Establishing Organoids from Human Tooth as a Powerful Tool Toward Mechanistic Research and Regenerative Therapy
Summary
We present a protocol to develop epithelial organoid cultures starting from human tooth. The organoids are robustly expandable and recapitulate the tooth’s epithelial stem cells, including their ameloblast differentiation capacity. The unique organoid model provides a promising tool to study human dental (stem cell) biology with perspectives for tooth-regenerative approaches.
Abstract
Teeth are of key importance in life not only for food mastication and speech but also for psychological well-being. Knowledge on human tooth development and biology is scarce. In particular, not much is known about the tooth’s epithelial stem cells and their function. We succeeded in developing a novel organoid model starting from human tooth tissue (i.e., dental follicle, isolated from extracted wisdom teeth). The organoids are robustly and long-term expandable and recapitulate the proposed human tooth epithelial stem cell compartment in terms of marker expression as well as functional activity. In particular, the organoids are capable to unfold an ameloblast differentiation process as occurring in vivo during amelogenesis. This unique organoid model will provide a powerful tool to study not only human tooth development but also dental pathology, and may pave the way toward tooth-regenerative therapy. Replacing lost teeth with a biological tooth based on this new organoid model could be an appealing alternative to the current standard implantation of synthetic materials.
Introduction
Teeth have essential roles in food mastication, speech, and psychological well-being (self-image). The human tooth consists of highly mineralized tissues of varying density and hardness1. Dental enamel, the main component of the tooth crown, is the highest mineralized tissue in the human body. During enamel formation (amelogenesis), when teeth develop, dental epithelial stem cells (DESCs) differentiate into enamel-forming cells (ameloblasts). Once formed, the enamel is rarely repaired or renewed due to the apoptotic loss of the ameloblasts at the onset of tooth eruption1. Restoration of damaged enamel tissue, as caused by trauma or bacterial disease, is currently accomplished using synthetic materials; however, these are troubled with important shortcomings such as microleakage, inferior osseointegration and anchorage, finite life span, and lack of fully functional repair2. Hence, a robust and reliable culture of human DESCs with the capacity to generate ameloblasts and the potential to produce mineralized tissue would be a major step forward in the dental regenerative field.
Knowledge on human DESC phenotype and biological function are scarce3,4,5. Interestingly, DESCs of human teeth have been proposed to exist in the Epithelial Cell Rests of Malassez (ERM), cell clusters present within the dental follicle (DF), which surrounds unerupted teeth, and remains present in the periodontal ligament around the root once the tooth erupts1. ERM cells co-cultured with dental pulp have been found to differentiate into ameloblast-like cells and generate enamel-like tissue6. However, profound studies of the specific role of ERM cells in enamel (re-) generation have been limited due to the lack of reliable study models7. Current ERM in vitro culture systems are hampered by limited life span and quick loss of phenotype in the 2D conditions standardly used8,9,10,11,12. Hence, a tractable in vitro system to faithfully expand, study, and differentiate human DESCs is strongly needed.
During the last decade, a powerful technique to grow epithelial stem cells in vitro has been successfully applied to several types of (human) epithelial tissues to study their biology as well as disease13,14,15,16. This technology enables the tissue epithelial stem cells to self-develop into 3D cell constructions (i.e., organoids) when seeded into an extracellular matrix (ECM)-mimicking scaffold (typically, Matrigel) and cultured in a defined medium replicating the tissue's stem cell niche signaling and/or embryogenesis. Typical growth factors needed for organoid development include epidermal growth factor (EGF) and wingless-type MMTV integration site (WNT) activators14,15,16. The resultant organoids are characterized by lasting fidelity in mimicking the tissue's original epithelial stem cells, as well as high expandability while retaining their phenotype and functional properties, thereby overcoming the often-limited primary human tissue availability as acquired from the clinic. To establish organoids, isolation of the epithelial stem cells from the heterogeneous tissue (i.e., comprising other cell types such as mesenchymal cells) prior to culturing is not required as mesenchymal cells do not attach to, or thrive in, the ECM, eventually resulting in purely epithelial organoids13,16,17,18,19. This promising and versatile technology has led to the development of manifold organoid models from various human epithelial tissues. However, human tooth-derived organoids, valuable for deep study of tooth development, regeneration and disease, were not established yet20,21. We recently succeeded in developing such a new organoid model starting from DF tissue from third molars (wisdom teeth) extracted from adolescent patients19.
Here, we describe the protocol to develop epithelial organoid cultures from the adult human tooth (i.e., from the DF of third molars) (Figure 1A). The resultant organoids express ERM-associated stemness markers while being long-term expandable. Intriguingly, opposite to most other organoid models, the typically needed EGF is redundant for robust organoid development and growth. Interestingly, the stemness organoids show ameloblast differentiation properties, thereby mimicking ERM/DESC features and processes occurring in vivo. The new and unique organoid model described here allows exploring DESC biology, plasticity, and differentiation capacity and opens the door for taking the first steps toward tooth-regenerative approaches.
Protocol
All methods described here have been approved by the Ethics Committee Research UZ/KU Leuven (13/0104U). Extracted third molars (wisdom teeth) were obtained after patients' informed consent.
1. Preparations
- Prewarm a 48-well culture plate for 15-20 h in a 1.9% CO2 incubator at 37 °C.
- Liquefy a Matrigel aliquot (growth factor-reduced; phenol red-free; further referred to as basement membrane matrix; BMM) on ice (4 °C) for a minimum of 2 h prior to step 2.1.
NOTE: Avoid freeze/thaw cycles of the BMM. Liquefy the BMM for 15-20 h on ice and aliquot at 1 mL in microcentrifuge tubes and store at -20 °C. - Cool the centrifuge to 4 °C.
- Prepare media and filter the solution through a 0.22 µm filter. The following volumes are based on the collection of the DFs from all four third molars typically simultaneously extracted.
- Prepare 20 mL of DF collection medium (Table 1): minimum essential medium eagle (αMEM) containing 10% fetal bovine serum (FBS), 0.5% Amphotericin B and 1% penicillin-streptomycin. Transfer 4 mL of DF collection medium to a 15 mL tube and transfer the tubes on ice.
NOTE: One 15 mL tube is sufficient per patient for sample collection (i.e., for four third molars). - Make 20 mL of tooth organoid medium (further referred to as TOM; Table 2) using serum-free defined medium (SFDM; Table 3). Store TOM at 4 °C for a maximum of 2 weeks.
- Prepare 8 mL of dissociation medium (Table 4), i.e., phosphate-buffered saline (PBS) containing collagenase VI (3 mg/mL ) and dispase II (4 mg/mL). Prewarm the dissociation medium in a 37 °C water bath for at least 10 min before use and transfer it to two 15 mL tubes (4 mL per tube) to dissociate four DF tissues.
- Prepare a washing plate (12-well) containing: three wells with 70% EtOH, three wells with PBS, and three wells with DF collection medium.
- Make 15 mL of medium A per sample (i.e., 5 mL per two DFs per patient; Table 5).
NOTE: The following steps should be performed under sterile conditions.
- Prepare 20 mL of DF collection medium (Table 1): minimum essential medium eagle (αMEM) containing 10% fetal bovine serum (FBS), 0.5% Amphotericin B and 1% penicillin-streptomycin. Transfer 4 mL of DF collection medium to a 15 mL tube and transfer the tubes on ice.
2. Dental follicle dissociation
- Once the third molars with associated DFs are collected in the collection medium (on ice), transfer the content of the tubes to a Petri dish.
