Visualizing Subcellular Localization of a Protein in the Heart Using Quantum Dots-Mediated Immuno-Labeling Followed by Transmission Electron Microscopy
Summary
The present protocol describes a method of immuno-labeling a protein in the heart tissue sections using quantum dots. This technique provides a useful tool to visualize any protein's subcellular localization and expression at the ultrastructural level.
Abstract
The subcellular localization is critical to delineating proper function and determining the molecular mechanisms of a particular protein. Several qualitative and quantitative techniques are used to determine the subcellular localization of proteins. One of the emerging techniques in determining the subcellular localization of a protein is quantum dots (QD)-mediated immunolabeling of a protein followed by imaging them with transmission electron microscopy (TEM). QD is a semiconductor nanocrystal with a dual property of crystalline structure and high electron density, which makes them applicable to electron microscopy. This current method visualized the subcellular localization of Sigma 1 receptor (Sigmar1) protein using QD-TEM in the heart tissue at ultrastructural level. Small cubes of the heart tissue sections from a wild-type mouse were fixed in 3% glutaraldehyde, subsequently osmicated, stained with uranyl acetate, followed by sequential dehydration with ethanol and acetone. These dehydrated heart tissue sections were embedded in low-viscosity epoxy resins, cut into thin sections of 500 nm thickness, put on the grid, and subsequently subjected to antigen unmasking with 5% sodium metaperiodate, followed by quenching of the residual aldehydes with glycine. The tissues were blocked, followed by sequential incubation in primary antibody, biotinylated secondary antibody, and streptavidin-conjugated QD. These stained sections were blot dried and imaged at high magnification using TEM. The QD-TEM technique allowed the visualization of Sigmar1 protein's subcellular localization at the ultrastructural level in the heart. These techniques can be used to visualize the presence of any protein and subcellular localization in any organ system.
Introduction
The human body is composed of many proteins responsible for numerous bodily functions. The function of proteins largely depends on their localization in the organ and cellular organelles. Several techniques, including subcellular fractionation, immunofluorescence, and detergent-mediated protein extraction, are commonly used to determine the protein's subcellular localization1,2. Microscopy using immunofluorescent dye is the most widely used method among these techniques. However, the fluorescent dyes used in this technique are less stable and prone to photobleaching3. Other techniques involved high-resolution microscopy to visualize the protein at an ultrastructural level by immunolabeling proteins with electron-dense, heavy metals (gold, ferritin) or quantum dots nanocrystals and followed by visualizing them using transmission electron microscopy (TEM)4,5.
QD is a semiconductor nanocrystal composed of semiconductor metal compounds with controllable photoluminescence properties holding a great significance in biological systems3. QD nanocrystals are made in a core-shell format where a nanocrystal is encapsulated in the formation of nanocrystals to assure their proper stability and functioning. Commonly used core-shell nanocrystals combination are CdSe/ZnS, CdSe/CdS CdSe/ZnSe, CdTe/CdS, CdTe/ZnS, and CdTe/CdS/ZnS (core/shell/shell)3. Among these nanocrystal combinations, CdSe/ZnS and CdSe/CdS are most vigorously studied and frequently used as secondary antibody conjugates3,6. These QD nanoparticles also possess fluorescent properties with different excitation and emission spectra than traditional fluorophores. QD employs the excitation of electrons from the bulk valence band to exhibit higher fluorescence quantum yields compared to traditional fluorophores. The nanocrystal arrangement of semiconductor metals makes QD-mediated labeling more stable and resistant to photobleaching6. In addition, the nanocrystal core in QD and its crystalline structure allows QD of different sizes to have a wide range of absorption spectra and very narrow emission peaks7. Moreover, these QD particles are large enough to yield high electron density, making them useful in high-resolution microscopy techniques, including transmission electron microscopy5,8,9. These QD nanocrystals are also commercially available in multiple sizes with different fluorescence emission spectra and shapes, making them a great candidate for labeling with multiple antibodies10,11.
