动态核磁共振波谱线形分析,用于表征手性铼多氢化物配合物的配位球重排
Summary
在一系列温度下收集的NMR波谱的线形分析可指导手性,八坐标,铼(V)多氢化物配合物ReH5(PPh3)2(仲丁胺)的内配位球原子的重排。线形分析还用于确定这些原子重排的活化参数ΔH‡,ΔS‡和ΔG‡。
Abstract
动态溶液核磁共振(NMR)波谱是表征过渡金属多氢化物配合物配位球内原子动态重排的典型方法。动态核磁共振波谱的线形拟合可以估计动态重排过程的活化参数。金属结合磷原子的动态31 P-{1 H} NMR波谱与氢化物配体的动态1H-{31P} NMR波谱的组合可以识别与磷原子重排一起发生的氢化物配体重排。对于表现出这种偶联重排对的分子,动态核磁共振波谱可用于测试配体重排的理论模型。动态 1H-{31P} NMR 波谱和线形拟合还可以识别是否存在交换过程,该过程通过与溶剂分子(如不定水)的质子交换将特定氢化物配体移出金属的内部配位球。介绍了一种新化合物ReH5(PPh 3)2(仲丁胺)的制备方法,该化合物体现了多种动态重排过程,并介绍了该配合物动态NMR波谱的线形拟合。线形拟合结果可以通过艾林方程进行分析,以估计已识别动态过程的活化参数。
Introduction
核磁共振波谱通常用于表征分子内部或分子之间发生的动态过程。对于许多简单的分子内重排,ΔG‡ 的估计就像测量慢交换极限下两个共振之间的频率差 Δν 并确定这些相同共振的聚结温度一样简单(图 1)1。关系,
ΔG‡ = 4.575 x 10-3 大卡/摩尔 x T c [9.972 + 对数 (Tc/Δν)]
其中Tc 是一对共振的聚结温度,代表动态样品的慢交换形式,可用于求解这种动态重排的活化自由能。更复杂的动态系统需要动态核磁共振波谱的线形拟合或其他核磁共振技术,如二维交换波谱(2D-EXSY)或二维旋转框架Overhauser效应波谱(2D-ROESY)来估计活化参数。
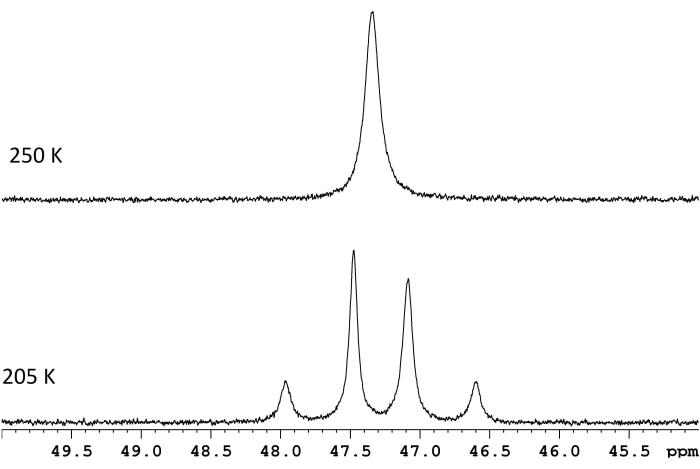
图 1:ReH5(PPh3)2(仲丁胺)的d 8-甲苯溶液在两个温度下的 NMR 波谱。两个慢交换双峰(下迹线,117.8 Hz)和聚结温度250 K(上迹线)之间的频率差对应于11.8 kcal/mol的能量势垒(ΔG‡)。 请点击此处查看此图的大图。
动态核磁共振波谱的线形拟合是一种常用技术,长期以来一直用于估计活化参数,这些参数描述活化能约为5至25 kcal/mol2,3,4,5的物质的动态重排。确定水和胺分子之间质子交换的能量势垒6,二甲基甲酰胺7中围绕C-N键旋转的能量势垒,或有机部分的一般尺寸8,只是通过动态NMR波谱线形拟合评估的许多特性中的几个例子。本手稿演示了使用线形拟合来表征复合物ReH 5(PPh3)2(仲丁胺)发生的分子间和分子内动力学过程。该实验和类似的线形拟合核磁共振实验的目标是:1)表征所有核磁共振可观察到的分子内动态原子交换过程(如果存在),2)识别和表征核磁共振可观测的分子内动态原子交换过程(如果存在),3)识别在本例中氢原子和磷原子发生的相关分子内原子交换,以及4)对于此处介绍的示例, 比较两个已发表的模型,了解复合物ReH5(PPh3)2(仲丁胺)中发生的动态过程。
