In Situ Visualization of Axon Growth and Growth Cone Dynamics in Acute Ex Vivo Embryonic Brain Slice Cultures
Summary
This protocol demonstrates a straightforward and robust method to study in situ axon growth and growth cone dynamics. It describes how to prepare ex vivo physiologically relevant acute brain slices and provides a user-friendly analysis pipeline.
Abstract
During neuronal development, axons navigate the cortical environment to reach their final destinations and establish synaptic connections. Growth cones -the sensory structures located at the distal tips of developing axons- execute this process. Studying the structure and dynamics of the growth cone is crucial to understanding axonal development and the interactions with the surrounding central nervous system (CNS) that enable it to form neural circuits. This is essential when devising methods to reintegrate axons into neural circuits following injury in fundamental research and pre-clinical contexts. Thus far, the general understanding of growth cone dynamics is primarily founded on studies of neurons cultured in two dimensions (2D). Although undoubtedly fundamental to the current knowledge of growth cone structural dynamics and response to stimuli, 2D studies misrepresent the physiological three-dimensional (3D) environment encountered by neuronal growth cones in intact CNS tissue. More recently, collagen gels were employed to overcome some of these limitations, enabling the investigation of neuronal development in 3D. However, both synthetic 2D and 3D environments lack signaling cues within CNS tissue, which direct the extension and pathfinding of developing axons. This protocol provides a method for studying axons and growth cones using organotypic brain slices, where developing axons encounter physiologically relevant physical and chemical cues. By combining fine-tuned in utero and ex utero electroporation to sparsely deliver fluorescent reporters along with super-resolution microscopy, this protocol presents a methodological pipeline for the visualization of axon and growth cone dynamics in situ. Furthermore, a detailed toolkit description of the analysis of long-term and live-cell imaging data is included.
Introduction
Neurons are highly polarized cells that represent the basic computational unit in the nervous system. They receive and emit information that relies upon compartmentalization of input and output sites: dendrites and axons, respectively1. During development, axons extend while navigating an incredible complex environment to reach their destination. Axon navigation is guided by the growth cone, a sensory structure located at the tip of the developing axon. The growth cone is responsible for detecting environmental cues and translating them into the dynamical spatial reorganization of its cytoskeleton2,3. The resulting morpho-mechanical reactions instruct the growth cone to extend or retract from the triggering cue, leading to specific axon maneuvers.
The current understanding of axon extension and growth cone dynamics stems from studies evaluating axon growth over two-dimensional (2D) substrates2,4,5,6,7. These pioneering studies identified sophisticated interplay between growth cones and growth substrates and revealed striking differences dependent on substrate characteristics such as adhesiveness and stiffness8,9. Led by these insights, extracellular environmental cues were hypothesized to dictate axon growth, with the growth cone cytoskeleton executing this growth2,10,11,12. Notably, neurons can extend axons in non-adhesive substrates (e.g., poly-lysine, poly-ornithine)13. Moreover, substrate rigidity can influence axon growth rate independent of cell adhesive complexes8. Hence, studying growth cone dynamics in 2D substrates alone cannot accurately model the balance of forces that arise from the interaction of axonal growth cones with physiologically relevant three-dimensional (3D) environments, such as those found in vivo.
To overcome the limitations of the 2D assays, axon growth and growth cone dynamics have been studied in 3D matrices8,9. These matrices pose more physiological context yet allow studying cell-intrinsic mechanisms of axon growth. It enables growth cone examination in a single-cell fashion in a variety of conditions and pharmacological treatments9. In such 3D environments, axons displayed distinct cytoskeletal dynamics and grew faster than those observed in 2D cultured neurons9. These elegant studies demonstrated the influence of an extra dimension on the reorganization of the growth cone cytoskeleton and, consequently, on its behavior.
Despite apparent advantages presented by 3D matrices over 2D surfaces in supporting native-like neuronal development and axon growth, they remain a simplified synthetic scaffold that cannot reflect the complexity of dynamics observed in central nervous system (CNS) tissue. Here, delivery of reporter plasmids by ex utero and in utero electroporation was combined with brain organotypic slice culture and in situ live super-resolution imaging to analyze growth cone dynamics within a physiological context. This methodology allows visualization of developing axons while experiencing the 3-dimensionality of in vivo environments and the complexity of its physicochemical composition. Lastly, user-friendly procedures to measure axon growth and growth cone dynamics using commonly licensed and publicly available software are described.
Protocol
Animal experiments must comply with the relevant institutional and federal regulations. Embryonic day 15.5 and 12.5 (E15.5 and E12.5) pregnant female C57BL/6JRj mice were used in this protocol. Experiments were performed in accordance with the Animal Welfare Act of North Rhein-Westphalia State Environmental Agency (Landesamt für Natur, Umwelt und Verbraucherschutz (LANUV)).
1. Preparation of plasmids for injection
- Isolate DNA using endotoxin-free Maxiprep kit according to manufacturer's protocol (see Table of Materials).
- Mix selected DNA at the desired concentration (Table 1) and 10% Fast Green solution (see Table of Materials) to visualize the delivery of DNA mixture into brain ventricles.
NOTE: Specific plasmids were used for sparse labeling of the cortical neurons (Figure 1A), filamentous actin (F-actin) structures in the growth cone (Figure 1B), and dual-labeling of the growth cones within the same cortex (Figure 1C). All plasmids (Table 1) used in this protocol have been deposited in Addgene (see Table of Materials). - Prepare glass capillaries using a capillary electrode puller set as per the following program: pressure: 500, heat: 800, pull: 30, and velocity: 40.
- Load 15 µL of DNA/Fast Green mix into each glass capillary using micro loader pipette tips.
NOTE: Ensure that no bubbles form. - Store DNA-filled capillaries in a 10 cm dish with a piece of modeling clay across the diameter of the dish. Capillaries can be loaded and stored at 4 °C the day before the experiment. Seal the back end of the capillary with flexible film to prevent drying.
2. Preparation of solutions
- Prepare Hank's Buffered Salt Solution-supplemented with glucose (HBSS-G).
- Add 0.5% of 20% glucose stock to a bottle of 1x HBSS. Mix well and store at 4 °C for up to 2 weeks. For the embryo extraction, bubble HBSS-G solution with carbogen (95% O2 and 5% CO2) using bubbling stone shortly before embryo collection.
- Slice media solution
- Prepare fresh slice media containing Neurobasal 1x, 5% horse serum, 5% foetal calf serum, B27 supplement 1:50, L-glutamin supplement 1:400, penicillin-streptomycin 1:200, and Neuropan-2 supplement 1:100 (at pH = 7.3), under sterile conditions (see Table of Materials).
- Prepare 3 cm dishes with 1 mL of slice media each. Place in the incubator at 35 °C with 5% CO2 for at least 1 h before the experiment to equilibrate the media's pH through gas exchange.
NOTE: Media pH equilibration is caused by acidification of the media by the CO2 from the incubator. Slice media can be stored at 4 °C for up to 1 week.
- Low melting point agarose solution (3%)
- Weigh the desired amount of low melting point agarose powder and dissolve in an appropriate volume of 1x HBSS-G in a glass bottle. Approximately 7 mL of agarose solution per brain is needed.
- Place the bottle in a microwave for 2-3 min, with the cap loosely placed, and shake every 10-20 s.
- Once the powder has completely dissolved, place the bottle in a water bath or bead bath set to 37 °C at least 1 h before the experiment to allow agarose to cool down.
NOTE: It is recommended to heat the agarose twice over 15 min to ensure the agarose powder is dissolved. This is crucial for the proper adhesion of agarose to brain tissue. A thermometer should be used to measure the temperature of the agarose solution while embedding brains, making sure it is between 37-40 °C. Brains from different aged animals have different rigidity. It is recommended to test a range of agarose concentrations to find homogeny between tissue and agarose. - Prepare Phosphate Buffered Saline with 0.3% Triton X-100 (PBS-T).
- Prepare Phosphate Buffered Saline with 0.2% Sodium Azide (PBS-NaN3).