QD technology attracted great significance in biological research due to multiple functional properties, including use in live-cell imaging, the study of transport mechanisms in the cell, membrane transport of protein's diffusion movement, functional heterogeneity of cells, and marking of intracellular organelle3,12,13,14,15,16. QD is also useful in molecular diagnostics for targeting and detecting cancer tissues, characterizing the tumor's molecular profile and immune status, and visualizing vitreous body and epiretinal membranes3,17,18. In addition, QD can also be used in medical therapies to treat tumor malignancies via photodynamic therapy and ophthalmic anomalies by delivering medicines to eyes3,17,18,19.
Using these highly useful QD nanocrystals, the present study determined the subcellular localization of a protein named Sigma 1 receptor (Sigmar1). Sigmar1 is a ubiquitously expressed multi-tasking molecular chaperone protein. Extensive studies focusing on Sigmar1's subcellular localization in different tissues and organs reported a cell and tissue-type specific subcellular localization eliciting molecular function20. In different cells (neuronal, photoreceptor, and immune cells) and tissues (liver and brain) using different biochemical approaches, studies reported the localization of Sigmar1 on endoplasmic reticulum (ER), mitochondria-associated ER membrane (MAM), nuclear envelope, plasma membrane, nucleoplasmic reticulum, nucleus, and mitochondria. Despite all these studies, the subcellular localization of Sigmar1 in the heart remained unknown20. Therefore, the subcellular localization of Sigmar1 in cardiac tissue was determined using the QD-mediated immunolabeling followed by TEM imaging.
Protocol
The animal handling procedures in this protocol complied with the Guide for the Care and Use of Laboratory Animals (Eighth Edition, National Institutes of Health, Bethesda, MD) and were approved by the Animal Care and Use Committee of Louisiana State University Health Sciences Center-Shreveport. Six-month-old male mice with FVB/N background, were used for the present study. The mice were obtained from commercial sources (see Table of Materials). The mice used were housed in a well-regulated environment in cages allowing a 12 h light-dark-cycle, provided with water and a regular chow diet ad libitum, and were cared for according to the NIH Guide for the Care and Use of the Laboratory Animals. The overall process is illustrated in Figure 1.
1. Animal preparation
- Anesthetize the mice using 3% isoflurane. Open the chest by making a horizontal incision in the region above the middle abdomen and pulling the skin. Carefully, make another incision to open the chest cavity without puncturing any other organs21,22,23.
- Perfuse24 the heart through the apex first, and then the right ventricle using cold 3% glutaraldehyde in cardioplegic solution (50 mM KCl, 5% dextrose in PBS, see Supplementary File 1) for 2 min to ensure complete myocardial relaxation.
- Perfuse the heart using ice-cold 3% glutaraldehyde in 0.1 M of sodium cacodylate buffer (pH 7.4, step 2.1.1) for another 2 min. Using gravity pressure, use a 25 G 5/8" needle to introduce the fixatives into the heart. Immediately after the heart starts to fill up with the fixative, lift up the apex of the heart and cut the vessels underneath at 1-2 mm from the heart to relieve pressure and allow the liquids to drain.
- Dissect the heart, remove the atria24, and drop the ventricles into ice-cold 3% glutaraldehyde/0.1 M sodium cacodylate in a Petri dish. Make a butterfly cut after 30-60 min of fixation and place it in a Petri dish containing 3% glutaraldehyde/cacodylate (see Table of Materials). Chop the heart using a surgical blade in small cubes of 1 mm3 while it is in glutaraldehyde/cacodylate solution.
- Fix/immerse the dissected heart tissue in glutaraldehyde/cacodylate solution for 24 h at 4 °C.
2. Heart tissue processing
- After 24 h of fixation, wash the tissues in 0.1 M of sodium cacodylate buffer for 20 min.
- Prepare 0.1 M cacodylate buffer following the steps below.
- To prepare 0.1 M sodium cacodylate buffer, prepare a stock of 1 M sodium cacodylate buffer by dissolving sodium cacodylate (21.4 g) and of 1% calcium chloride solution (3 mL) in 90 mL of distilled water. Volume up the solution to 100 mL by adding the distilled water. Stir the solution well and leave it overnight to dissolve the solute.
- Next, take 10 mL of 1 M sodium cacodylate stock solution and add it to 80 mL of distilled water. Adjust the pH to 7.4 using HCl and volume it up to 100 mL by adding distilled water.