- Hold the tooth with a tweezer and carefully isolate the DF using a surgical blade.
NOTE: This step requires practice; be careful with the blade. - To wash the remaining blood off the DFs, place the DF tissues briefly in the first well of 70% EtOH (washing plate) for 20 s, and then transfer to the next EtOH well for 20 s, and further to the third EtOH well (maximum 1 min in 70% EtOH in total; longer incubation time will result in reduced cell viability).
- Next, continue rinsing in the three PBS wells (maximum 2 min in total).
- Rinse the DFs in the three remaining DF collection medium wells (up to a maximum of 20 min in total).
- Transfer the rinsed DFs to a new Petri dish.
- Cut a small piece of one of the DFs (~5 mm2) for paraformaldehyde (PFA) fixation (see section 7) and store it on ice (for a maximum of 6 h) in a microcentrifuge tube containing 500 µL of DF collection medium until PFA fixation.
- Mince the rest of the DFs in small pieces (~1 mm2).
NOTE: It is advised to immediately process the freshly isolated DF tissues for the optimal organoid formation and growth efficiency, rather than to start from cryopreserved tissue, which results in lower efficiency. Nevertheless, it is possible to cryopreserve primary DF tissue at this step (see cryopreservation medium and protocol at section 5). - Transfer the minced DFs to a 15 mL tube containing 4 mL of prewarmed dissociation medium and incubate in a 37 °C water bath for 2 h.
NOTE: Add two DFs per 4 mL prewarmed dissociation medium (one tube) to assure optimal dissociation. - Every 15 min, pipet the DF-dissociation medium mix up and down using a glass Pasteur pipet to speed up the tissue disintegration. When DF pieces are no longer observed (usually after 1 h), proceed with DF dissociation using a narrowed fire-polished Pasteur pipet.
NOTE: For optimal DF dissociation, switch to a Pasteur pipet narrowed by fire-polishing no later than 1 h of DF isolation.- In the meantime, prepare 10 mL of medium A containing 50 µL of DNase (0.2 mg/mL; Table 5).
- Add 5 mL of medium A (containing the DNase) to each tube with dissociated DF, and incubate for 1 min at room temperature (RT).
- Filter the cell suspension (containing single cells and small cell clumps) through a 40 µm cell strainer to remove the remaining large fragments and (most of) the fibrous tissue.
- Combine the several tubes with dissociated DF from one patient at this step.
- Rinse the filter with 1 mL of medium A. Centrifuge the filtered cell suspension at 200 x g for 10 min at 4 °C.
- Remove the supernatant, resuspend the pellet in 1 mL of SFDM (Table 3), and transfer the cell suspension to a 1.5 mL microcentrifuge tube.
- Calculate the cell concentration using an automated cell counter. Neglect the cell clumps that remain.
- Centrifuge at 200 x g for 5 min at 4 °C.
3. Establishment of tooth organoid culture (Figure 1A and Figure 2A)
- Based on the obtained cell number, calculate the number of wells that can be seeded. One 20 µL droplet should contain 20,000 cells. The final mix is composed of cell suspension and BMM at a ratio of 30:70.
- Remove the appropriate amount of supernatant and resuspend in ice-cold BMM to obtain a 70:30 ratio of BMM: cell suspension for plating. For example, when 200,000 cells are obtained, plate 10 droplets of 20 µL. Thus, remove the supernatant until 60 µL of the cell suspension is left, add 140 µL of ice-cold BMM and resuspend.
- Resuspend slowly to avoid air bubble formation. Once resuspended in BMM, keep the microcentrifuge tube on ice.
NOTE: Keeping the microcentrifuge tube on ice is crucial to avoid BMM solidification.
- Resuspend slowly to avoid air bubble formation. Once resuspended in BMM, keep the microcentrifuge tube on ice.
- Pipet the 20 µL BMM droplets in the center of the wells of the preheated 48-well culture plate.
- Flip the plate upside down, put it in a 1.9% CO2 incubator (% CO2 according to SFDM buffer), and let it solidify for at least 20 min at 37 °C.
- Add ROCK inhibitor (RI; 10 µM) and Amphotericin B (0.1%) to TOM and prewarm the medium in a 37 °C water bath.
NOTE: Amphotericin B is light-sensitive. - Take the 48-well plate from the incubator, place upright and add 250 µL of the prepared prewarmed medium to each well with BMM droplet/cells and return the plate to the 1.9% CO2 incubator.
- To refresh the medium (preferably every 2-3 days), tilt the 48-well plate at a 45° angle, gently remove the previous medium while avoiding touching the BMM droplet, and add 250 µL of new prewarmed TOM medium.
NOTE: It is recommended to refresh at least three times a week to avoid depletion of key nutrients and growth factors. RI only needs to be added to TOM at initial seeding and the first days of each passaging (see section 4) to prevent cell death by anoikis and enhance organoid outgrowth. Amphotericin B is only added to the medium during passage 0 (P0) to block possible fungal contamination.
4. Amplification and passaging of tooth organoid culture (Figure 1B and Figure 2B)
- Passage the organoids between 10 and 14 days of culture.
NOTE: Avoid culturing for a longer period as prolonged culture may limit organoid regrowth and passageability. Only at initial development (P0) organoids may be cultured for up to 20 days depending on their growth rate (till maximum ±200 µm in diameter is reached). - Remove the medium from the wells with organoids that need to be passaged. Within one culture condition, pool up to four confluent wells (see Figure 2C).
- To collect the organoids, add 400 µL of ice-cold SFDM per well directly onto the BMM droplet and repeatedly pipet the medium up and down until the entire BMM droplet is dislodged.
- In case wells are being pooled, transfer the 400 µL from the first well to the next (and so on) to dislodge the organoid-containing BMM droplets of all wells that need to be pooled.
- Transfer the dislodged BMM-organoid assembly to a 1.5 mL microcentrifuge tube and repeat step 4.3 again until all the organoid structures are collected from the wells.
- Centrifuge at 200 x g for 5 min at 4 °C.
- Prewarm an aliquot of TrypLE Express (supplemented with RI at 5 µM) in a 37 °C water bath. Per microcentrifuge tube of organoids, a volume of 400 µL TrypLE Express is needed.
- After centrifugation, remove the supernatant and resuspend the pellet in 400 µL of TrypLE. Incubate the suspension in a 37 °C water bath for 12 min.
- Add 400 µL of ice-cold SFDM to inactivate the enzyme and centrifuge at 200 x g for 5 min at 4 °C. Remove the supernatant.
- Pre-coat a tip (for volume see below) with ice-cold SFDM and resuspend the organoid pellet.
- To avoid organoid loss, re-use the same (pre-coated) tip as much as possible without jeopardizing sterility.
NOTE: The volume of SFDM required for resuspending the pellet depends on the number of organoid structures obtained and the current passage number. - As a rule of thumb, for passages, P0 to P2-3 (generally still growing a low number of organoids) use a volume of 200 µL SFDM. From passages, P3-4 or higher (generally yielding a larger number of organoids) use a volume of 700 µL of SFDM.
NOTE: Both the procedures are referred to as 'low passage' and 'higher passage' methods, respectively (see steps 4.10 and 4.11). Correctly applying the distinct methods is crucial for efficient passaging of the organoids. With the higher passage method, organoids are more easily lost and should not be applied to low numbers of organoids (i.e., at P0 to P2-3). However, the higher passage method is needed to efficiently dissociate larger quantities of organoids.
