced into data by collecting other muscles outside the muscle of interest. Utilizing a single researcher will ensure the same muscle will be collected according to consistent landmarks used to identify the muscle. Second, when placing muscles on the wood surface for measurement, care must be taken in laying all muscles in a natural position. This is especially true for the length measurement, as it can be manipulated by stretching the muscle when laying it down on the measurement surface. No nicotinamide riboside effects were seen in the current study for pectoralis major muscle morphometrics at incubation day 18. Xu et al.10 reported no pectoralis major muscle weight and length differences at incubation day 19; thus, indicating nicotinamide riboside's effect on whole muscle morphometrics may not manifest until after incubation day 19 in these two genetic broiler lines.
Compared to previously published studies, one of the major modifications in the current study was the use of commercially available capsule-form nicotinamide riboside. In the previous studies9, 10, pure nicotinamide riboside was secured from a manufacturer. With assistance from the manufacturer, the research group was informed that the commercial product utilized in the current study also had cellulose ingredients mixed into the product, reducing the calculated concentration of nicotinamide ribose by 34%. Therefore, in the present study, pectoralis major muscle weight from hatched chicks injected with 500 and 1,000 mM nicotinamide riboside was greater than chicks from embryos injected with 0 mM nicotinamide riboside by 15 and 10%, respectively. This weight increased mainly due to these treatments' pectoralis major muscle depth increasing by 17 and 7%, respectively. This response was less than half of the previous responses. Xu et al.10 reported nicotinamide riboside supplementation, 250 to 1,000 mM concentrations, increased pectoralis major muscle weight by 35% due to increased muscle length, width, and depth. While reduced response could be primarily due to supplementing less nicotinamide riboside than calculated, it is also unknown if cellulose material hindered myogenesis. Therefore, the authors recommended all future research utilize pure nicotinamide riboside and not commercially available products.
Regardless of the present results, following the methodologies outlined in this publication will ensure robust execution of in ovo feeding studies. Future researchers can utilize the above methods to test other compounds that may positively affect broiler chicken in ovo muscle development and growth.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Cobb Vantress, Inc. for donation of the eggs and providing technical assistance on egg incubation. The authors would like to thank ChromaDex, Inc. for nicotinamide riboside technical assistance.
Materials
| Air-Tite™ Sterile Hypodermic Needles- 20 G; 1 inch | Fisher Scientific | 14-817-208 | https://www.fishersci.com/shop/products/sterile-hypodermic-needles-32/p-7182916#?keyword= |
| Analytical Balance | VWR | VWR-214B2 | https://us.vwr.com/store/product/20970740/vwr-b2-series-analytical-and-precision-balances |
| Complete Dissection Set | DOCAZON | DK1001 | https://www.amazon.com/DOCAZON-Complete-Dissection-Set-Dissecting/dp/B07VBHKSW3 |
| Fisherbrand™ Isotemp™ General Purpose Deluxe Water Baths | Fisher Scientific | FSGPD02 | https://www.fishersci.com/shop/products/isotemp-general-purpose-water-baths/p-6448020 |
| Fisherbrand™ Sterile Syringes for Single Use | Fisher Scientific | 14-955-464 | https://www.fishersci.com/shop/products/sterile-syringes-single-use-12/p-7114739#?keyword= |
| HIGH INTENSITY EGG CANDLER | Titan Incubators | N/A | https://www.titanincubators.com/collections/egg-candlers/products/egg-candler-high-intensity |
| Infrared Forehead Thermometer | HALIDODO | XZ-001 | |
| Microsoft Excel | Microsoft | N/A | |
| Neiko Tools Digital Caliper | Neiko Tools | 01408A | https://www.amazon.com/Neiko-01407A-Electronic-Digital-Stainless/dp/B000NEA0P8?th=1 |
| Nexcare Absolute Waterproof Tape | Nexcare Brand | 732 | https://www.nexcare.com/3M/en_US/nexcare/products/catalog/~/Nexcare-Absolute-Waterproof-Tape/?N=4326+3294529207+3294631805 &rt=rud |
| Pen Size Temperature and Humidity USB Data Logger with Display | Omega | OM-HL-SP-TH | https://www.omega.com/en-us/temperature-measurement/temperature-and-humidity-data-loggers/p/OM-HL-SP-Series |
| SAS 9.4 for Windows | SAS Institute | N/A | https://www.sas.com/en_us/home.html |
| Sportsman 1502 Incubator | GQF Manufacturing | 1502 | https://www.gqfmfg.com/item/1502-digital-sportsman/ |
| Tru Niagen (Nicotinamide riboside) | ChromaDex, Inc. | N/A | https://www.truniagen.com/truniagen-300mg/ – note, contact company for pure product |
| Wood Craft Sticks | Creatology | M20001547 | https://www.michaels.com/wood-craft-sticks-by-creatology/M20001547.html |
References
- Biressi, S., Molinaro, M., Cossu, G. Cellular heterogeneity during vertebrate skeletal muscle development. Biologie du développement. 308 (2), 281-293 (2007).
- Chal, J., Pourquie, O. Making muscle: Skeletal myogenesis in vivo and in vitro. Development. 144 (12), 2104-2122 (2017).
- Sharma, J., Burmester, B. Resistance of Marek’s disease at hatching in chickens vaccinated as embryos with the Turkey herpesvirus. Avian Diseases. 26 (1), 134-149 (1982).
- Al-Murrani, W. K. Effect of injecting amino acids into the egg on embryonic and subsequent growth in the domestic fowl. British Poultry Science. 23 (2), 171-174 (1982).
- Ohta, Y., Kidd, M. T., Ishibashi, T. Embryo growth and amino acid concentration profiles of broiler breeder eggs, embryos, and chicks after in ovo administration of amino acids. Poultry Science. 80 (10), 1430-1436 (2001).
- Zhao, M. M., et al. In ovo feeding of creatie pyruvate increases hatching weight, growth performance, and muscle growth but has no effect on meat quality in broiler chickens. Livestock Science. 206, 59-64 (2017).
- Bieganowski, P., Brenner, C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss Handler independent route to NAD1 in fungi and humans. Cell. 117 (4), 495-502 (2004).
- Chi, Y., Sauve, A. Nicotinamide riboside, a trace nutrient in foods, is a Vitamin B3 with effects on energy metabolism and neuroprotection. Current Opinion in Clinical Nutrition and Metabolic Care. 16 (6), 657-661 (2013).
- Gonzalez, J. M., Jackson, A. R. In ovo feeding of nicotinamide riboside affects pectoralis major muscle development. Translational Animal Science. 4 (3), 1-7 (2020).
- Xu, X., Jackson, A. R., Gonzalez, J. M. The effects of in ovo nicotinamide riboside dose on broiler myogenesis. Poultry Science. 100 (3), 100926 (2021).