NOTE: Solutions described in steps 2.4-2.5 are for use in the later immunohistochemistry step.
3. Preparation of the surgery station
- Clean the surgery station with 70%-96% ethanol and place operation underlay on the station surface.
- Sterilize the surgery instruments by rinsing with 70%-96% ethanol, followed by dry sterilization in a hot bead sterilizer.
- Clean platinum tweezer electrodes (see Table of Materials) with 70%-96% ethanol before connecting to the pulse generator.
- Insert a DNA/Fast Green-filled glass capillary into the capillary holder. Immediately before use, gently break off the capillary tip using fine scissors and test solution flow inside a 1.5 mL microcentrifuge tube filled with pre-warmed saline or water.
NOTE: Figure 2A,B show surgery station set-up and tools used for ex utero electroporation (EUE) and in utero electroporation (IUE). - For IUE, warm-up saline solution to 37 °C in a water bath.
4. Embryo extraction
- Place pregnant mouse in anesthesia inducer chamber with 5% isoflurane until the mouse is deeply anesthetized. Confirm anesthesia by the absence of pedal withdrawal reflex.
- Transfer the mouse to an operation underlay and maintain isoflurane at 1.5%-2% through a nose cone.
- Apply ointment to both eyes to prevent corneal drying.
- Shave the mouse's abdomen, and then use 70%-96% ethanol-soaked gauze to remove shaved hair. Clean the area with betadine.
- Using sterile small surgical scissors, make a 2 cm skin incision along the abdominal midline, followed by a 1.5 cm muscle incision.
NOTE: Incision size depends on the embryo size. Indeed, larger embryos will require a larger incision to accommodate their extraction. - Cut a hole in the middle of the gauze sufficiently wide to fit the skin incision (~2 cm in diameter), soak it with warm saline, and place it around the abdominal opening.
- Pull out both uterine horns using a cotton bud soaked in warm saline or using forceps, carefully grabbing the spaces between embryos to pull them out. Place embryos on wet gauze (Figure 2C).
NOTE: Even a small damage to blood vessels and capillaries around the uterine horns will likely result in profuse bleeding. Therefore, avoid direct handling of these vascularised areas at all times. - Cut open the uterine sack and remove each embryo (Figure 2D).
- Euthanize each embryo immediately after extraction via a descending diagonal cut, ensuring complete spinal cord transection (Figure 2E).
- Place the embryos in a 10 cm dish containing HBSS-G on ice.
NOTE: Beheading the embryo is avoided to prevent DNA/Fast Green mixture leakage from the brain and facilitate easy embryo positioning in the holder (see ex utero electroporation, step 5). - Sacrifice the mother immediately after extraction of embryos by performing cervical dislocation.
NOTE: Here, the mother is euthanized under anesthesia to spare it from any further post-procedure pain or suffering in compliance with the protocol approved by the Animal Welfare Act of North Rhein-Westphalia State Environmental Agency (LANUV).
5. Ex utero electroporation (EUE)
- Pick up an embryo and place it in the holder.
NOTE: A cut 1 mL pipette tip attached to the end of a cell scraper is used as an embryo holder. It is essential to keep arms of embryos outside the tip during the procedure to prevent them from sliding into the tip (Figure 2F-I). The diameter of the pipette tip is easily adjustable to accommodate embryos of various sizes. Cut a second tip at length where the diameter of the tip matches embryo size and use it as an adapter insert to the holder mentioned above. - Carefully insert DNA/Fast Green-filled glass capillary through the embryo's skull into the lateral ventricle and inject 2-3 µL of DNA plasmid mix (Figure 1A,B; Table 1) into each ventricle (Figure 2F).
NOTE: Use the lambdoidal and sagittal sutures as a guide for the location of DNA injection. The lambdoidal and sagittal sutures are fibrous joints connecting the bone plate of the skull. The former joins the parietal bone with the occipital bone, and the latterjoins the two parietal bones. - Hold the embryo's head between platinum tweezer electrodes at the appropriate angle to target the desired brain area (60° angle in this case), with the cathode facing the area where DNA transfer is intended (Figure 2G-H).
- Apply five pulses at 30 mV with an interval of 1 s and a duration of 50 ms using a square wave pulse generator.
NOTE: Bear in mind that brains in EUE experience a more effective electric field than those in IUE. Hence, at a given DNA concentration, EUE results in higher efficiency of DNA transfer than IUE, and DNA concentrations need to be adjusted accordingly. - If bilateral electroporation is desired, repeat steps 5.3-5.4 with the cathode and anode mirroring the previous position to target the contralateral cortex.
NOTE: Since both ventricles were injected with DNA, cortices of both hemispheres were targeted. - Place the electroporated embryo in a 6 cm dish containing ice-cold HBSS-G. Repeat steps 5.1-5.6 for all embryos required.
6. In utero electroporation (IUE)
- Inject pregnant mouse with analgesic; 50 µL of buprenorphine (0.1 mg/kg) (see Table of Materials) subcutaneously, 20 min before the procedure.
- Perform steps 4.1-4.8 from the embryo extraction section.
NOTE: Avoid leaving embryos exposed unnecessarily by covering them with sterile gauze soaked in warm saline. - Using fingertips, gently rotate the embryo inside the uterus until lambdoidal and sagittal sutures are located (Figure 2J). Carefully insert DNA/Fast Green glass capillary through the uterine wall and embryo's skull into the lateral ventricle and inject 2-3 µL of DNA plasmid mix (Figure 1A,C) into either one or both ventricles as desired (Figure 2K-L).
NOTE: Excessive finger pressure on uterine horns could lead to amniotic sack collapse. - Hold the embryo's head between platinum tweezer electrodes at the appropriate angle to target the desired brain area (60° angle in this case), with the cathode facing the area where the DNA transfer is intended. Avoid squeezing the uterus since it may cause the collapse of the amniotic sack (Figure 2M).
- Apply five pulses at 35 mV with an interval of 600 ms and a duration of 50 ms using a square wave pulse generator.
- If both lateral ventricles were injected, repeat steps 6.5-6.6 with the cathode and anode mirroring the previous position to target the contralateral cortex.
- Repeat steps 6.3-6.6 for all embryos required.
- Once all the required embryos have been electroporated, use a saline-soaked cotton bud to place uterine horns back inside the abdominal cavity gently.
NOTE: The addition of saline solution into the peritoneal cavity will help uterine horns slide back into position. - Suture muscle and skin incisions using 5-0 suture material. Use suture clips to secure the wound and disinfect the suture wound by spraying it with betadine (Figure 2N-P).
- Inject the mouse with 200 µL of 5% glucose subcutaneously.
- Inject the mouse with an antibiotic; 50 µL of Enrofloxacin (5 mg/kg) subcutaneously (see Table of Materials).
- Place the mouse back in the recovery cage and maintain warmth using a far-infrared warming light or heating pad for at least 20 min post-procedure (Figure 2Q).
- Monitor the mouse daily, and inject meloxicam after the procedure for pain relief following institutional and federal guidelines.
- Extract embryos 2 days after the procedure (i.e., E17.5) following step 4.
7. Brain extraction and embedding in agarose
NOTE: It is recommended to carry out the following steps under a dissection microscope for better precision. Avoiding damage to the brain is critical for the success of the procedure.
- Set up extraction tools in a sterile working space under a dissection hood (Figure 3A).
- Separate the head of an embryo from the rest of the body using dissection scissors.
- Fix the head as shown in Figure 3C, and then remove the skin and skull by cutting along the midline, starting from the base of the head toward the nose (Figure 3D).
- Peel the skin and skull laterally, making a big enough gap (~1 cm) for the brain to be excised.
- To remove the brain, insert the closed tip of sterile dissection scissors, starting from under the olfactory bulb moving toward the brain stem (Figure 3E).
- Cut off the brain stem and trim any loose pieces of meninges around the brain (Figure 3F).