- Prepare 0.1 M cacodylate buffer following the steps below.
- Repeat the wash in 0.1 M sodium cacodylate buffer for another 20 min. Remove the tissues from sodium cacodylate buffer and immerse them in 2% osmium tetroxide solution for 4 h at room temperature. This process is called osmication.
- Prepare 2% Osmium tetroxide solution following the steps below.
- To prepare 2% Osmium tetroxide solution, take 4% Osmium tetroxide solution, 4 mL (see Table of Materials), 1 M sodium cacodylate stock solution (0.8 mL), and distilled water (3.2 mL) to make a total of 8 mL solution.
NOTE: This whole process and the steps from hereon must be carried out in the fume hood.
- To prepare 2% Osmium tetroxide solution, take 4% Osmium tetroxide solution, 4 mL (see Table of Materials), 1 M sodium cacodylate stock solution (0.8 mL), and distilled water (3.2 mL) to make a total of 8 mL solution.
- Prepare 2% Osmium tetroxide solution following the steps below.
- After osmication, immerse the tissues in 2% sodium acetate solution for 10 min at room temperature.
- Prepare 2% sodium acetate solution by dissolving 4 g of sodium acetate in 20 mL of distilled water.
- Next, immerse the tissues in 2% uranyl acetate solution for 1 h at room temperature.
- Prepare 2% uranyl acetate solution by dissolving 4 g of uranyl acetate in 20 mL of distilled water.
- After uranyl acetate staining, dehydrate the tissues sequentially through the graded alcohols and acetone in the order mentioned in Supplementary File 1.
3. Heart tissue embedding
- Embed the dehydrated tissues in low viscosity epoxy resin following the steps below.
- To make low-viscosity epoxy resin, mix 10.24 mL of vinyl cyclohexene dioxide (ERL 4221) epoxy monomer, 6.74 mL of diglycidyl ether of polypropylene glycol (DER 732), and 30.05 mL of nonenyl succinic anhydride (NSA) (see Table of Materials) in a 50 mL tube. Stir the suspension well by hand for 2 min.
- Add 18 drops of 2-dimethylaminoethanol (DMAE, see Table of Materials) epoxy accelerator and stir the suspension to mix the components thoroughly.
- Impregnate the tissues in epoxy resin in the following sequence of mixture.
- Replace the 100% acetone with 1:1 resin: acetone suspension and immerse the tissues in it for 1 h at room temperature.
- Replace 1:1 resin: acetone suspension with 6:1 resin: acetone suspension and immerse the tissues in it for 3 h.
- Finally, replace the 6:1 resin: acetone suspension with 100% resin suspension and leave tissues immersed in it overnight at room temperature.
- Put the tissues into fresh resin in 8 mm micro molds (see Table of Materials) and cure the embedded tissues at 70 °C overnight.
NOTE: Ensure that the resin is hard but not brittle after curing.
4. Tissue sectioning using an ultramicrotome
- Trim the resin blocks with the tissue to no larger than 1 mm before mounting on the ultramicrotome (see Table of Materials). Place the mold precisely as possible in the segmented arm of the ultramicrotome and manually advance the sample mold toward the diamond knife.
- Cut the sections at a thickness of 500 nm (one-half micron) with a Histo knife (see Table of Materials), and using the EM loop tool, pick them up and place them on a glass slide.
- Place the glass slide on a hot plate for toluidine blue stain25 to find the area of interest.
- After finding the area of interest, use an Ultra 45° knife (see Table of Materials) to produce pale gold ultrathin sections (100 nm). Place these ultrathin sections on the dull side of a 200 mesh copper grid.
5. Ultrathin heart section staining
- Start the staining by unmasking the antigen using an etching solution, i.e., 5% sodium metaperiodate solution.
- Prepare 500 µL of 5% sodium metaperiodate (see Table of Materials) solution in distilled water.
NOTE: Prepare fresh solution before use. - Put 20 µL of sodium metaperiodate solution on a clean paraffin film. Place the completely dried grids with tissue sections on the droplet of the etching solution.
NOTE: The dull side of the grid with the tissue section must face toward the etching solution. - Leave the section grid on the solution for 30 min at room temperature.