- To avoid organoid loss, re-use the same (pre-coated) tip as much as possible without jeopardizing sterility.
- Low passage method: Resuspend the pellet in 200 µL of ice-cold SFDM. Push the completely emptied pipet tip against the bottom of the microcentrifuge tube to reduce its diameter. Check whether the diameter is small enough (slower aspiration, flow is skewed but not blocked). Pipet up and down for 5 min to mechanically disrupt the organoids.
- Higher passage method: Resuspend the pellet in 700 µL of ice-cold SFDM with a P1000 tip. Add a P200 tip (no filter) on top of this P1000 tip and pre-coat with ice-cold SFDM. Prevent air bubble formation by adjusting the pipet's volume setting in order to aspire at least 90% of the medium volume (with organoids). Pipet up and down for 5 min to mechanically disrupt the organoids.
- Check under the light microscope (at 4x magnification) if mainly single cells with (only) a few undispersed structures are obtained.
- Add 500 µL of ice-cold SFDM to the microcentrifuge tube and gently mix the dissociated cell mixture with the fresh SFDM by pipetting.
- Let large undispersed structures sediment for 10 min by vertically placing the microcentrifuge tube on ice.
NOTE: It is necessary to remove the undispersed structures because they negatively influence organoid passageability. For organoid initiation (P0), this sedimentation step is not needed. - Collect the supernatant (~500-1,000 µL depending on the passaging method) containing single cells and small cell clusters; transfer into a new microcentrifuge tube, and centrifuge at 190 x g for 10 min at 4 °C.
- Calculate the cell concentration using an automated cell counter. Neglect the cell clumps that remain.
- Count how many wells can be seeded and calculate the appropriate cell suspension/BMM ratio as described above (section 3).
- Add 70% of BMM to the cell pellet and maintain the microcentrifuge tube on ice.
NOTE: It is crucial to keep the microcentrifuge tube on ice to avoid BMM solidification. - Proceed with step 3.3 until 3.7 and passage again between day 10 and day 14 of culture.
5. Cryopreservation of tooth organoids
- Collect and dissociate the organoids as mentioned above for passaging (step 4). Centrifuge the cell suspension at 200 x g for 5 min at 4 °C.
- Discard the supernatant and resuspend the pellet in 1 mL of cryopreservation medium containing SFDM (70%), FBS (20%), and DMSO (10%).
- Transfer the suspension into a cryovial and put it on ice. Place the cryovials in a cryo box and transfer to -80 °C (for at least 4 h).
- Within 1 month, transfer the frozen samples to liquid nitrogen for long-term (>12 months) storage.
6. Thawing of cryopreserved tooth organoids
- Before starting the thawing procedure, place one 15 mL tube per cryovial on ice containing 10 mL of SFDM with 20% FBS.
- Remove the cryovial from the liquid nitrogen and put it on ice.
NOTE: Perform the following steps as rapidly as possible and avoid too long thawing time (>5 min) as well as too long intervals between the steps (>5 min), as such prolongation will decrease cell survival. - Place the cryovial in a warm water bath (37 °C) until thawed (~1-2 min).
- Immediately transfer the content of the cryovial to the ice-cold 15 mL tube and centrifuge at 200 x g for 5 min at 4 °C.
- Remove 9 mL of the supernatant and centrifuge the remaining 1 mL at 200 x g for 5 min at 4 °C. Remove the supernatant and wash the pellet with 1 mL ice-cold SFDM.
- Transfer cell suspension to a 1.5 mL microcentrifuge tube and count the cells using an automated cell counter.
- Count how many wells can be seeded and calculate the appropriate cell suspension/BMM ratio as described above (section 3).
- Centrifuge at 200 x g for 5 min at 4 °C. Resuspend the pellet in a 70:30 ratio of BMM:TOM and keep on ice.
- Proceed with step 3.3 until 3.7 and passage between day 10 and day 14 of culture.
7. Fixation and paraffin-embedding of tooth organoids
NOTE: This procedure (including sections 8 and 9) can also be applied to the primary DF tissue.
- Fixation of tooth organoids in PFA
- Remove the medium from each well as in step 4.2.
- Collect the organoids by dislodging the BMM droplet (step 4.3). Transfer the BMM-organoid mixture to a 1.5 mL microcentrifuge tube.
- Centrifuge at 200 x g for 5 min at 4 °C.
- Remove the supernatant and add 500 µL of 4% PFA and incubate for a minimum of 30 min (maximum 1 h) at RT with gentle mixing on an orbital shaker.
CAUTION: Use the chemical hood when working with PFA. - Centrifuge at 200 x g for 5 min at 4 °C. Remove the supernatant and rinse the organoid pellet with PBS for 10 min at RT with gentle shaking.
- Wash the organoid pellet (step 7.1.5) an additional two times. Spin the fixed organoids down at 200 x g for 5 min at 4 °C.
- Resuspend the pellet in 500 µL of 70% EtOH (in deionized water). Store the organoids for up to 1 month in 70% EtOH at 4 °C.
- Agarose- and paraffin-embedding of tooth organoids
NOTE: To efficiently embed organoids in paraffin, an additional step of embedding in agarose is needed.- Centrifuge the PFA-fixed organoids in 70% EtOH at 200 x g for 5 min at 4 °C. Remove the supernatant.
- Prepare a 2% agarose solution in 30 mL of PBS in a glass bottle. Warm up the agarose-PBS mixture in a microwave until a gel-like structure is observed (approximately 2.5 min at 600 W).
- In parallel, add 30 mL of PBS to another glass bottle and warm up in the microwave (approximately 2.5 min at 600 W). Let the agarose solution cool down for 1 min.
- Cut the end of a P200 tip to allow working with the agarose solution precisely and to avoid air bubbles.
- Prewarm the P200 tip in the warm PBS by pipetting up and down a few times.
- Add 150 µL of prewarmed agarose solution to the organoid pellet and gently pipet up the organoid-agarose mixture (with minimal resuspension) while avoiding air bubbles.
NOTE: The minimal resuspension will allow better locating the organoids in the agarose gel at microtome sectioning since the organoids are then closer to each other. - Immediately transfer the organoid-agarose mixture to the same microcentrifuge tube cap, put the tube horizontally, and let the agarose solidify (~20 min at RT). In the meantime, label the cassettes.
- Once solidified, remove the agarose gel from the microcentrifuge cap using a tweezer and transfer it to a labeled cassette.
- Transfer the agarose-containing cassette to a beaker containing 50% EtOH in deionized water. Cover the beaker with parafilm to avoid evaporation of the EtOH.
NOTE: The volume of the beaker depends on the number of cassettes. - Process the samples in a tissue processor overnight.
NOTE: As paraffin solidifies at RT, the following steps must be performed rapidly. - Prewarm a heating block in the embedding workstation for 15 min.
- Remove the organoid-containing agarose gel enclosed in the cassette and place it in the prewarmed heating block. Remove the cap from the cassette and put it aside for later use.
- Fill the remaining heating block with paraffin.
NOTE: Check with a tweezer whether the organoid-containing agarose gel is still located at the bottom of the heating block. Due to its lightweight, it might start floating, resulting in sample loss. - Place the cassette cap on top of the heating block. Let it solidify for 30 min on a cold plate. Remove the heating block and store the paraffin blocks at 4 °C.
8. Microtome sectioning and staining of tooth organoids (Figure 2B and Figure 3A-C)
- Microtome sectioning of organoid-containing paraffin blocks
- Slice 5 µm sections of the tooth organoids in the paraffin blocks using a microtome.