八配位铼(V)多氢化物体系是复杂的动态系统,其中配体参与多个动态过程,磷原子可以参与单个动态过程,这是氢化物配体交换过程的第二个方面9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,
27,28,29.八坐标、伪十二面体、铼(V)多氢化物配合物采用分子几何形状(图2),可描述为配体17,26的一对正交梯形。梯形长边上的顶点通常标记为B位点,并且在铼多氢化物配合物中,通常是中性双电子供体配体(如叔膦或胺配体)占据的位点。梯形短边上的顶点通常标记为A位点,通常由阴离子,双电子供体,氢化物配体占据。由于室温溶液中发生的几个动态过程,铼(V)多氢化物配合物的室温NMR波谱通常看似简单。
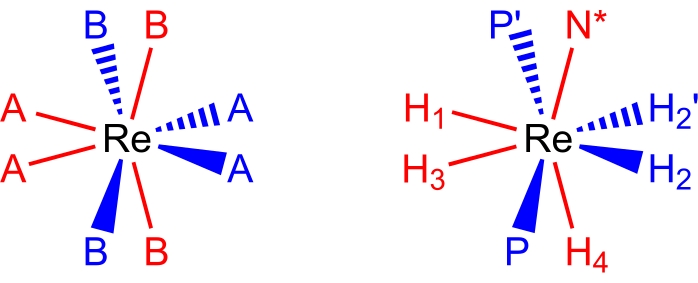
图 2:十二面体配位集(左)和复合物 ReH5(PPh3)2(仲丁胺)从同一角度(右)。红色位点表示形成垂直梯形的配位点,蓝色位点表示形成水平梯形的配位点。请点击此处查看此图的大图。
形式的ReH5(PPh3)2(胺)的配合物是关于动态过程9,10,12,13,16,30,31的研究最彻底的一类多氢化铼配合物。ReH5(PPh 3)2(胺)配合物的三个动态过程(图3)已被鉴定:1)唯一的B位氢化物配体与来自水分子的质子之间的质子交换(偶然或有意)9,13,2)一对A位氢化物配体与相邻B位氢化物配体9的旋转门交换, 11,13,30,31和3)空间位阻反转(或伪旋转),表现为A位点氢化物配体的成对交换和B位点原子成对向铼中心另一侧的运动(如图4所示)4,5,6,8,26,27.通过动态核磁共振波谱可以观察到B位点原子向铼另一侧的运动:1)在室温下使N=吡啶的不等价3和5质子的过程10,30,31,2)导致N =不对称取代的芳香胺配体的E和Z异构体在室温下进行快速交换的过程9, 10,13,30,31或3)引起非对映素磷原子对的空间位阻视角相对于位于胺配体上的手性中心快速交换的过程9,30,31。以前未报道的手性配合物ReH5(PPh3)2(仲丁胺)提供了一个机会,可以大致描述可用于鉴定和表征铼多氢化物配合物动态重排的方法。
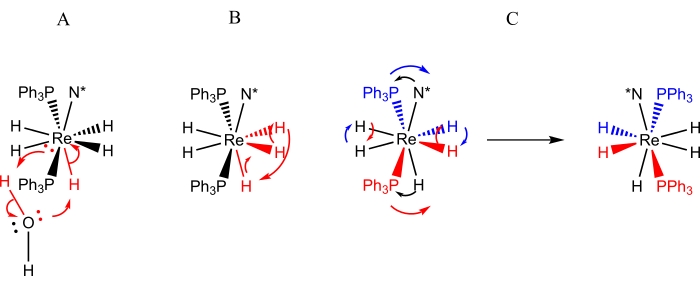
图 3:通过 NMR 波谱观察到的 ReH5(PPh3)2(仲丁胺)溶液动态过程的表示。表示A描述了单个不定水质子与独特的B位氢化物配体的交换。表示B描述了三个相邻氢化物配体的旋转门交换,其中两个位于A位点,而第三个位点是唯一的B位点氢化物配体。表示C描述了A位点氢化物配体的成对交换以及磷原子相对于手性胺配体(N*)的空间位阻反转。应该注意的是,A位点氢化物配体成对交换不需要将A位点氢化物配体移动到铼中心的另一侧。请点击此处查看此图的大图。