- Prepare 500 µL of 5% sodium metaperiodate (see Table of Materials) solution in distilled water.
- Wash the etched tissue section by placing it on a droplet of distilled water for 60 s.
- Block the residual aldehydes by placing the section grids on a droplet of 0.05 M glycine solution for 10 min at room temperature. Blot the grid's edges on filter paper to remove the residual glycine solution.
- Prepare 0.05 M glycine solution (see Table of Materials) by dissolving 3.75 mg of glycine in 1 mL of 1x PBS (pH 7.4).
- Place the section grids in 10-20 µL of blocking solution for 25 min at room temperature.
NOTE: Blocking solution composition: 2 µL of 1% normal goat serum (NGS) + 20 µL of 1% BSA (final concentration: 10%) + 178 µL of 1x PBS (pH 7.4) to make a final volume of 200 µL. - Blot the grid edges on filter paper and place the grid section on antibody diluent for conditioning at room temperature for 10 min.
- Incubate the grid sections with primary antibody (Sigmar1 in this case, see Table of Materials; diluted 1:10 in antibody diluent) for 1 h 30 min in a humidified chamber.
- Blot dry the grid and wash the grid sections with antibody diluent twice for 5 min each.
- Incubate the grid sections with biotinylated secondary antibody (in this case, biotinylated goat anti-rabbit polyclonal secondary antibody, see Table of Materials; diluted 1:10 in antibody diluent) for 1 h in a humidified chamber.
- Blot dry the grid and wash the grid sections with antibody diluent twice for 5 min each.
- Incubate the grid sections in commercially available streptavidin-conjugated QD (QD655nm, see Table of Materials; diluted 1:10 in antibody diluent) for 1 h in a humidified chamber at room temperature. Prevent exposure to light by covering the chamber with aluminum foil.
- Blot dry the grid edges using filter paper and wash the grid sections by placing them on water droplets for 2 min.
- Blot the edges of the grid to dry.
6. Transmission Electron Microscopy (TEM) imaging
- Place the ultrathin sections on copper grids in a specimen quartet holder and clamp them in a position to allow the selection of grids in the electron beam.
- Insert the specimen holder in the microscope column and engage the pump switch to evacuate the goniometer, followed by full insertion of the specimen holder in the microscope column (see Table of Materials).
- Set the voltage to 80 kV before generating the beam for image observation.
- Focus well on the desired area, capture the image using a high-speed digital camera, and save the file in .tif format.
NOTE: The microscope setting to capture image in this study was accelerating voltage or high tension voltage of 80 kV and a magnification of 20,000x.
Representative Results
The present QD-TEM methodology visualized the presence of Sigmar1 and its localization on the subcellular heart compartments by performing anti-Sigmar1 targeted QD labeling on adult mouse ultra-thin heart sections. Electron-dense anti-Sigmar1 labeled QD on mitochondrial membranes (inner and outer), lysosomes, and the endoplasmic reticulum / sarcoplasmic reticulum (ER/SR) membrane-mitochondrial interface (Figure 2A,B) were illustrated by the QD-TEM images. In addition, heart sections were also labeled with rabbit IgG and QD as an Isotype control for anti-Sigmar1 rabbit primary antibody (Figure 2C,D). Therefore, QD-TEM imaging highlighted the localization of endogenous Sigmar1 and its enrichment mostly on the lysosomes and the mitochondrial membranes.
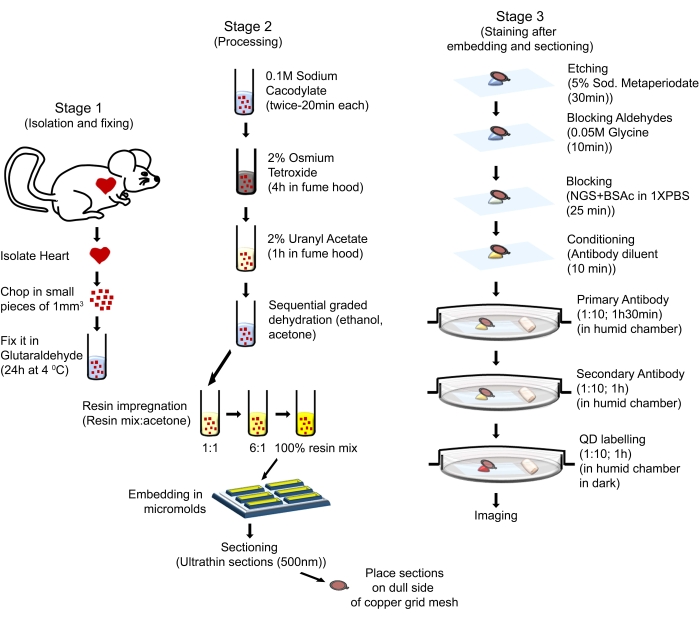
Figure 1: Schematic illustration showing the sequential steps and procedures used to perform QD-TEM. Please click here to view a larger version of this figure.