- Place the paraffin slices on top of a microscope glass slide. Place the microscope glass slide on a warm plate at 37 °C and cover it with deionized water using a Pasteur pipet.
- Let the microscope glass slide dry overnight.
- Staining of tooth organoid sections
- Deparaffinize the organoid sections (on the microscope glass slide) in an oven for 1 h at 58 °C.
- Rehydrate the organoid sections (on the microscope glass slide) in decreasing EtOH series inside the chemical hood in the following order:
Xylene 2x for 3 min each
100% EtOH 2x for 3 min each
95% EtOH 2x for 3 min each
90% EtOH 2x for 3 min each
70% EtOH 3x for 3 min each
CAUTION: Use the chemical hood when working with Xylene. - Rinse the microscope glass slide in tap water for 5 min at RT. Then, rinse it in PBS for 5 min at RT.
- Perform antigen retrieval by placing the organoid sections (on the microscope glass slide) in prewarmed citrate buffer (10 mM of citric acid in deionized water with pH 6; in a plastic container; prewarmed for 10 min in a 95 °C water bath) for 30 min in a 95 °C water bath.
- Let the microscope glass slide cool down for 20 min at RT. Rinse the microscope glass slide in PBS for 5 min at RT, and then in PBS containing 0.1% Triton-X (PBT) for 5 min at RT.
- Use a marking pen to create a hydrophobic barrier at the borders of each slide.
- Block for a minimum of 1 h at RT in blocking buffer (containing 1.5 mg/mL glycine, 2 mg/mL albumin bovine serum (BSA) dissolved in PBT) plus 10% donkey serum.
NOTE: Typically, 300 µL of blocking buffer plus 10% donkey serum is needed per microscope glass slide. - Place the microscope glass slide in a humid chamber. Use an airtight box where all slides fit and wet a few paper tissues with water to place at the bottom of the box. This will prevent the slides from drying out during the subsequent staining steps.
- Remove the blocking buffer, add the primary antibody (Table 6) prepared in blocking buffer plus 1% donkey serum and incubate the slide covered with antibody solution overnight in the humid chamber at 4 °C. Redo the marking pen border if needed.
- Wash the microscope glass slide three times in PBT at RT for 10 min with gentle mixing on an orbital shaker (75-150 rpm).
- Add the secondary antibody (Table 6), prepared in blocking buffer plus 1% donkey serum, and incubate for 1 h in the humid chamber at RT.
- Remove the blocking buffer and wash the microscope glass slide three times in PBT at RT for 10 min with gentle mixing on an orbital shaker.
- Add antifade mounting medium with DAPI (1 to 3 droplets) on a glass coverslip and mount this on top of the organoid sections (on the microscope glass slide). Proceed with imaging; store slides for a maximum of 1 week at 4 °C.
9. RNA Extraction and RT-qPCR of tooth organoids (Figure 2B and Figure 3D)
- RNA extraction of tooth organoids
- Remove the medium from each well (step 4.2).
- Collect the organoids by dislodging the BMM droplet (step 4.3), transfer the BMM-organoid mixture to a 1.5 mL microcentrifuge tube, and centrifuge at 200 x g for 5 min at 4 °C.
- Remove the supernatant, resuspend vigorously in 350 µL of 1% β-mercaptoethanol dissolved in lysis buffer (Table of Materials), and put on ice.
CAUTION: Perform all the steps with β-mercaptoethanol in a chemical hood.
NOTE: Samples can be stored at this step for up to 1 month at -80 °C. Thaw the samples on ice before proceeding with step 9.1.4. - Vortex the samples until no organoid structures are observed anymore.
- Proceed with RNA extraction using the RNA extraction kit (Table of Materials) according to the manufacturer's instructions.
- RT-qPCR of tooth organoids (Table 7)
- Reverse-transcribe (RT) the RNA19 using the reverse transcription kit (Table of Materials) according to the manufacturer's instructions.
- Analyze the resulting cDNA samples with SYBR Green-based quantitative PCR (qPCR)19 using a Real-Time PCR System (Table of Materials).
Representative Results
Tooth organoid development
We provide a detailed protocol to establish organoid cultures from human DF tissue acquired following wisdom tooth extraction (Figure 1A). Isolated DF is enzymatically and mechanically dissociated. The obtained cells are cultured within BMM in media that were empirically defined for optimal organoid development and growth (tooth organoid medium; TOM)19.
The organoids typically develop within 2 weeks after DF cell seeding (P0; Figure 2A). The organoids are long-term expandable (up to 11 passages so far) (Figure 2B, shown at P4). Seeding around 20,000 cells per BMM droplet (at both P0 and further passages) yields an optimal density of organoids (Figure 2C), whereas seeding higher cell numbers leads to suboptimal organoid outgrowth (i.e., smaller organoids at too high density) as there is insufficient space to grow (Figure 2D). The eventually optimized culture conditions allow the development of organoids from DF samples at 100% efficiency19.
Tooth organoid characterization and validation
The developed organoids show a dense appearance and contain cells displaying a high nucleo-cytoplasmic ratio, as similarly observed in ERM cells7 (Figure 3A). Moreover, and in further analogy, the organoids express the ERM marker cytokeratin 14 (CK14)22, thereby confirming their epithelial origin (Figure 3B), as well as other proposed ERM markers (such as P63, CD44 and ITGα612,22,23 (Figure 3B). Moreover, organoids express SOX2, a well-known DESC marker in mice and also present in the epithelium of developing human teeth (Figure 3B)1. Interestingly, amelogenin (AMELX), the main component of the enamel matrix, also found expressed in the organoids, is also detected in the ERM24 (Figure 3C). Expression of still other ERM/stemness markers is described in our recent study19 and can be used to further certify the obtained organoids. Furthermore, the organoids retain their ERM/stemness phenotype during passaging, among others shown by stable expression of ERM/stem cell markers (Figure 3D). Finally, the tooth-derived organoids show differentiation capacity to ameloblast(-like) cells, which can also be applied to validate the obtained organoid cultures, showing expression of mature ameloblast markers such as odontogenic-ameloblast associated protein (ODAM) and amelotin (AMTN) after transfer to differentiation medium (see19).
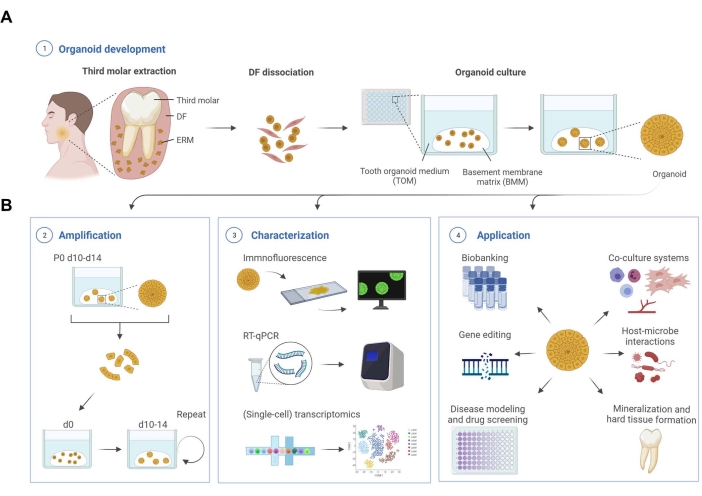
Figure 1: Schematic workflow of tooth organoid development, characterization, and applications. (A) Tooth organoid development from dental follicle (DF) tissue isolated from unerupted third molars. (B) Amplification, characterization, and application potential of tooth organoids. d, day; P, passage. Created with BioRender.com. Please click here to view a larger version of this figure.