NOTE: Loose meninges often cause slices to remain attached to the agarose block after cutting, leading to detaching of tissue from the agarose during slice collection. - Repeat steps 7.1-7.6 for all embryos and keep the brains on ice (ideally no longer than 30 min) until the embedding step.
NOTE: The following steps 7.7.1-7.7.4 refer to brain extraction of E12.5 brains.- Isolate the top of the head just below the eye, as shown in Figure 3G.
- Cut the skin and skull on top of the brainstem, following the dashed line as shown in Figure 3H, without removing the brainstem.
- Make a 2 mm skin-skull incision at the back of the head, as shown in Figure 3I (see drawings for clarity).
NOTE: This incision provides initial gripping points to peel off the layers of skin and skull. Typically, they come off as one layer. The typical incision size is 2 mm, corresponding to the length of the cutting edge of the used micro spring scissors. - Start peeling off skin-skull layers by securing one side of the incision and pulling the other carefully. With the same care, finalize by peeling off the base of the head until freeing the brain (Figure 3J).
NOTE: This must be done with great care, observing that the brain is not being pulled along the tissue layers. Alternate sides to remove the tissue covering the brain.
- Pour warm agarose (at 37-40 °C) in a 3 cm dish.
- Pick up the brain using a perforated spoon and remove excess liquid by dabbing the bottom of the spoon against dry tissue paper. Place the brain in an agarose dish.
NOTE: It is essential to remove as much liquid from around the brain as possible to allow for better adhesion of agarose to the tissue. - Put the dish with liquid agarose on ice. Using a smaller spoon, mix agarose for 10 s for even cooling. Maneuver the brain to the middle of the dish. Place the brain horizontally in the dish with the dorsal side up, ensuring it is completely covered with agarose from all directions (Figure 3K).
NOTE: Brains will often sink to the bottom of the dish once placed in agarose; lift the brain using a small spoon until a gap of 1-2 mm under the brain is established. - Repeat steps 7.8-7.10 for all brains.
- Once agarose has polymerized, add 500 µL of HBSS-G on top of the agarose block to prevent drying. Then, cover the dish with ice.
NOTE: Keep the sample on ice for 5 min before sectioning to allow the brain temperature to reach 4 °C.
8. Organotypic slice culture
NOTE: Clean vibratome and surrounding surfaces with 70%-96% ethanol to avoid slice contamination. The set-up of the vibratome workstation (see Table of Materials) is shown in Figure 3B.
- Fill the vibratome buffer tray with cold HBSS-G and the outer tray with ice to maintain the HBSS-G cold throughout the procedure.
- Continuously supply HBSS-G in the buffer tray with carbogen using a bubbling stone.
- Using a fresh blade, make a large cut (~2 x 2 cm) around the brain and remove a block of agarose containing the brain, with enough surrounding agarose to trim the agarose into a small rectangle block.
NOTE: This step allows adjusting the block's angle so that the sagittal axis of the brain is perpendicular to the vibratome plate and the coronal axis aligns parallel to the blade. Leave around 5 mm of agarose at the dorsal side of the brain for easy handling of the slices. - Place a small drop of fast-adhesive solvent-free superglue in the middle of the specimen holder and spread to an area that will cover the bottom of the agarose block.
- Gently pick up the agarose block and dry the bottom by dabbing against tissue paper. Place the block on the glued area of the specimen holder, with the rostral side of the brain up. Put the specimen holder on ice and allow the glue to dry for 1 min.
- Once the glue has dried, place the specimen holder in the buffer tray.
- Cut the brain in coronal slices at an angle of 15°.
NOTE: The thickness of the slices can vary depending on the application. Here, brains were sliced at a thickness of 150 µm. Set vibratome speed to 1.0-1.5 mm/s for trimming excess agarose on top and trimming olfactory bulbs. Reduce cutting speed to 0.5 mm/s for collecting cortical slices for analysis. Most vibratomes can be paused to collect each slice. If reduced quality of slices or detaching of tissue from agarose are experienced, reducing the cutting speed or replacing the vibratome blade can help. - Using clean spatulas, collect brain slices and place them on Polytetrafluoroethylene (PTFE) membrane, immobilized in a 35 mm glass-bottomed dish using paraffin (up to five brain slices/ membrane) (Figure 3L-M).
NOTE: Fix the PTFE membrane inside a 35 mm glass-bottomed dish using wax. This will stabilize the membrane when adding the slice culture media and also during imaging. - Using a 200 µL pipette, remove excess HBSS-G from around the slices on the PTFE membrane, leaving the slices semi-dry.
- Add 500 µL of slice media (pre-warmed to 35 °C) directly to the space under the PTFE membrane.
NOTE: No bubbles must form under the membrane when adding the media. This will leave whole or partial slices with no media exchange. Replace 200 µL of media every 2 days in culture or after every imaging session. - Incubate the slices at 35 °C with 5% CO2.
9. Immunohistochemistry
- Fix slices with 1 mL of 4% paraformaldehyde (PFA)-supplemented with 4% sucrose-per dish. Incubate at RT for 30 min.
CAUTION: When handling PFA, wear a lab coat and gloves. Perform fixation steps under a chemical hood, and dispose of PFA waste appropriately. - Wash the slices twice with 300 µL PBS for 5 min. Transfer the slices to a 24-well plate.
NOTE: The experiment can be paused at this stage. Add PBS-NaN3 to the slices and store at 4 °C. NaN3 is a toxic compound; when handling solutions with it, wear a lab coat and gloves. Steps 9.3-9.10 areto be performed in an orbital shaker. - Quench the slices with 300 µL of 0.1 M glycine at 4 °C overnight.
- Wash out glycine with PBS at RT 3x for 10 min.
- Permeabilise the slices with 300 µL of PBS-T at RT for 2 h.
- Block using 10% goat serum in PBS-T at RT for 2 h.
- Add 300 µL of primary antibody (anti-vimentin antibody at a dilution of 1:200; see Table of Materials) diluted in 10% goat serum in PBS-T solution at 4 °C overnight.
NOTE: In steps 9.8-9.12, slices were light-protected to prevent loss of fluorescence. - Wash primary antibody with PBS at RT 3x for 20 min.
NOTE: PBS was used instead of PBS-T to wash out Triton X-100. - Add 300 µL of secondary antibody (either Alexa Fluor 488 or 647 at a dilution of 1:400; see Table of Materials) in PBS at RT for 2 h.
NOTE: DAPI is added immediately after removing the secondary antibody at a dilution of 1:10,000 for 5 min. - Wash the secondary antibody with PBS at RT 3x for 20 min. Wash with distilled water 2x for 1 min.
- Transfer the slices to a glass slide using a fine brush, and then dry at 30 °C for 20 min.
- Mount the slices using an aqueous mounting medium. Keep the slides at RT overnight for the mounting media to curate.
10. Imaging acquisition
NOTE: Regardless of the DNA delivery approach (IUE or EUE), slices were analyzed at the same developmental age range (E17.5-E18.5). IUE allows neuronal progenitors to divide and develop for two further days in vivo. EUE, on the other hand, allows for the tracking of early developmental events.
- Switch on the chamber incubator and set it to 35 °C with 5% CO2-ideally 4 h before imaging-to allow microscope components to equilibrate at 35 °C.
- For deep imaging of slices, use water-immersion objectives to reduce the mismatch in refractive index between the tissue and objective.
NOTE: Here, super-resolution imaging mode was used. Imaging through the PTFE membrane requires an objective with a long working distance (~1 mm). If a long working distance objective is unavailable, slices may be transferred to an 8-well glass-bottomed dish. To transfer slices, add 1 mL of slice media to the top of the membrane, and then use a spatula to lift a slice and transfer it to a well containing 200 µL of media. Remove excess media using a 1 mL pipette tip leaving the slices semi-dry. - For imaging axon growth, locate a region of the cortex with low to medium cell density. For imaging growth cone dynamics, locate a growth cone in the cortex's intermediate zone or subventricular zone.