对于具有一系列复杂动态过程的化学系统,例如多氢化铼配合物,动态NMR波谱的线形拟合是表征过程最常用的NMR技术9,11,13,16,21,29。二维EXSY9,32或2D-ROESY11是替代的动态NMR技术,也可用于定量表征动态过程。二维EXSY光谱通常在慢交换温度域中测量;二维ROESY光谱通常在快速交换温度域中测量。这两种二维技术都可能需要在光谱仪中花费相当长的时间来获取数据,因为每种技术在给定温度下获取的数据集都比线形拟合分析所需的一维数据集大得多。众所周知的简单动态过程,例如二甲基甲酰胺的两个甲基的动态交换,可以很容易地通过三种NMR技术中的任何一种来表征。更复杂的系统,例如ReH5(PPh3)2(仲丁基胺),其中单个氢化物配体参与多个动态过程,或不一定很好理解的系统,例如可能在氢化物配体和外源水之间交换质子的新型过渡金属多氢化物复合物,比二维NMR方法更容易通过线形拟合NMR方法进行定量表征。 与二维核磁共振方法不同,线形拟合方法提供了易于解释的测试模型与实验数据之间匹配的可视化,以及将氢化物配体移出铼内部配位球的交换的视觉证据。基于慢速交换光谱中的峰高和峰形,即使是复杂的动态系统,如ReH5(PPh 3)2(仲丁胺),也可以产生易于测试的初始交换模型集。此外,当已经报告了多个分子转化的理论模型时,动态NMR波谱的线形拟合可以允许将每个模型与观察到的光谱进行视觉比较。
除了上述三种核磁共振技术之外,涉及D2O或HD的同位素取代核磁共振实验已被用于定性证明复杂铼多氢化物系统的原子间交换,但尚未用于定量表征9,33,34,35。理论计算为表征复杂动态系统的动态过程提供了一种附加方法30,31,36。理论计算相对于线形拟合的优势在于,理论计算可用于区分线形拟合分析无法区分的可能性。例如,理论计算已被用于描述涉及某些铼(V)配合物上的三个相邻氢化物配体的交换,作为所有三个氢化物配体的旋转门交换,而不是与每个成对交换的交替成对交换,包括一个独特的氢化物配体和两个化学等效的氢化物配体之一30,31.理论计算的结果通常与上述三种核磁共振技术之一的实验观察定量表征进行比较,以检查计算结果的有效性。
动态核磁共振波谱的线形拟合利用了核磁共振测量期间核磁共振活性核在不同化学环境之间移动时核磁共振波谱外观的变化。慢交换核磁共振波谱(每组交换原子核具有独立洛伦兹共振的波谱)发生在交换原子核的共振与原子核交换速率相比较大的温度下37.快速交换核磁共振波谱(具有单个洛伦兹共振以交换原子核的波谱)发生在原子核交换速率远大于慢交换共振之间的频率差的温度下37。对于慢交换温度域和快速交换温度域之间的温度,中间交换速率发生在37之间。如果已知拉莫尔频率、交换核的化学位移、交换核的耦合常数(如果有的话)以及每种核类型的相对种群的基本参数,则可以通过将模拟光谱与几个中间温度下的观测光谱进行比较来确定原子核之间推定交换的速率常数。在多个温度下进行仿真的良好拟合会产生温度和速率常数数据,这些数据可以与艾林方程一起使用,以估计假定交换的激活参数。已发现该方法的结果既准确又可重复。
Protocol
Representative Results
Discussion
ReH7(PPh 3)2的制备中有四个项目会影响所生产材料的数量和纯度。首先,在反应的前15分钟内使用冰浴对于从硼氢化钠和水之间发生的反应中去除热量很重要。较高的初始温度导致ReH7(PPh 3)2产物的产率降低,这是由于热分解产物Re2H8(PPh 3)4的形成。其次,反应混合物的颜色比反应的时间量更重要。当反应混合?…
Divulgations
The authors have nothing to disclose.