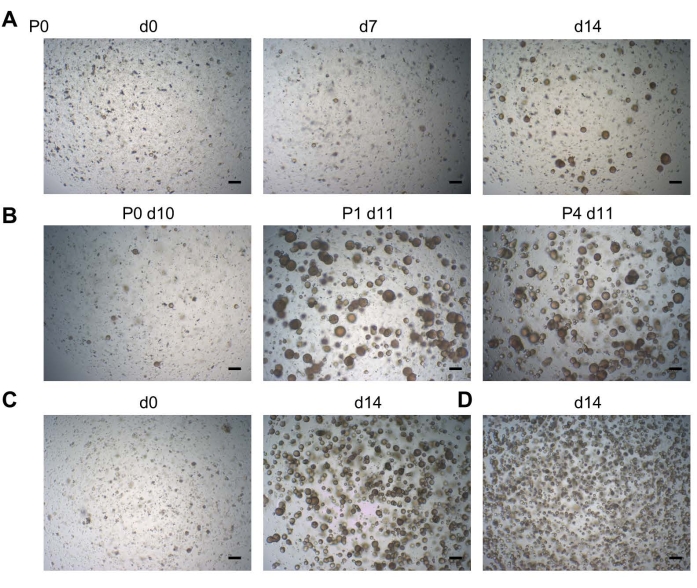
Figure 2: Tooth organoid development. (A) Organoid development from DF tissue. Representative brightfield images shown at different days (d) after seeding (P0; P, passage; 2.5x). (B) Brightfield images showing robust passageability of a tooth organoid line (2.5x). (C) Brightfield images showing a tooth organoid line immediately at passaging (d0; left; 2.5x) seeded at a density of 20,000 cells per well, and the resultant confluent organoid culture ready to be passaged (d14; right; 2.5x). (D) Brightfield images showing a tooth organoid line, which had been seeded at a density of >20,000 cells, leading to smaller organoids at too high density at d14 (2.5x). Scale bars: 200 µm. Please click here to view a larger version of this figure.
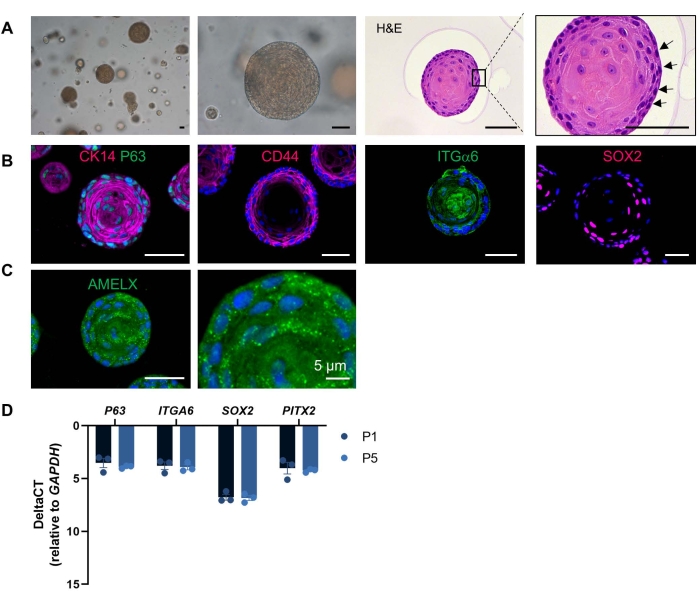
Figure 3: Tooth organoid characterization. (A) Brightfield images of DF-derived organoid cultures at different magnifications showing dense structures developed at 14 days in TOM (P4; 5-20x)). Hematoxylin and eosin staining of an organoid (P1, day 11). Box is enlarged. Arrows indicate cells with a high nucleo-cytoplasmic ratio. (B) Immunofluorescent staining for epithelial/ERM/stemness markers in TOM-grown organoids (20x). (C) Immunofluorescent staining for amelogenin (AMELX) in TOM-grown organoid (20x). DAPI (blue) was used to label nuclei. (D) Gene expression levels (relative to GAPDH) of ERM/stemness markers in P1 and P5 TOM-grown organoids at day 14 of culture (mean ± SEM; n = 3 biological replicates). Scale bars: 50 µm, unless indicated otherwise. Please click here to view a larger version of this figure.
| Dental follicle (DF) collection medium | |
| Name | Concentration |
| Minimum essential medium eagle (αMEM) | |
| Fetal bovine serum (FBS) | 10% |
| Amphotericin B | 0.5% |
| Penicillin-streptomycin (Pen/Strep) | 1% |
Table 1: Dental follicle (DF) collection medium. The table lists the constituents of the DF collection medium.
| Tooth organoid medium (TOM) | |
| Name | Concentration |
| Serum-free defined medium (SFDM) | See Table 3 for composition |
| A83-01 | 0.5 µM |
| B27 (without vitamin A) | 2% |
| Cholera Toxin | 100 ng/mL |
| FGF2 (= basic FGF) | 20 ng/mL |
| FGF8 | 200 ng/mL |
| FGF10 | 100 ng/mL |
| L-Glutamine | 2 mM |
| IGF-1 | 100 ng/mL |
| N2 | 1% |
| N-acetyl L-cysteine | 1.25 mM |
| Nicotinamide | 10 mM |
| Noggin | 100 ng/mL |
| RSPO1 | 200 ng/mL |
| SB202190 (p38i) | 10 µM |
| SHH | 100 ng/mL |
| WNT3a | 200 ng/mL |
Table 2: Tooth organoid medium (TOM). The table lists the constituents and their respective concentrations required to prepare the tooth organoid medium.
| Serum-free defined medium (SFDM) (pH 7.3) | |
| Name | Concentration |
| Sterile H2O | |
| DMEM 1:1 F12 without Fe | 16.8 g/L |
| Transferrin | 5 mg/L |
| Insulin from bovine pancreas | 5 mg/L |
| Penicillin G sodium salt | 35 mg/L |
| Streptomycin sulfate salt | 50 mg/L |
| Ethanol absolute, ≥99.8% (EtOH) | 600 µL/L |
| Catalase from bovine liver | 50 µL/L |
| Sodium Hydrogen Carbonate (NaHCO3) | 1 g/L |
| Albumin Bovine (cell culture grade) | 5 g/L |
Table 3: Serum-free defined medium (SFDM) (pH 7.3). The table lists the composition of the serum-free defined medium.
| Dissociation medium | |
| Name | Concentration |
| Phosphate buffered saline (PBS) | |
| Collagenase IV | 3 mg/mL |
| Dispase II | 4 mg/mL |
Table 4: Dissociation medium. List of constituents and their required concentrations for preparing the dissociation medium.
| Medium A (pH 7.3) | |
| Name | Concentration |
| Sterile H2O | |
| DMEM powder high glucose | 13.38 g/L |
| HEPES | 5.958 g/L |
| Sodium-pyruvate (C3H3NaO3) | 110 mg/L |
| Penicillin G sodium salt | 35 mg/L |
| Streptomycin sulfate salt | 50 mg/L |
| Sodium Chloride (NaCl) | 0.5 g/L |
| Sodium Hydrogen Carbonate (NaHCO3) | 1 g/L |
| Albumin Bovine (cell culture grade) | 3 g/L |
| Dnase* | 0.2 mg/mL |
| *add when mentioned |
Table 5: Medium A (pH 7.3). The table lists the concentration of the constituents used to prepare Medium A.