- Define a z-stack size in the image processing software (see Table of Materials). For axon growth in a large z-stack, set a step size of 2 µm. For growth cones in a smaller z-stack, set a step size of 1 µm.
NOTE: Always account for potential movement of the growth cone and axon through the x, y, and z planes. Axons grow at a much higher rate in organotypic cultures than in in vitro cultures. Here, a z-stack of around 80 µm to image axon growth was sufficient. For growth cone dynamics, a z-stack of ~6 µm was adequate. - For imaging axon growth of neurons in a larger area, define a tile scan.
- Use the lowest laser power possible to minimize the chances of bleaching growth cones during acquisition.
- For imaging axon growth, acquire time-lapses for 2 h with an interval of 5 min. For imaging growth cone dynamics, acquire time-lapses for 2-5 min with an interval of 2.5-3 s.
11. Data analysis
- Measure the speed of axon growth using kymographs
- Open the image file in Fiji14 through File > Open and select the image.
- Obtain the maximum intensity projection of the time-lapse through Image > Stacks > Z-Projection > Maximum Intensity Projection.
- Go through the time-lapse and locate a growing axon.
- Once located, draw a line through the growing axon. Start from the tip of the axon in the first frame, and follow the axon through the entire time-lapse.
- Generate a kymograph using the plugin KymoResliceWide.
- Set the scale of the kymograph by going to Image > Properties. Set the distance in µm in Pixel Width and set the time in s or min in Pixel Height.
- Go to Analyze > Measure.
NOTE: An angle relative to the x-axis will be given. - Calculate the speed of axon growth by substituting the angle in the following equation: SIN(RADIANS(θ))/COS(RADIANS(θ)) in a spreadsheet.
- Measure the volume of the growth cone using an image analysis software (see Table of Materials).
- Open the image file in the image analysis software through File > Open and select the file of interest.
- Select the Add New Surfaces wizard.
NOTE: A section on the lower-left corner will appear with six steps for manual editing. - In step 1 -under Algorithm Settings- select Segment Only A Region Of Interest. In step 2, crop the frame to fit the entire growth cone in all frames.
- Keep thresholding to Absolute Intensity in step 3, and ensure the entire growth cone region is thresholded in step 4.
- In step 5, select Number of Voxels lmg = 1 under Filter Type.
NOTE: In the last step, multiple measurement sets may be created. Here, only one measurement was created for volume. - Select the Execute button to perform all the creation steps and terminate the Add New Surfaces Wizard.
- In the Statistiques tab at the top of the wizard window, select Specific Values and Volume under the Detailed tab.
Representative Results
Representative results obtained with the described method workflow are shown. E15.5 mice were used in the present demonstration, though this protocol is easily adaptable to virtually all embryonic ages ranging from E11 to late E17. In this protocol, either ex utero electroporation (EUE; Figure 2A, 2C-I) or in utero electroporation (IUE; Figure 2B,C, and 2J–Q) were used to deliver plasmids into the progenitor neurons lining the lateral ventricles. These progenitors are the source of future cortical projecting neurons (CPN)15,16. Plasmid mixes were prepared to drive sparse neuron-specific expression of either membrane-targeted (Lyn)-mNeonGreen (Figure 1A) or LifeAct-enhanced (E)GFP (Figure 1B) to evaluate the overall behavior and actin dynamics in growth cones, respectively. Furthermore, a plasmid mix aimed to label individual neurons with either turbo(t)-RFP or zoanthus sp. (Zs) green fluorescent protein (ZsGreen) (Figure 1C) was included. This facilitates the monitoring of growth cone behavior from independent neighboring neurons.
Brain dissection from electroporated embryos is a crucial step that needs to be carefully performed to obtain high-quality slices, preserving the native brain structure. Dissection instruments and vibratome were prepared beforehand and carefully ethanol-sterilized (Figure 3A,B). Next, the heads of electroporated embryos were carefully dissected and the brains were extracted. Here, representative dissection of brains from the embryos subjected to EUE at E15 (Figure 3C–F) and E12.5 (Figure 3G–J) are shown. Brains are immediately encased in an agarose matrix, sliced, and placed on PTFE membrane inserts within a bottom-glass dish for incubation (Figure 3K–M).
The health status of brain slices is a significant point for control to ensure reliable results. A visual inspection for any contamination was performed daily. Additionally, once the culture was finalized, the brain slices are fixed and subjected to immunohistochemistry. Here,4′,6-diamidino-2-phenylindole (DAPI) was used to control the overall cellular organization and vimentin staining to reveal glial organization; particularly, radial glia (RG) scaffold. Typically, successfully cultured brain slices derived from either IUE or EUE show normal cellular distribution as revealed by DAPI and a somewhat organized array of RG with apically oriented pial-contacting processes17 (Figure 4A,B respectively). Occasionally, marked disturbances in the RG scaffolding in cultured brain slices are observed, especially in those derived from EUE electroporation (Figure 4C). Brain slices with extremely disorganized RG scaffold show impaired neuronal migration and defective axon growth (not shown). Hence, controlling the RG scaffold is an easy post-culture method to sort the data obtained from reliable brain slices.
Brain slices derived from either IUE or EUE with Lyn-mNeonGreen-expressing plasmid mix result in similar sparse neuron labeling. A representative pyramidal CPN expressing Lyn-mNeonGreen and the dynamic behavior of its growth cone is shown as an example (Figure 5A and Supplementary Video 1, top left). In addition, neurons were labeled using a plasmid expressing an actin probe to analyze actin dynamics of axonal growth cones in situ (Figure 5B and Supplementary Video 1, bottom left). In situ experiments were also performed with a dual-Cre/Dre fluorophore-expressing plasmid design (Figure 1C and Supplementary Video 1, right). tRFP or ZsGreen fluorophores in this plasmid could be specifically and individually activated by either Dre or Cre recombinases, respectively, in neighboring neurons (Figure 5C). This experimental line-up allows side-by-side analysis of growth cones from control neurons with neighboring modified neurons (any given loss or gain of function). This circumvents variability arising from the use of different slices to test control and experimental conditions.
Kymographs generated from the recorded movie were analyzed, from which dynamic growth parameters such as protrusive activity over time and growth length can be easily obtained (Figure 6A). Note that a simple adjustment in the temporal resolution of the time-lapse allows measurement of axon elongation speed for 2 h (Figure 6A). Furthermore, the variation of growth cone volume over time-a measure of general growth cone dynamic activity-can be easily obtained, in this case with licensed software (Figure 6B and Figure 6E,F). This can be used to evaluate the speed of actin treadmilling and the balance of filopodia/lamellipodia during growth cone exploring activity.
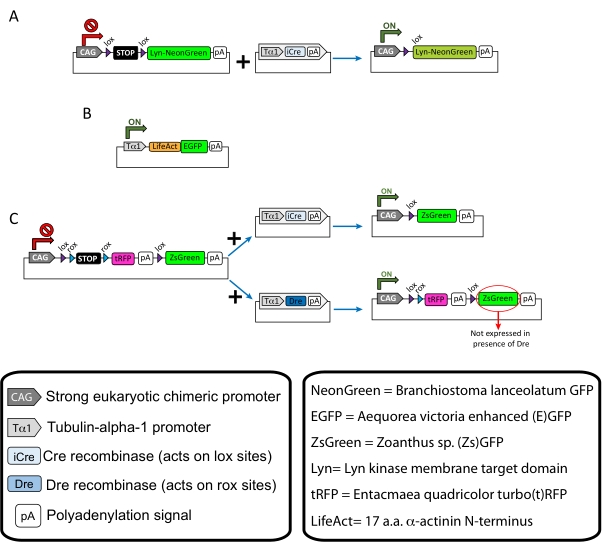
Figure 1: Schemes of the plasmids used in the protocol. (A) pCAG-lox-STOP-lox-Lyn-mNeonGreen. (B) p-Tub-alpha-1-LifeAct-GFP. (C) pCAG-lox-rox-STOP-rox-tRFP-pA-lox-ZsGreen-pA. Relevant information regarding plasmid components and fluorophore's origin is found in the boxes. Please click here to view a larger version of this figure.