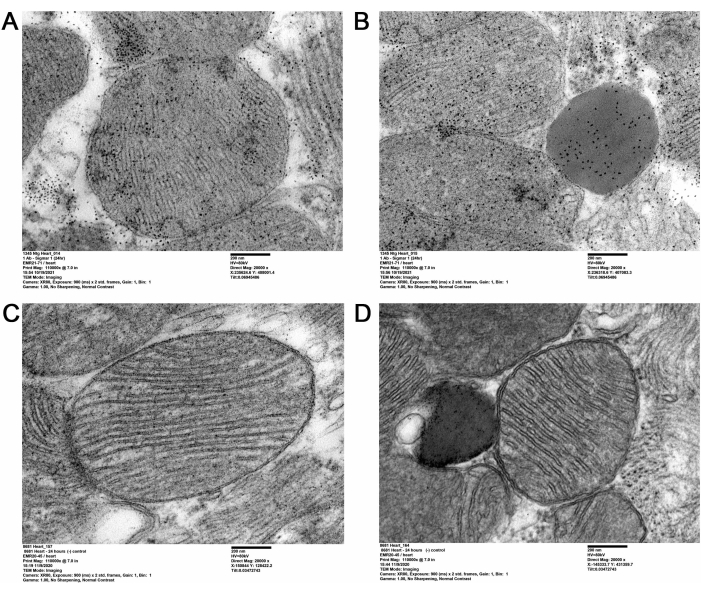
Figure 2: Sigmar1 immuno-labeled QD in adult mice heart tissues. (A,B) Representative TEM images showing Sigmar1 labeled QD on mitochondria (outer- and inner-membrane), mitochondria-associated endoplasmic reticulum (ER) / sarcoplasmic reticulum (SR) membranes, and lysosomes. (C,D) TEM image of the heart sections of isotype control labeled with QDs and rabbit IgG. Scale bar: 200 nm. Please click here to view a larger version of this figure.
Supplementary File 1: Composition of PBS and other solutions used for tissue dehydration. Please click here to download this File.
Discussion
In the present study, QD-mediated immunolabeling was used to distinctively show the subcellular localization of Sigmar1. Using QD, Sigmar1's localization on the mitochondrial membrane, especially the inner mitochondrial membrane, was depicted in cardiac tissue. Additionally, Sigmar1 was also found to be located on sarcoplasmic/endoplasmic reticulum (S/ER) and lysosomes at the ultrastructural level, as shown in Figure 2A-D.
A critical step in this protocol is the etching or antigen unmasking step using highly concentrated sodium metaperiodate solution to unmask the antigen after glutaraldehyde fixation and osmication. This protocol used a single treatment with a high concentration (5%) of sodium metaperiodate for 30 min at room temperature. Extra care is needed in this step as a longer duration or higher concentration for sodium metaperiodate incubation will result in aggregation of structures, loss of membrane definition for the organelles, and cause perforations in the section, making it tough to visualize the protein or the structure. Alternatively, a lower concentration of (3%) metaperiodate solution in two steps for 30 min can also be used instead of 5% metaperiodate. Studies have shown this option to exhibit similar results as with 5% metaperiodate solution for one-step 30 min incubation. However, a 3% metaperiodate solution for 30 min of incubation for two times provides better control over the process26,27,28. Initially, this protocol used incubation of the sections with 10% metaperiodate solution for 30 min. However, due to too many perforations created in the tissue section by this concentration, the final concentration and incubation duration of the metaperiodate solution was tapered down and optimized to 5% for 30 min.