| Primary Antibodies | ||
| Name | Host | Concentration |
| AMELX | mouse | 1:100 |
| CD44 | mouse | 1:200 |
| CK14 | mouse | 1:200 |
| ITGA6 | rabbit | 1:200 |
| P63 | rabbit | 1:1000 |
| SOX2 | rabbit | 1:2000 |
| Secundary Antibodies | ||
| Name | Host | Concentration |
| mouse IgG (Alexa 555) | donkey | 1:1000 |
| rabbit IgG (Alexa 488) | donkey | 1:1000 |
Table 6: List of antibodies and their dilutions. The table lists the antibodies and their respective dilutions used in this study.
| Primers | ||
| Gene | Forward primer | Reverse primer |
| GAPDH | GGTATCGTGGAAGGACTCATGAC | ATGCCAGTGAGCTTCCCGTTCAG |
| P63 | CAACGCAGTAGACACCATTTCC | CCCAAAACCTTCTCGCTTGTT |
| ITGA6 | GGCGGTGTTATGTCCTGAGTC | AATCGCCCATCACAAAAGCTC |
| SOX2 | GCTGGGACATGTGAAGTCTG | CCCTGTGGTTACCTCTTCCT |
| PITX2 | CAGCGGACTCACTTTACCAG | ATTCTTGAACCAAACCCGGAC |
Table 7: List of primers. The table lists the primers of GAPDH, P63, ITGA6, SOX2, and PITX2 used in this study.
Discussion
This protocol describes the efficient and reproducible generation of organoids starting from the human tooth. To our knowledge, this is the first methodology for establishing current-concept (epithelial) organoids starting from human dental tissue. The organoids are long-term expandable and display a tooth epithelial stemness phenotype, duplicating DESCs previously reported in the ERM compartment of the DF7. Moreover, the organoids replicate functional DESC/ERM characteristics, including the unfolding of an ameloblast differentiation process7,25,26. Findings are robust since comparable results were found with independent patient organoid lines19.
When executing this tooth organoid protocol, several critical points need to be taken into account. First, the addition of Rho-associated kinase (ROCK) inhibitor Y-27632 at initial seeding and immediately after each passaging is essential to prevent the single cells from undergoing anoikis27. In addition, Amphotericin B is required in all media refreshments during P0 to avoid (oral) fungal outgrowth. Second, it is advised to immediately process the freshly isolated DF tissues for the optimal organoid formation and growth efficiency, rather than to start from cryopreserved tissue, which results in lower efficiency. Third, when thawing a cryopreserved organoid line for culturing, perform steps as rapidly as possible and avoid too long thawing time as well as too long intervals between the steps as time prolongation decreases cell survival. Fourth, it should be noted that the number of organoids at early passage (P0-P3) may remain limited, also because only limited numbers of ERM (stem) cells may be present in the specific isolated DF tissue samples. Hence, the organoid cultures at early passage should be handled with care and consideration. Therefore, it is recommended to (i) avoid rapid expansion of the organoid culture (i.e., only start to split at 1:3 or more starting from P3-4, and before at 1:0.5 or 1:1); (ii) use the appropriate splitting method (low passage – higher passage) as described. Within this context, it is advised to collect unerupted wisdom teeth of young adolescent patients (15 to 19 years old) since ERM cells decrease in number with tooth development and age28. Fifth, remaining undispersed hard tissue fragments from the DF tissue in the cell suspension (even after filtering) cause the BMM drop to be less stable and more likely to dislodge during culture. A higher percentage of BMM (such as 80%) is recommended if multiple undispersed hard tissue fragments are observed in the dissociated DF cell suspension. Sixth, it is strongly advised to passage the organoids between day 10 and day 14 of culture since longer culture will negatively affect organoid expandability because of less optimal dissociation. If for one or the other reason, organoids are cultured longer than 14 days, TryplE Express quantity and incubation time for organoid dissociation could be extended for efficient dissociation, although 15 min of enzymatic exposure should not be surpassed. Within the same context, the culture medium must be refreshed every 2-3 days to prevent nutrient and growth factor exhaustion. In case organoids do not expand properly, regardless of the critical points mentioned above, one should focus on keeping all tools (BMM, ice-cold SFDM for pre-coating tip, microcentrifuge tubes) used during passaging on ice. In addition, it is crucial to correctly apply the distinct passaging methods (low passage and higher passage method) for efficient passaging of the organoids.
Before, other groups have reported in vitro growth of primary human DESC/ERM tissue8,9,10,11,12,21. However, cultures were mainly 2D (monolayers) and not 3D, such as this organoid model, moreover only showing short-term growth and phenotype retention. Alternatively, often (spontaneously) immortalized cells were used, which, however, are not physiological and show only limited resemblance to the tissue or cells of origin. Moreover, these cell lines were derived from embryonic tissue and/or from animals. Furthermore, ameloblast differentiation is either not described or only limitedly documented. Thus, the organoid model presented here offers several advantages, being (i) faithful recapitulation of the tissue/cells of origin, (ii) long-term expandable, (iii) cultured in 3D more closely representing the in vivo configuration, (iv) of human origin and postnatal age, and (v) capable of differentiating into mature dental cells (ameloblast cell type) (see19).
Thus, we generated a valuable research tool, not reported before, holding several interesting applications (Figure 1B). The organoids can be applied to study human DESC/ERM stemness and plasticity. It provides the opportunity to gain further insight into the biology of the still enigmatic ERM cell population by means of immunofluorescent, gene expression, and (single-cell) transcriptomic analyses. In addition, organoids are particularly suited for human disease modeling to decipher pathogenetic mechanisms, identify (new) therapeutic targets and generate drug discovery and screening tools29. More specifically, this model can be applied to odontogenic cysts (for which no reliable research model is available), which can be compared to healthy tooth-derived organoids. In addition, this tooth organoid approach may be harnessed to model and study tooth diseases ranging from the impact of bacteria to genetic mutations associated with tooth anomalies (such as mutations in P63, which could be introduced using cutting-edge gene-editing methods such as CRISPR-Cas)30, eventually leading to potential, and novel, therapeutic targets, and treatments. Other applications of the tooth organoid protocol may include biobanking (currently already available for dental pulp, such as the Future Health Biobank)31 to collect organoid lines from manifold persons and diseases (e.g., for basic and translational research such as drug screening). Furthermore, several reports on composite organoid models containing not only epithelial but also other cell types of the tissue of origin have recently been published32,33. As tooth composition is rather complex, accommodating mesenchymal, immune, and endothelial cells, applying this epithelial organoid model in combination with these cell types to more in detail represent their in vivo counterpart is an appealing perspective. Also, this system allows to explore amelogenesis in the human tooth, at present only poorly understood, but certainly relying on epithelial-mesenchymal interactions. Deciphering ameloblast development is expected to represent an important leap forward in the dental scientific and clinical world since the production of enamel, the quintessential component of our teeth, is a highly chased goal in dental tissue repair. Moreover, the organoid modeling detailed in this study may signify the start toward the formation of mineralized tissues in vitro and pave the way toward developing a bioengineered tooth (or at least parts) for replacement therapy.