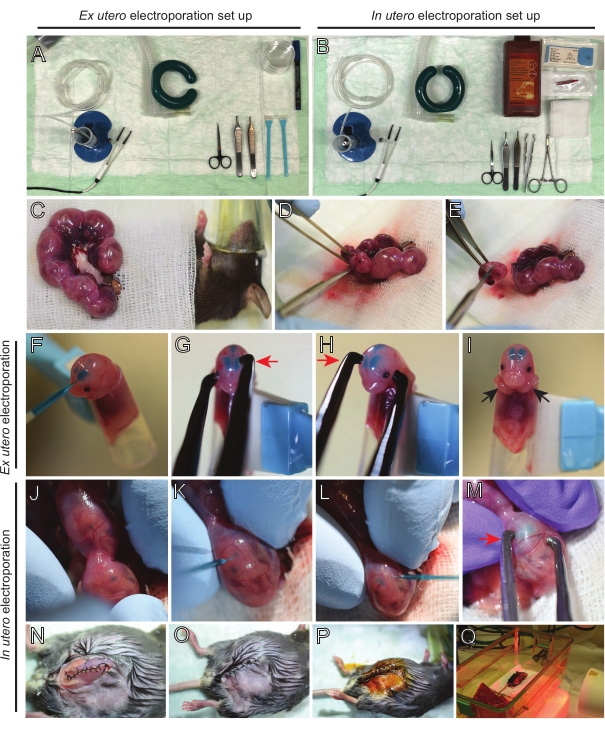
Figure 2: Workflow of ex utero and in utero electroporation of E15.5 mice. (A) Set up of surgery station for ex utero electroporation. (B) Set up of surgery station for in utero electroporation. (C) Uterine horns pulled outside the abdominal cavity of the anesthetized mouse. (D) Extraction of an embryo from the uterine sack. (E) Embryo sacrifice by complete spinal cord transection via a diagonal incision; note that beheading was avoided. (F) Placement of embryo in the holder and injected with DNA/Fast Green mixture into the left lateral ventricle. (G,H) Position the embryo's head between platinum tweezer electrodes with the cathode (red arrow) over the cortex at a 60° angle. (I) Placement of embryo's arms (black arrows) outside the holder to prevent sliding of the embryo during the procedure. (J) Rotation of the embryo inside the uterine sack to expose the head. (K,L) Injection of DNA/Fast Green mixture into embryo's lateral ventricles through the uterine wall. (M) Position the embryo's head between platinum tweezer electrodes with a cathode (red arrow) over the cortex at a 60° angle. (N) Sutured muscle incision via running locking suture. (O) Sutured skin incision via an interrupted suture. (P) Securing of the wound using surgical wound clips and disinfection using betadine. (Q) Placement of the mouse in the recovery cage with far infrared warming light. Please click here to view a larger version of this figure.
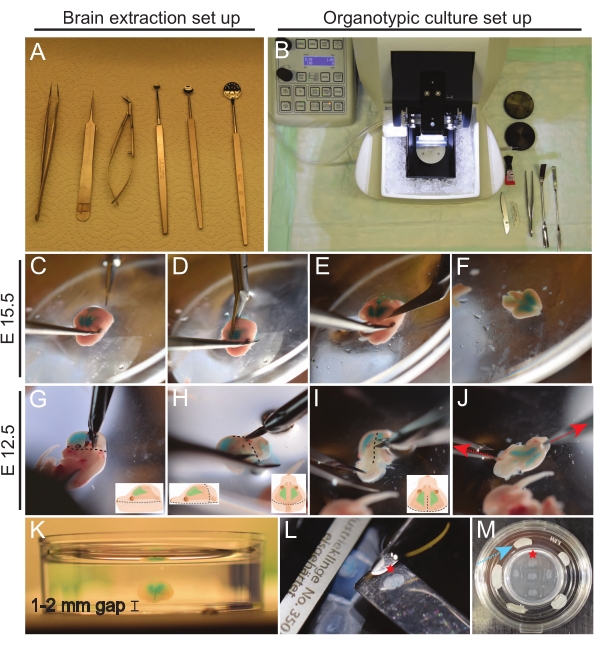
Figure 3: Extraction of E15.5 and E12.5 brains and organotypic slice culture procedure. (A) Tools used for the brain extraction procedure. (B) Set up of organotypic culture station. (C–F) Extraction of E15.5 brain. (G–J) Extraction of E12.5 brain. Dotted lines highlight the location of incisions. Red arrows are pointing out the direction of pulling by forceps. (K) Embedding the brain in a 3 cm dish containing 3% low melt agarose, leaving a 1-2 mm agarose spacing gap under the brain. (L) Collection of 150 µm brain slice. (M) Placement of brain slices on PTFE membrane inserts immobilized in a 35 mm dish using paraffin film (blue arrow). Red star marking indicates a given brain slice collection from vibratome (L) and its transfer to PTFE membrane (M). Please click here to view a larger version of this figure.
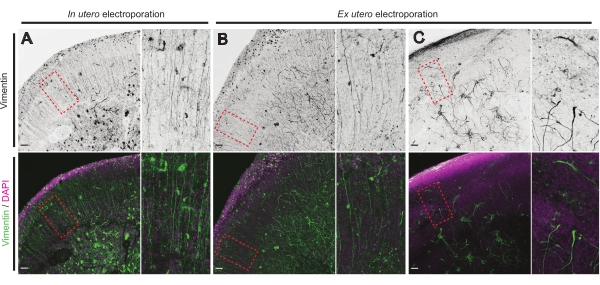
Figure 4: Conserved radial glial cell structure in healthy organotypic slices. Confocal images of E17.5 brain slices revealing RG array (vimentin; green) and overall cell organization (DAPI; magenta) following IUE (A) and EUE (B,C). Note the strong disturbances in the RG array that may occasionally result from EUE (C). Magnifications correspond to the red dotted frames in the main figure: scale bars, 10 µm. Please click here to view a larger version of this figure.
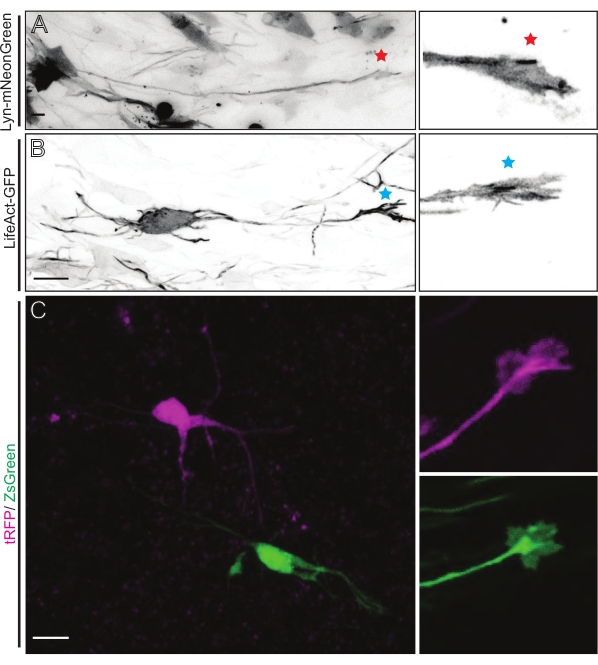
Figure 5: In situ visualization of the growth cone dynamics in acute organotypic slices. (A,B) Neurons and their corresponding growth cones labeled with Lyn-mNeonGreen and LifeAct-GFP, respectively. Red star marking growth cone of Lyn-mNeonGreen expressing neuron. Blue asterisk marking growth cone of LifeAct-GFP expressing neuron. (C) Neighboring neurons labeled with the dual plasmid system containing tRFP (magenta) and ZsGreen (green) and their corresponding growth cones. Growth cones imaged (right) were outside the captured frame (left), obtained shortly after acquiring the growth cone time-lapse; scale bars, 5 µm. Please click here to view a larger version of this figure.