Another step required optimization of the fixation time with glutaraldehyde. Suboptimal fixation of tissues results in inadequate QD labeling, whereas over fixation of tissues results in higher non-specific labeling. Therefore, careful consideration must be given in determining and titrating an optimal level of tissue fixation for proper and specific labeling of proteins. In this method using heart tissues, the fixation time with glutaraldehyde was titrated using 24 h and 48 h as timepoints. Based on the staining images of the sections fixed for both the timepoints, it was found that sections fixed for 24 h displayed better results. To date, QD nanocrystals are available in multiple sizes, including 525, 565, 585, 605, 655, and 705 nm11,29. Each of these QD has its own emission spectra and emits fluorescence at different wavelengths. Additionally, these commercially available QDs of different sizes display different shapes; for example, QD 525, 565, and 585 are virtually spherical with different sizes, whereas QD 605, 655, and 705 are irregular oblong shaped. Of these different QD nanocrystals, QD 525, 565, and 655 are easily distinguishable from one another11,29. These differences in emission spectra and shapes make QD a great candidate for multi-labeling of proteins and visualization by fluorescence and electron microscopy. In this study, a commercially available QD, QD 655, was used to label the Sigmar1 protein to distinguish it from any non-specific background in the stained sections.
Another counterpart of QD for protein labeling in high-resolution microscopy is the immunogold particle. The immunogold particles are traditionally used to label proteins for high-resolution microscopy. These gold particles are highly electron-dense and easily identifiable compared to QD nanocrystals. However, QD exhibits better efficiency with better penetration in tissues, higher stability and shelf life, and better retention of ultrastructural components, making them a better candidate for protein labeling4,5. QD also has a unique ability to be detected by both light and electron microscopy, which adds to its value over immunogold labeling10.
One limitation of this QD-mediated immunolabeling is the use of osmium tetroxide during processing. Osmium tetroxide is used to increase the electron density, conductivity, and contrast of otherwise less electron-dense and less contrasting biological membrane structures5,30. However, the use of osmium tetroxide instantaneously and irreversibly destroys the property of the specimen to create fluorescence when labeled with QD6. This limits the use of QD in fluorescence microscopy. An alternative approach omitting the use of osmium tetroxide will be advantageous in retaining the fluorescent properties and hence the dual application of QD-mediated immunolabeling. Some of the newer models of TEM have the option of attaching the Energy Dispersive X-Ray Analysis (EDX) system that allows identification of the elemental composition of materials. Another limitation of the study is the lack of elemental mapping of a sample and image analysis using EDX. Therefore, future studies should focus on the EDX analysis of the QD spectra to analyze the elemental composition.
QD labeling of proteins has gained a lot of attention in recent times. QD offers several applications and advantages in both biological research and medical therapeutics. QD being additionally wrapped with polydentate ligand exhibits increased stability maintaining the quantum yield. Further, encapsulating QD with these bio-favorable agents also increases its bioavailability in tissues making it a good candidate for potential application in detecting tumors, live-cell imaging, drug delivery, and tissue imaging3,31,32.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Institutes of Health grants: R01HL145753, R01HL145753-01S1, R01HL145753-03S1, and R01HL152723; LSUHSC-S CCDS Finish Line Award, COVID-19 Research Award, and LARC Research Award to MSB; LSUHSC-S Malcolm Feist Cardiovascular and AHA Postdoctoral Fellowship to CSA (20POST35210789); and LSUHSC-S Malcolm Feist Pre-doctoral Fellowship to RA.