One of the limitations of the organoid model is that it solely represents the epithelial component of tissue. However, as described in detail above, this shortcoming could be solved by the addition of other cell/tissue types, such as the dental mesenchyme19. Another aspect that may be recognized as a limitation is the origin of the BMM used here (Matrigel). This BMM is derived from a sarcoma (Engelbrecht-Holm-Swarm) of a mouse and therefore must be replaced before translating the organoid approach to the clinic. Recently, several efforts have been made to replace Matrigel with synthetic hydrogels34,35. However, more research is needed to successfully grow organoids in such non-natural gels. Although the organoid technology provides an interesting approach for future dental regenerative therapy – for instance, the development of a bioengineered tooth – ethical questions should be raised regarding the privacy of cell donors as well as the commercialization of human organoids and tissues derived thereof. So far, no conclusions regarding organoid commercialization for regenerative purposes have been reached36. Dental pulp biobanks have been on the rise31, as well as organoid biobanks from several, mainly cancerous tissues, for drug screening purposes. Given that organoids cannot be categorized as cells, gametes, tissues, or organs (which all are regulated by law), there is an urgent need for depicting its juridical status for its use in clinical, scientific, or commercial settings. Even though the organoids have shown to deposit mineralized tissue when subcutaneously transplanted in vivo19, further studies are required to analyze their potential to deposit enamel similar to that of a natural human tooth.
Altogether, the new organoid model developed presents a promising, valuable tool to study human tooth (stem cell) biology and amelogenesis, both at present only poorly explored, with future perspectives toward tooth disease modeling and regenerative therapies.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We are grateful to all staff members of the Oral and Maxillofacial Surgery (MKA) of UZ Leuven, as well as the patients, for their invaluable help in collecting freshly extracted third molars. We would also like to thank Dr. Reinhilde Jacobs and Dr. Elisabeth Tijskens for their help with the sample collection. This work was supported by grants from KU Leuven (BOF) and FWO-Flanders (G061819N). L.H. is an FWO Ph.D. Fellow (1S84718N).
Materials
| 1.5 mL Microcentrifuge tube | Eppendorf | 30120.086 | |
| 15 mL Centrifuge tube | Corning | 430052 | |
| 2-Mercaptoethanol | Sigma-Aldrich | M-6250 | |
| 48-well flat bottom plates | Corning | 3548 | |
| 50 mL Centrifuge tube | Corning | 430290 | |
| A83-01 | Sigma-Aldrich | SML0788 | |
| Agarose | Lonza | 50004 | |
| Albumin Bovine (cell culture grade) | Serva | 47330.03 | |
| AMELX antibody | Santa Cruz | sc-365284 | |
| Amphotericin B | Gibco | 15200018 | |
| B27 (without vitamin A) | Gibco | 12587-010 | |
| Cassette | VWR | 7202191 | |
| Catalase from bovine liver | Sigma-Aldrich | C100 | |
| CD44 antibody | Abcam | ab34485 | |
| Cell strainer, 40 µm | Falcon | 352340 | |
| Cholera Toxin | Sigma-Aldrich | C8052 | |
| Citric acid | Sigma-Aldrich | C0759 | |
| CK14 antibody | Thermo Fisher Scientific | MA5-11599 | |
| Collagenase IV | Gibco | 17104-019 | |
| Cover glass | VWR | 6310146 | |
| Cryobox | Thermo Scientific | 5100-0001 | |
| Cryovial | Thermo Fisher Scientific | 375353 | |
| Dimethylsulfoxide (DMSO) | Sigma-Aldrich | D2650 | |
| Dispase II | Sigma-Aldrich | D4693 | |
| DMEM 1:1 F12 without Fe | Invitrogen | 074-90715A | |
| DMEM powder high glucose | Gibco | 52100039 | |
| Dnase | Sigma-Aldrich | D5025-15KU | |
| Donkey serum | Sigma-Aldrich | D9663 – 10ML | |
| Embedding workstation, 220 to 240 Vac | Thermo Fisher Scientific | 12587976 | |
| Ethanol absolute, ≥99.8% (EtOH) | Fisher Chemical | E/0650DF/15 | |
| Fetal bovine serum (FBS) | Sigma-Aldrich | F7524 | |
| FGF10 | Peprotech | 100-26 | |
| FGF2 (= basic FGF) | R&D Systems | 234-FSE-025 | |
| FGF8 | Peprotech | AF-100-25 | |
| GenElute Mammaliam Total RNA Miniprep Kit | Sigma-Aldrich | RTN350-1KT | Includes 1% β-mercaptoethanol dissolved in lysis buffer |
| Glass Pasteur pipette | Niko Mechanisms | 170-40050 | |
| Glycine | VWR | 101194M | |
| HEPES | Sigma-Aldrich | H4034 | |
| IGF-1 | PeproTech | 100-11 | |
| InSolution Y-27632 (ROCK inhibitor, RI) | Sigma-Aldrich | 688001 | |
| Insulin from bovine pancreas | Sigma-Aldrich | I6634 | |
| ITGA6 antibody | Sigma-Aldrich | HPA012696 | |
| L-Glutamine | Gibco | 25030024 | |
| Matrigel (growth factor-reduced; phenol red-free) | Corning | 15505739 | |
| Microscope slide | Thermo Fisher Scientific | J1800AMNZ | |
| Millex-GV Syringe Filter Unit, 0.22 μm | Millipore | SLGV033R | |
| Minimum essential medium eagle (αMEM) | Sigma-Aldrich | M4526 | |
| mouse IgG (Alexa 555) secondary antibody | Thermo Fisher Scientific | A-31570 | |
| N2 | Gibco | 17502-048 | |
| N-acetyl L-cysteine | Sigma-Aldrich | A7250 | |
| Nicotinamide | Sigma-Aldrich | N0636 | |
| Noggin | PeproTech | 120-10C | |
| P63 antibody | Abcam | ab124762 | |
| Pap Pen | Sigma-Aldrich | Z377821-1EA | Marking pen |
| Paraformaldehyde (PFA), 16% | Merck | 8.18715 | |
| Penicillin G sodium salt | Sigma-Aldrich | P3032 | |
| Penicillin-streptomycin (Pen/Strep) | Gibco | 15140-122 | |
| Petri dish | Corning | 353002 | |
| Phosphate buffered saline (PBS) | Gibco | 10010-015 | |
| Pipette (P20, P200, P1000) | Eppendorf or others | 2231300006 | |
| Plastic transfer pipette (3.5 mL) | Sarstedt | 86.1171.001 | |
| Rabbit IgG (Alexa 488) secondary antibody | Thermo Fisher Scientific | A21206 | |
| RSPO1 | PeproTech | 120-38 | |
| SB202190 (p38i) | Biotechne (Tocris) | 1264 | |
| Scalpel (surgical blade) | Swann-Morton | 207 | |
| SHH | R&D Systems | 464-SH-200 | |
| Silicone molds (Heating block) | VWR | 720-1918 | |
| Sodium Chloride (NaCl) | BDH | 102415K | |
| Sodium Hydrogen Carbonate (NaHCO3) | Merck | 106329 | |
| Sodium-pyruvate (C3H3NaO3) | Sigma-Aldrich | P-5280 | |
| SOX2 antibody | Abcam | ab92494 | |
| StepOnePlus | Thermo Fisher Scientific | Real-Time PCR System | |
| Stericup-GP, 0.22 µm | Millipore | SCGPU02RE | |
| Steriflip-GP Sterile Centrifuge Tube Top Filter Unit, 0.22 μm | Millipore | SCGP00525 | |
| Sterile 1000 μL pipette tips with filter | Greiner | 740288 | |
| Sterile 20 μL pipette tips with filter | Greiner | 774288 | |
| Sterile 200 μL pipette tips with and without filter | Greiner | 739288 | |
| Sterile H2O | Fresenius | B230531 | |
| Streptomycin sulfate salt | Sigma-Aldrich | S6501 | |
| Superscript III first-strand synthesis supermix | Invitrogen | 11752-050 | Reverse transcription kit |
| Tissue processor | Thermo Scientific | 12505356 | |
| Transferrin | Serva | 36760.01 | |
| Triton X-100 | Sigma | T8787-50ML | |
| TrypLE express | Gibco | 12605-010 | |
| Vectashield mounting medium+DAPI | Labconsult NV | H-1200 | Antifade mounting medium with DAPI |
| WNT3a | Biotechne (Tocris) | 5036-WN-500 | |
| Xylenes, 99%, for biochemistry and histology | VWR | 2,89,75,325 |
References
- Yu, T., Klein, O. D. Molecular and cellular mechanisms of tooth development, homeostasis and repair. Development (Cambridge). 147 (2), (2020).