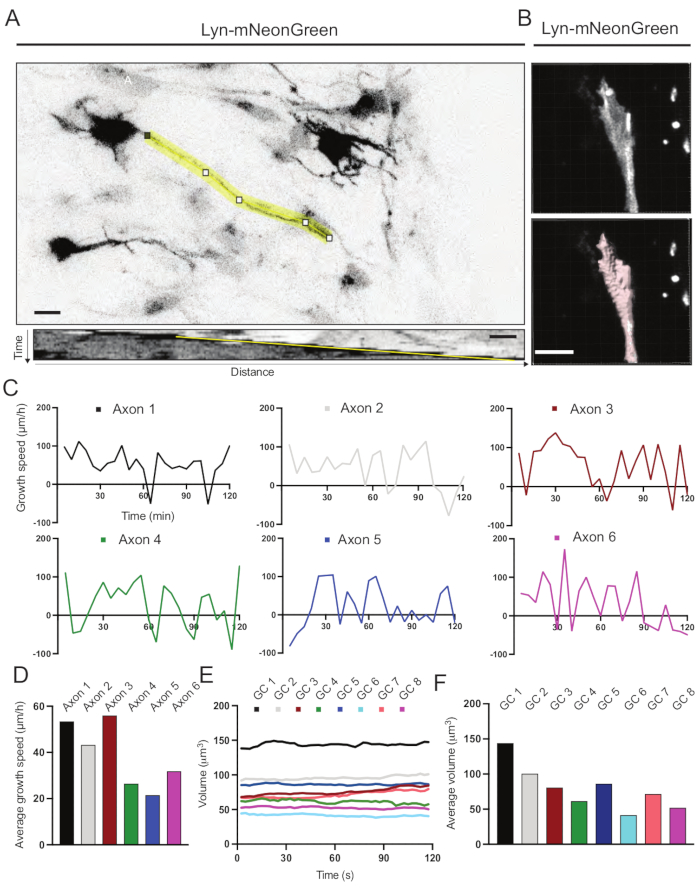
Figure 6: Analysis of axon growth speed and growth cone volume. (A) Axon tracing on a neuron expressing Lyn-mNeonGreen (top) and its corresponding kymograph (below) generated using ImageJ. (B) Reconstruction of z-stack video of growth cone using the image analysis software (top) and the same growth cone highlighted using the surfaces measurement tool (below). (C) Graphs showing changes in growth speed over time for several axons. (D) The average growth speed of axons is quantified in (C). (E) Graph showing the changes in the growth cone volume over time. (F) The average volume of growth cones is quantified in (E); scale bar, 5 µm. Please click here to view a larger version of this figure.
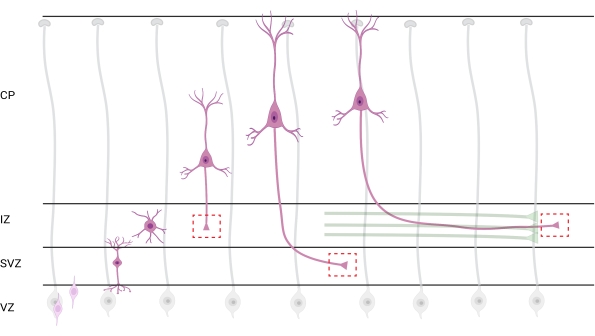
Figure 7: Radial migration and neuronal polarization of pyramidal cortical neurons. Diagram illustrating developing pyramidal cortical neurons (pink) migrating radially from the germinal ventricular zone (VZ) toward the pia surface. Guided by radial glia processes (gray), migrating polarized neurons establish a leading process, future dendrite, and trailing process, future axon, that continue to extend downward toward the intermediate zone (IZ). Dashed red boxes represent the cortical areas where growth cones were imaged. Specifically in the IZ, subventricular zone (SVZ), or joining axon bundles (green). The illustration was created with a web-based tool, BioRender.com. Please click here to view a larger version of this figure.
| Plasmid | Concentration (µg/µL) | Intended use |
| pCAG-lox-STOP-lox-Lyn-mNeonGreen | 0.25 | Labelling of membrane targeted protein (Lyn) |
| + | + | |
| p-Tub-alpha-1-iCre | 0.08 | |
| p-Tub-alpha-1-LifeAct-GFP | 0.125 | Filamentous actin (F-actin) labelling in growth cones |
| pCAG-lox-rox-STOP-rox-tRFP-lox-Lyn-ZsGreen | 1 | Independent labelling of two populations of neighboring neurons |
| + | + | |
| p-Tub-alpha-1-iCre | 0.004 | |
| + | + | |
| p-Tub-alpha-1-Dre | 0.2 |
Table 1: List of plasmids used in the Protocol. Name, concentration, and intended use of each utilized plasmid.
Supplementary Video 1: In situ visualization of the growth cone dynamics in acute organotypic slices. Dynamics of growth cones labeled with Lyn-mNeonGreen (top left) and LifeAct-GFP (bottom left). Neighboring growth cones are differentially labeled with the dual plasmid system containing tRFP (magenta; top right) and ZsGreen (green; bottom right). Imaging interval, 2.5 s. Scale bars, 5 µm. Please click here to download this File.
Discussion
How the growth cones sense and react to their surrounding environment to coordinate simultaneous axon extension and guidance is still a matter of debate3,18. Pioneering studies in 2D substrates provided a glimpse into the fundamental molecular mechanisms generating the forces that drive growth cone dynamics during axon formation, outgrowth, and navigation2,10,11,12,19. More recently, studies in 3D matrices revealed how much influence a third dimension has in the behavior of the growth cone and consequently in axon growth8,9. Nevertheless, the intricate mechanisms instructing growth cone dynamics in vivo remain to be thoroughly examined.
Preparation of organotypic slice cultures from IUE or EUE brains is widely utilized and well-documented. It has become a golden standard allowing scientists to gain insights into the development and behavior of neurons in the living brain tissue20,21. Indeed, this technique has been successfully utilized in combination with various high-resolution imaging techniques to visualize specific molecular processes and morphological events in situ. Such studies include, but are not limited to axon formation and extension19,22, cortical neuronal migration19,22,23,24, centrosome dynamics25,26, microtubule dynamics27, as well as the functional dynamics of pre-and postsynaptic compartments28,29.
This protocol addresses a gap in experimental neurobiology, visualizing the growth cone dynamics of developing cortical neurons in situ, in ex vivo acute brain slice cultures, and the tools to analyze the data obtained.
Acute brain slice cultures were utilized to establish this protocol because they (1) with some practice, are easy to generate; (2) present an accessible system to study growth cones embedded in a quasi-fully–physiological environment, yet transparent enough to allow high-resolution live-cell imaging; (3) can be expanded for its use with a myriad of transgenic mouse lines; (4) combined with either IUE or EUE, provide virtually unlimited potential to deliver molecular tools to evaluate the performance of growth cones and axons in vivo under loss/gain of function regimes, along with fluorescent reporters and cytoskeleton probes.
This methodology was described in the context of both EUE and IUE. Although still a highly reliable method, EUE resulted in an increased incidence of brain slices showing a disorganized RG network compared to those obtained with IUE as a delivery method (Figure 4C). Disturbances in the RG array strongly affect neuronal migration and the pattern of axon elongation30,31. These are key parameters that predict where to find axons for analysis at a given time and the type of environment they are navigating. Brain slices with a significantly disrupted RG network typically have impaired cortical neuron stratification. This, in turn, produces axons with chaotic trajectories. Therefore, it is strongly recommended to control for the structural integrity of the RG network. Interestingly, poor structural integrity correlates with increased age of the embryonic brain. Indeed, such effects in younger E12.5-E13.5 embryos were typically not observed19.