Materials
| 200 Mesh copper grid | Ted Pella | G200HH | |
| 6-month-old male mice with FVB/N background | Jackson Laboratory, Bar Harbor, ME | ||
| Acetone 100% | Fisher Chemicals | A949 | |
| Antibody diluent | Dako | S3022 | |
| anti-Sigmar1 antibody | Cell Signaling | 61994S | |
| Biotinylated goat anti-rabbit IgG antibody | Sigma Aldrich | B7389 | |
| BSAc (10%) | Electron Microscopy Sciences | 25557 | |
| Calcium chloride | Sigma Aldrich | C7902 | |
| Cytoseal Xyl | Thermo fisher | 8312-4 | |
| DER 732C36AA10:C33 | Electron Microscopy Sciences | 13010 | |
| Dextrose | Sigma Aldrich | G7528 | |
| Diamond Knife | Diatome | Histo; Ultra 450 | |
| DMAE | Electron Microscopy Sciences | 13300 | |
| Electron microscope | JEOL | JEOL-1400 Flash | |
| ERL 4221 | Electron Microscopy Sciences | 15004 | |
| Ethanol 100% | Fisher Chemicals | A405P | |
| Glutaraldehyde 3% | Electron Microscopy Sciences | 16538-15 | |
| Glycine | Alfa Aesar | A13816 | |
| Hydrochloric acid | Fisher Scientific | SA56 | |
| Micromolds | Ted Pella | 10505 | |
| Microtome | Leica Microsystem | EM UC7 | |
| Normal goat serum | Invitrogen | PCN5000 | |
| NSA | Electron Microscopy Sciences | 19050 | |
| Osmium tetroxide | Electron Microscopy Sciences | 19150 | |
| Parafilm | Genesse Scientific | 16-101 | |
| Potassium Chloride | Sigma Aldrich | P5655 | |
| Potassium Phosphate monobasic | Sigma Aldrich | 71640 | |
| Qdot 655 Streptavidin Conjugate | Invitrogen | Q10121MP | |
| Sodium Acetate | Fisher Scientific | BP334 | |
| Sodium Cacodylate | Electron Microscopy Sciences | 12300 | |
| Sodium Chloride | Fisher Scientific | BP358 | |
| Sodium metaperiodate | Sigma Aldrich | 71859 | |
| Sodium Phosphate dibasic | Sigma Aldrich | P9541 | |
| Surgical blade (size 10) | Aspen surgical | 371110 | |
| TEM image software | AMT-V700 | AMT TEM imaging systems | |
| TEM imaging camera | XR80 TEM series | AMT TEM imaging systems | |
| Toluidine Blue O solution (0.5%) | Fisher Scientic | S25612 | |
| Uranyl acetate | Polysciences | 21447 |
References
- Lidke, D. S., Lidke, K. A. Advances in high-resolution imaging – techniques for three-dimensional imaging of cellular structures. Journal of Cell Science. 125 (11), 2571-2580 (2012).
- de Duve, C. Tissue fraction-past and present. The Journal of Cell Biology. 50 (1), 20 (1971).
- Pleskova, S., Mikheeva, E., Gornostaeva, E., Saquib, Q., Faisal, M., Al-Khedhairy, A. A., Alatar, A. A. . Cellular and Molecular Toxicology of Nanoparticles. , 323-334 (2018).
- Mayhew, T. M., Mühlfeld, C., Vanhecke, D., Ochs, M. A review of recent methods for efficiently quantifying immunogold and other nanoparticles using TEM sections through cells, tissues and organs. Annals of Anatomy – Anatomischer Anzeiger. 191 (2), 153-170 (2009).
- Kuipers, J., de Boer, P., Giepmans, B. N. G. Scanning EM of non-heavy metal stained biosamples: Large-field of view, high contrast and highly efficient immunolabeling. Experimental Cell Research. 337 (2), 202-207 (2015).
- Deerinck, T. J. The application of fluorescent quantum dots to confocal, multiphoton, and electron microscopic imaging. Toxicologic Pathology. 36 (1), 112-116 (2008).
- Nisman, R., Dellaire, G., Ren, Y., Li, R., Bazett-Jones, D. P. Application of quantum dots as probes for correlative fluorescence, conventional, and energy-filtered transmission electron microscopy. Journal of Histochemistry & Cytochemistry. 52 (1), 13-18 (2004).
- Michalet, X., et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 307 (5709), 538-544 (2005).
- Killingsworth, M. C., Bobryshev, Y. V. Correlative light- and electron microscopy using quantum dot nanoparticles. Journal of Visualized Experiments. (114), e54307 (2016).
- Deerinck, T. J., Giepmans, B. N. G., Smarr, B. L., Martone, M. E., Ellisman, M. H., Bruchez, M. P., Hotz, C. Z., Ellisman, M. H. . Quantum Dots: Applications in Biology. , 43-53 (2007).