- Arrow, P. Dental enamel defects, caries experience and oral health-related quality of life: a cohort study. Australian Dental Journal. 62 (2), 165-172 (2017).
- Mitsiadis, T. A., Orsini, G., Jimenez-Rojo, L., Zavan, B., Bressan, E. Dental Stem Cells for Tooth Regeneration. Dental Stem Cells: Regenerative Potential. Stem Cell Biology and Regenerative Potential. Stem Cell Biology and Regenerative Medicine. , (2016).
- Mitsiadis, T. A., Orsini, G. Editorial: a new era in dentistry: stem cell-based approaches for tooth and periodontal tissue regeneration. Frontiers in Physiology. 7, 357 (2016).
- Miran, S., Mitsiadis, T. A., Pagella, P. Innovative dental stem cell-based research approaches: the future of dentistry. Stem Cells International. 2016, 7231038 (2016).
- Shinmura, Y., Tsuchiya, S., Hata, K. I., Honda, M. J. Quiescent epithelial cell rests of malassez can differentiate into ameloblast-like cells. Journal of Cellular Physiology. 217 (3), 728-738 (2008).
- Davis, E. M. A review of the epithelial cell rests of Malassez on the bicentennial of their description. Journal of Veterinary Dentistry. 35 (4), 290-298 (2018).
- Athanassiou-Papaefthymiou, M., Papagerakis, P., Papagerakis, S. Isolation and characterization of human adult epithelial stem cells from the periodontal ligament. Journal of Dental Research. 94 (11), 1591-1600 (2015).
- Kim, G. -. H., et al. Differentiation and establishment of dental epithelial-like stem cells derived from human ESCs and iPSCs. International Journal of Molecular Sciences. 21 (12), 1-16 (2020).
- Nam, H., et al. Establishment of Hertwig’s epithelial root sheath/ epithelial rests of malassez cell line from human periodontium. Molecules and Cells. 37 (7), 562-567 (2014).
- Nam, H., et al. Expression profile of the stem cell markers in human hertwig’s epithelial root sheath/Epithelial rests of Malassez cells. Molecules and Cells. 31 (4), 355-360 (2011).
- Tsunematsu, T., et al. Human odontogenic epithelial cells derived from epithelial rests of Malassez possess stem cell properties. Laboratory Investigation; A Journal of Technical Methods and Pathology. 96 (10), 1063-1075 (2016).
- Artegiani, B., Clevers, H. Use and application of 3D-organoid technology. Human Molecular Genetics. 27 (2), 99-107 (2018).
- Boretto, M., et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nature Cell Biology. 21 (8), 1041-1051 (2019).
- Cox, B., et al. Organoids from pituitary as a novel research model toward pituitary stem cell exploration. Journal of Endocrinology. 240 (2), 287-308 (2019).
- Sato, T., et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 459 (7244), 262-265 (2009).
- Boretto, M., et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development (Cambridge). 144 (10), 1775-1786 (2017).
- Schutgens, F., Clevers, H. Human organoids: tools for understanding biology and treating diseases). Annual Review of Pathology. 15, 211-234 (2020).
- Hemeryck, L., et al. Organoids from human tooth showing epithelial stemness phenotype and differentiation potential. Cellular and Molecular Life Sciences. 79 (3), 153 (2022).
- Gao, X., Wu, Y., Liao, L., Tian, W. Oral organoids: progress and challenges. Journal of Dental Research. 100 (5), 454-463 (2021).
- Binder, M., et al. Novel strategies for expansion of tooth epithelial stem cells and ameloblast generation. Scientific Reports. 10 (1), 4963 (2020).
- Xiong, J., Mrozik, K., Gronthos, S., Bartold, P. M. Epithelial cell rests of malassez contain unique stem cell populations capable of undergoing epithelial-mesenchymal transition. Stem Cells and Development. 21 (11), 2012-2025 (2012).
- Luan, X., Ito, Y., Diekwisch, T. G. H. Evolution and development of Hertwig’s epithelial root sheath. Developmental Dynamics. 235 (5), 1167-1180 (2006).
- Fukumoto, S., et al. New insights into the functions of enamel matrices in calcified tissues. Japanese Dental Science Review. 50 (2), 47-54 (2014).
- Consolaro, A., Consolaro, M. F. M. O. ERM functions, EGF and orthodontic movement or Why doesn’t orthodontic movement cause alveolodental ankylosis. Dental Press Journal of Orthodontics. 15 (2), 24-32 (2010).
- Guajardo, G., et al. Immunohistochemical localization of epidermal growth factor in cat paradental tissues during tooth movement. American Journal of Orthodontics and Dentofacial Orthopedics. 118 (2), 210-219 (2000).
- Watanabe, K., et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nature Biotechnology. 25 (6), 681-686 (2007).
- Gonçalves, J., Sasso-Cerri, E., Cerri, P. Cell death and quantitative reduction of rests of Malassez according to age. Journal of Periodontal Research. 43 (4), 478-481 (2008).
- Kim, J., Koo, B. -. K., Knoblich, J. A. Human organoids: Model systems for human biology and medicine. Nature Reviews. Molecular Cell Biology. 21 (10), 571-584 (2020).
- Razmi, M. T., Narang, T., Handa, S. ADULT (acro-dermato-ungual-lacrimal-tooth) syndrome: a case report from India. Indian Dermatology Online Journal. 9 (3), 194 (2018).
- . Future Health Biobank Available from: https://futurehealthbiobank.com/ch-en/ (2022)
- Schreurs, R. R. C. E., Baumdick, M. E., Drewniak, A., Bunders, M. J. In vitro co-culture of human intestinal organoids and lamina propria-derived CD4+ T cells. STAR Protocols. 2 (2), 100519 (2021).
- Fiorini, E., Veghini, L., Corbo, V. Modeling cell communication in cancer with organoids: Making the complex simple. Frontiers in Cell and Developmental Biology. 8, 166 (2020).
- Gjorevski, N., et al. Designer matrices for intestinal stem cell and organoid culture. Nature. 539 (7630), 560-564 (2016).
- Zhang, Y., et al. Polyisocyanide hydrogels as a tunable platform for mammary gland organoid formation. Advanced Science. 7 (18), 2001797 (2020).
- Mollaki, V. Ethical challenges in organoid use. BioTech. 10 (3), 12 (2021).