The present protocol is thorough and straightforward. Nevertheless, there are a few critical steps where special care and attention must be taken to obtain optimal results. These have been expressly noted in the protocol and include (1) tuning the amount of DNA used in the electroporation to get sparse labeling; (2) avoiding damage during the extraction of brains; (3) controlling the temperature of the agarose during brain casing; (4) troubleshooting the ideal percentage of agarose for brains of a given age; and (5) selection of fluorophores, the experience of which follows. During protocol optimization, the performance of several fluorophores in live-cell in situ imaging was tested. Monomeric GFP variants EGFP and NeonGreen for the preparation of the LifeAct- and Lyn-tagged plasmids were chosen for this protocol (Figure 5A,B). Additionally, the RFP variant mScarlet was tested and found highly suitable for this set-up (data not shown). Multimeric tRFP (dimer) and ZsGreen (tetramer) (Figure 5C and Supplementary Video 1, right) were also tested. These fast-folding super-bright fluorophores are recommended when the method requires rapid fluorescent signal generation after DNA delivery.
A common practice in using slice cultures is to utilize slices from different brains to test control and experimental conditions. This represents an inherent source of unwanted variability. Here, an expression system that enables independent modification of neighboring neurons and reporters' expression for identification was used. Note that in this demonstration (Figure 5C), there were no differences between neurons expressing either of the fluorophores. However, as an example, such a plasmid mix combined with a transgenic mouse line harboring a Cre-sensitive gene will label with tRFP (Dre-sensitive) neurons that remained as wild type. In contrast, the ZsGreen (also Cre-sensitive) will label the recombined neurons. Hence, growth cones of the two different genotypes, and likely also phenotypes, could be studied side-by-side simultaneously in the same brain slice.
Localization of axons and growth cones for analysis is an important consideration. Cortical neurons polarize while radially migrating from the ventricular zone (VZ) toward the cortical plate (CP). During this process, neurons form a leading process (a future dendrite) and a trailing process that will become the axon, eventually joining pioneering axons in the intermediate zone (IZ), establishing axon tracts32. Therefore, to capture axonal growth cones, imaging was done on axonal fibers in the IZ, including axons exiting the CP and early-generated axons already associated with axonal bundles; or eventually, in fibers that transverse the IZ and extend below it (Figure 7).
This protocol makes it feasible to perform super-resolution imaging of neurons within organotypic slices. Historically, light scattering was a significant problem faced when imaging thick specimens. Over the last two decades, extensive advances in optical technologies made imaging of thick specimens possible. Here, a long working distance objective was used to visualize smaller structures better, such as growth cones. Unavoidably, this protocol does not capture more detailed events such as retrograde actin flow or microtubule dynamics. The long-working distance objective, which necessitates a lower Numerical Aperture (NA), preserves information from thick slices. However, it was also possible to adapt this protocol to use with objectives of shorter working distance. This required a smooth transfer of slices to a glass-bottomed dish to preserve structural integrity. However, using this method resulted in shorter survival-~15 h-due to loss of gas exchange (data not shown). Unlike 2D cultures, growth cones in 3D occupy a larger volume and require movement-artifact compensation in the z-axis. To increase the ability to image detailed events, modern confocal technology must be utilized. Therefore, it is recommended to use a fast-scanning z-stack motor, such as the z-Galvo available on highly sensitive confocal microscopes33.
Of note, this protocol presents three main limitations. First, it is often challenging to control levels of expression/number of expressing cells of any given plasmid in vivo. This introduces variability between all slices even when maintaining the same plasmid concentration. Therefore, the selection of the regulatory elements in the expression vectors used must be predetermined with care. Second, imaging detailed events using membrane inserts is currently not feasible. This second limitation may be overcome with the methodological updates proposed in the previous paragraph. Lastly, growth cones are highly photosensitive and can quickly become photobleached. Therefore, frequent imaging of the growth cones, for as little as 5 min using laser scanning microscopes can often collapse the growth cones. In this regard, new advancement in light-sheet microscopy generated devices can be adapted for long-term imaging of the brain slices34.
Protocols of this like are envisioned to open new research avenues, allowing a better understanding of what it takes for a growth cone to read and react toward a complex in vivo environment, and more importantly, to unravel the mechanics of this sophisticated interplay.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We would like to thank Maria Eugenia Bernis for photographing the procedures. We also thank Emily Burnside, Emily Handley, Thorben Pietralla, Max Schelski, and Sina Stern for reading and discussing the manuscript. We are grateful to our outstanding technical assistants, Jessica Gonyer, Blanca Randel, and Anh-Tuan Pham. We acknowledge the valuable support of the DZNE's light microscope facility and animal facility. This work was supported by Deutsche Forschungsgesellschaft (DFG), the International Foundation for Research in Paraplegia (IRP), and Wings for Life (to F.B). F.B. is a member of the excellence cluster ImmunoSensation2, the SFBs 1089 and 1158, and is a recipient of the Roger De Spoelberch Prize.
Materials
| Adson Forceps | Fine Science Tools | 11006-12 | |
| Alexa Fluor 488 | Invitrogen | A21202 | Goat Anti-Mouse |
| Alexa Fluor 647 | Invitrogen | A21236 | Goat Anti-Mouse |
| Anti-Vimentin antibody | sigma-Aldrich | V2258-.2ML | Monoclonal mouse, clone LN-6, ascites fluid |
| B27 supplement | ThermoFisher Scientific | 17504044 | |
| Betadine | B. Braun | 3864154 | |
| Biozym Sieve GP Agarose | Biozyme | 850080 | |
| Braunol, Sprühflasche | B. Braun | 3864073 | |
| Buprenorphine (Temgesic) | GEHE Pharma | 345928 | |
| DAPI | sigma-Aldrich | D9542 | |
| DMZ unevirsal electrode puller | Zeitz | NA | |
| Electric razor | Andes | NA | ProClip UltraEdge Super 2-Speed model |
| Enrofloxacin (Baytril) | Bayer | 3543238 | 2,5% (wt/vol) |
| Eppendorf microloader pipette tips | FischerScientific | 10289651 | |
| Fast Green FCF | Sigma-Aldrich | F7252-5G | Dye content ≥ 85 % |
| Fetal Bovine Serum | ThermoFisher Scientific | 10500064 | |
| Fiji 2.1.0 | NIH | NA | https://imagej.net/software/fiji/downloads |
| Fine Scissors | Fine Science Tools | 14058-09 | ToughCut/Straight/9cm |
| FluoroDish Cell Culture Dish | World Precision Instruments | FD5040-100 | |
| Fluoromount Aqueous Mounting Medium | sigma-Aldrich | F4680-25ML | |
| Glucose | MedPex | 3705391 | 5% |
| GlutaMAX Supplement | ThermoFisher Scientific | 35050061 | |
| Glycine | Sigma-Aldrich | G8898 | |
| HBSS | Life Technologies | 14025092 | calcium, magnesium, no phenol red |
| Horse serum | Pan-Biotech | P30-0711 | |
| Imaris 9.7.2 | Bitplane | NA | https://imaris.oxinst.com/products/imaris-for-neuroscientists |
| Isoflurane | Virbac | NA | |
| Isotonic saline solution | B. Braun | 8609261 | 0.90% |
| Leica VT1200 S vibratome | Leica | 14048142066 | |
| LSM 880 with Airyscan | Zeiss | NA | |
| Metacam | Venusberg Apotheke | 8890217 | 5 mg/ml |
| Mice | Janvier Labs | NA | C57BL/6JRj |
| Micro-Adson Forceps | Fine Science Tools | 11018-12 | |
| Micropipette Storage Jar | World Precision Instruments | E210 | 16.16.27 |
| Microsoft Excel | Microsoft | NA | https://www.microsoft.com/en-us/microsoft-365/p/excel/cfq7ttc0k7dx?activetab=pivot:overviewtab |
| Millicell Cell Culture Insert | EMD Millipore | PICM0RG50 | 30 mm, hydrophilic PTFE, 0.4 µm |
| Moria Perforated Spoons | Fine Science Tools | 10370-18 | |
| Moria Spoon | Fine Science Tools | 10321-08 | |
| Neurobasal Medium, minus phenol red | ThermoFisher Scientific | 12348017 | |
| Neuropan-2 supplement | Pan-Biotech | P07-11010 | |
| Normal goat serum | Abcam | ab138478 | |
| Olsen-Hegar Needle Holder with Scissors | Fine Science Tools | 12002-12 | |
| p-Tub-alpha-1-Dre | Addgene | 133925 | |
| p-Tub-alpha-1-iCre | Addgene | 133924 | |
| p-Tub-alpha-1-LifeAct-GFP | Addgene | 175437 | |
| Parafilm | VWR | 52858-000 | |
| Paraformaldehyde | sigma-Aldrich | P6148 | |
| PBS | Sigma-Aldrich | P3813-10PAK | |
| pCAG-lox-rox-STOP-rox-tRFP-lox-Lyn-ZsGreen | Addgene | 175438 | |
| pCAG-lox-STOP-lox-Lyn-mNeonGreen | Addgene | 175257 | |
| Penicillin-Streptomycin | ThermoFisher Scientific | 15140122 | |
| PicoNozzle Kit v2 | World Precision Instruments | 5430-ALL | |
| Platinum Tweezertrodes | Harvard Apparatus | 45-0487 | 1 mm / 3 mm |
| QIAGEN Maxi kit | QIAGEN | 12162 | |
| Reflex wound closure Clip | World Precision Instruments | 500344-10 | 7 mm |
| Sekundenkleber Pattex Mini Trio | Lyreco | 4722659 | |
| Square wave electroporation system ECM830 | Harvard Apparatus | W3 45-0052 | |
| Sterile gauze | Braun Askina | 9031216 | |
| Sterile lubricant eye ointment | Bayer Vital | PZN1578675 | |
| Sterile surgical gloves | Sempermed | 14C0451 | |
| Sucrose | Roth | 4621.2 | |
| Supramid 5-0 surgical silk sutures | B. Braun | NA | |
| Thin-wall glass capillaries | World Precision Instruments | TW100-4 | |
| Triton X-100 | Sigma-Aldrich | X100 | |
| Vannas spring scissors | Fine Science Tools | 15000-03 | |
| µ-Slide 8 Well Glass Bottom | Ibidi | 80827 |
References
- Schelski, M., Bradke, F. Neuronal polarization: From spatiotemporal signaling to cytoskeletal dynamics. Molecular and Cellular Neurosciences. 84, 11-28 (2017).