- Giepmans, B. N. G., Deerinck, T. J., Smarr, B. L., Jones, Y. Z., Ellisman, M. H. Correlated light and electron microscopic imaging of multiple endogenous proteins using Quantum dots. Nature Methods. 2 (10), 743-749 (2005).
- Lidke, D. S., et al. Quantum dot ligands provide new insights into erbB/HER receptor-mediated signal transduction. Nature Biotechnology. 22 (2), 198-203 (2004).
- Pleskova, S. N., et al. Differences in the functional activity of human neutrophilic granulocytes in their interactions with semiconductor quantum dots. Morfologiia. 135 (3), 47-49 (2009).
- Crane, J., Haggie, P., Verkman, A. Quantum dot single molecule tracking reveals a wide range of diffusive motions of membrane transport proteins. Proceedings of SPIE. 7189, (2009).
- Hanaki, K. -. i., et al. Semiconductor quantum dot/albumin complex is a long-life and highly photostable endosome marker. Biochemical and Biophysical Research Communications. 302 (3), 496-501 (2003).
- Lim, Y. T., et al. . Selection of quantum dot wavelengths for biomedical assays and imaging. 2, 50-64 (2003).
- Gao, X., Cui, Y., Levenson, R. M., Chung, L. W. K., Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nature Biotechnology. 22 (8), 969-976 (2004).
- Åkerman, M. E., Chan, W. C. W., Laakkonen, P., Bhatia, S. N., Ruoslahti, E. Nanocrystal targeting in vivo. Proceedings of the National Academy of Sciences. 99 (20), 12617-12621 (2002).
- Sarwat, S. A. -. O. X., Stapleton, F., Willcox, M., Roy, M. A. -. O. Quantum dots in ophthalmology: A literature review. Current Eye Research. 44 (10), 1037-1046 (2019).
- Aishwarya, R., Abdullah, C. S., Morshed, M., Remex, N. S., Bhuiyan, M. S. Sigmar1’s molecular, cellular, and biological functions in regulating cellular pathophysiology. Frontiers in Physiology. 12, 705575 (2021).
- Bohne, B., Harding, G. . Processing the mouse temporal bone for gross, cellular and subcellular evaluation poster. , (2004).
- Roszkowski, M. . The effects of acute stress on Apold1 gene expression and blood-brain barrier permeability. , (2014).
- Wu, J., et al. Transcardiac perfusion of the mouse for brain tissue dissection and fixation. Bio-protocol. 11 (5), 3988 (2021).
- Jones, W. K., et al. Ablation of the murine alpha myosin heavy chain gene leads to dosage effects and functional deficits in the heart. Journal of Clinical Investigation. 98 (8), 1906-1917 (1996).
- Hunter, E. E. . Practical Electron Microscopy: A Beginner’s Illustrated Guide. , (1993).
- Morris, R. E., Ciraolo, G. M. A universal post-embedding protocol for immunogold labelling of osmium-fixed, epoxy resin-embedded tissue. Journal of Electron Microscopy. 46 (4), 315-319 (1997).
- Brorson, S. H. Deplasticizing or etching of epoxy sections with different concentrations of sodium ethoxide to enhance the immunogold labeling. Micron. 32 (2), 101-105 (2001).
- Lobo, M. V. T., et al. Ultrastructural Staining with Sodium Metaperiodate and Sodium Borohydride. Journal of Histochemistry & Cytochemistry. 50 (1), 11-19 (2002).
- Bruchez, M., Moronne, M., Gin, P., Weiss, S., Alivisatos, A. P. Semiconductor nanocrystals as fluorescent biological labels. Science. 281 (5385), 2013-2016 (1998).
- Tapia, J. C., et al. High-contrast en bloc staining of neuronal tissue for field emission scanning electron microscopy. Nature Protocols. 7 (2), 193-206 (2012).
- Smith, A. M., Duan, H., Mohs, A. M., Nie, S. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Advanced Drug Delivery Reviews. 60 (11), 1226-1240 (2008).
- Gour, A., Ramteke, S., Jain, N. K. Pharmaceutical applications of quantum dots. AAPS PharmSciTech. 22 (7), 233 (2021).