- Lowery, L. A., Van Vactor, D. The trip of the tip: understanding the growth cone machinery. Nature Reviews: Molecular Cell Biology. 10 (5), 332-343 (2009).
- Stoeckli, E. T. Understanding axon guidance: are we nearly there yet. Development. 145 (10), (2018).
- Bradke, F., Dotti, C. G. The role of local actin instability in axon formation. Science. 283 (5409), 1931-1934 (1999).
- Neukirchen, D., Bradke, F. Cytoplasmic linker proteins regulate neuronal polarization through microtubule and growth cone dynamics. Journal of Neuroscience. 31 (4), 1528-1538 (2011).
- Witte, H., Bradke, F. The role of the cytoskeleton during neuronal polarization. Current Opinion in Neurobiology. 18 (5), 479-487 (2008).
- Witte, H., Neukirchen, D., Bradke, F. Microtubule stabilization specifies initial neuronal polarization. Journal of Cell Biology. 180 (3), 619-632 (2008).
- Nichol, R. H., Catlett, T. S., Onesto, M. M., Hollender, D., Gomez, T. M. Environmental elasticity regulates cell-type specific RHOA signaling and neuritogenesis of human neurons. Stem Cell Reports. 13 (6), 1006-1021 (2019).
- Santos, T. E., et al. Axon growth of CNS neurons in three dimensions is amoeboid and independent of adhesions. Cell Reports. 32 (3), 107907 (2020).
- Mitchison, T., Kirschner, M. Cytoskeletal dynamics and nerve growth. Neuron. 1 (9), 761-772 (1988).
- Lin, C. H., Thompson, C. A., Forscher, P. Cytoskeletal reorganization underlying growth cone motility. Current Opinion in Neurobiology. 4 (5), 640-647 (1994).
- Myers, J. P., Gomez, T. M. Focal adhesion kinase promotes integrin adhesion dynamics necessary for chemotropic turning of nerve growth cones. Journal of Neuroscience. 31 (38), 13585-13595 (2011).
- Turney, S. G., et al. Nerve growth factor stimulates axon outgrowth through negative regulation of growth cone actomyosin restraint of microtubule advance. Molecular Biology of the Cell. 27 (3), 500-517 (2016).
- Schindelin, J., et al. Fiji: an open-source platform for biological-image analysis. Nature Methods. 9 (7), 676-682 (2012).
- Noctor, S. C., Flint, A. C., Weissman, T. A., Dammerman, R. S., Kriegstein, A. R. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 409 (6821), 714-720 (2001).
- Noctor, S. C., Martinez-Cerdeno, V., Ivic, L., Kriegstein, A. R. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nature Neuroscience. 7 (2), 136-144 (2004).
- Ferent, J., Zaidi, D., Francis, F. Extracellular control of radial glia proliferation and scaffolding during cortical development and pathology. Frontiers in Cell and Developmental Biology. 8, 578341 (2020).
- Dent, E. W., Gupton, S. L., Gertler, F. B. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harbor Perspectives in Biology. 3 (3), (2011).
- Dupraz, S., et al. RhoA controls axon extension independent of specification in the developing brain. Current Biology. 29 (22), 3874-3886 (2019).
- Azzarelli, R., Oleari, R., Lettieri, A., Andre, V., Cariboni, A. In vitro, ex vivo and in vivo techniques to study neuronal migration in the developing cerebral cortex. Brain Sciences. 7 (5), (2017).
- Humpel, C. Organotypic brain slice cultures: A review. Neurosciences. 305, 86-98 (2015).
- Namba, T., et al. Pioneering axons regulate neuronal polarization in the developing cerebral cortex. Neuron. 81 (4), 814-829 (2014).
- Shah, B., et al. Rap1 GTPases are master regulators of neural cell polarity in the developing neocortex. Cerebral Cortex. 27 (2), 1253-1269 (2017).
- Wiegreffe, C., Feldmann, S., Gaessler, S., Britsch, S. Time-lapse confocal imaging of migrating neurons in organotypic slice culture of embryonic mouse brain using in utero electroporation. Journal of Visualized Experiments: JoVE. (125), (2017).
- de Anda, F. C., Meletis, K., Ge, X., Rei, D., Tsai, L. H. Centrosome motility is essential for initial axon formation in the neocortex. Journal of Neuroscience. 30 (31), 10391-10406 (2010).
- Sakakibara, A., et al. Dynamics of centrosome translocation and microtubule organization in neocortical neurons during distinct modes of polarization. Cerebral Cortex. 24 (5), 1301-1310 (2014).
- Schatzle, P., Kapitein, L. C., Hoogenraad, C. C. Live imaging of microtubule dynamics in organotypic hippocampal slice cultures. Methods in Cell Biology. 131, 107-126 (2016).
- Qu, X., Kumar, A., Bartolini, F. Live imaging of microtubule dynamics at excitatory presynaptic boutons in primary hippocampal neurons and acute hippocampal slices. STAR Protocols. 2 (1), 100342 (2021).
- Tonnesen, J., Katona, G., Rozsa, B., Nagerl, U. V. Spine neck plasticity regulates compartmentalization of synapses. Nature Neuroscience. 17 (5), 678-685 (2014).
- Buchsbaum, I. Y., Cappello, S. Neuronal migration in the CNS during development and disease: insights from in vivo and in vitro models. Development. 146 (1), (2019).
- Rigby, M. J., Gomez, T. M., Puglielli, L. Glial cell-axonal growth cone interactions in neurodevelopment and regeneration. Frontiers in Neuroscience. 14, 203 (2020).
- Barnes, A. P., Polleux, F. Establishment of axon-dendrite polarity in developing neurons. Annual Review of Neuroscience. 32, 347-381 (2009).