Acknowledgements
作者感谢蒙茅斯大学化学与物理系以及创造力和研究资助计划(Naik,Moehring)对这项工作的财政支持。
Materials
| Bruker Avance II 400 MHz NMR spectrometer | Bruker Biospin | The instrument includes a two channel probe (1H and X) with the X channel tunable from 162 MHz to 10 Mhz. The instrument is also VT capable with a dewar and heat exchanger for VT work. | |
| d8-toluene | MilliporeSigma | 434388 | |
| Powerstat variable transformer | Powerstat | ||
| sec-butyl amine | MilliporeSigma | B89000 | |
| Sodium borohydride | MilliporeSigma | 452882 | |
| Tetrahydrofuran | MilliporeSigma | 186562 | |
| Thermowell C3AM 100 mL | Thermowell | ||
| Topspin 3.0 or 4.1.4 with dNMR | Bruker Biospin | Data was acquired with Topspin version 3.0 and data handling was performed on a second computer that was running Topspin version 4.1.4.. | |
| Trichlorooxobis(triphenylphosphine) rhenium(V) | MilliporeSigma | 370193 | |
| Vacuubrand PC3000 vacuum pump with a CVC 3000 controller | Vacuubrand |
References
- Zimmer, K. D., Shoemaker, R., Ruminski, R. R. Synthesis and characterization of a fluxional Re(I) carbonyl complex fac-[Re(CO)3(dpop’)Cl] with the nominally tri-dentate ligand dipyrido(2,3-α:3′,2′-j)phenazine (dpop). Inorganica Chimica Acta. 359 (5), 1478-1484 (2006).
- McGlinchey, M. J. Symmetry breaking in NMR spectroscopy: the elucidation of hidden molecular rearrangement processes. Symmetry. 6 (3), 622-654 (2014).
- Casarini, D., Luazzi, L., Mazzanti, A. Recent advances in stereodynamics and conformational analysis by dynamic NMR and theoretical calculations. European Journal of Organic Chemistry. 2010 (11), 2035 (2010).
- Palmer, A. G., Williams, J., McDermott, A. Nuclear magnetic resonance studies of biopolymer dynamics. Journal of Physical Chemistry. 100 (31), 13293-13310 (1996).
- Kern, D., Kern, G., Scherer, G., Fischer, G., Drakenberg, T. Kinetic analysis of cyclophilin-catalyzed prolyl cis/trans isomerization by dynamic NMR spectroscopy. Biochimie. 34 (41), 13594-13602 (1995).
- Menger, F. M., Lynn, J. L. Fast proton transfer at a micelle surface. Journal of the American Chemical Society. 97 (4), 948-949 (1975).
- Pines, A., Rabinovitz, M. A nuclear magnetic resonance total line-shape treatment of internal rotation in dimethylformamide. Tetrahedron Letters. 9 (31), 3529-3532 (1968).
- Mancinelli, M., Bencivenni, G., Pecorari, D., Mazzanti, A. Stereochemistry and recent applications of axially chiral organic molecules. European Journal of Organic Chemistry. 2020 (27), 4070-4086 (2020).
- Streisel, D. J., et al. Fluxionality, substitution, and hydrogen exchange at eight-coordinate rhenium(V) polyhydride centers. Inorganica Chimica Acta. 496 (1), 119028 (2019).
- Jimenez, Y., Strepka, A. M., Borgohain, M. D., Hinojosa, P. A., Moehring, G. A. Ortho-metalation, rotational isomerization, and hydride-hydride coupling at rhenium(V) polyhydride complexes stabilized by aromatic amine ligands. Inorganica Chimica Acta. 362 (9), 3259-3266 (2009).
- Lee, J. C., Yao, W., Crabtree, R. H., Ruegger, H. Fluxionality in [ReH5(PPh3)2(pyridine)]. Inorganic Chemistry. 35 (3), 695-699 (1996).
- Patel, B. P., Kavallieratos, K., Crabtree, R. H. Effects of dihydrogen bonding on fluxionality in ReH5(PPh3)2L. Journal of Organometallic Chemistry. 528 (1), 205-207 (1997).
- Geetha, B., et al. Chiral amine ligands at rhenium(V) pentahydride complexes allow for characterization of an energetically accessible and reversible steric inversion of diastereotopic phosphorus atoms. Inorganica Chimica Acta. 531 (1), 120741 (2022).
- Paulo, A., Ascenso, J., Domingos, A., Galvao, A., Santos, I. Rhenium-(III) and -(V) hydride complexes with modified poly(pyrazolyl)borates. Journal of the Chemical Society, Dalton Transactions. 1999 (8), 1293-1300 (1999).
- Bianchini, C., et al. Synthesis and characterization of rhenium polyhydrides stabilized by the tripodal ligand MeC(CH2PPh2)3. Journal of Organometallic Chemistry. 451 (1), 97-106 (1993).
- Scorzelli, A. G., Macalush, B. E., Naik, D. V., Moehring, G. A. Comparative study of fluxional processes at two different classes of eight-coordinate rhenium(V) polyhydride complexes. Inorganica Chimica Acta. 516 (1), 120120 (2021).
- Luo, X. -. L., Crabtree, R. H. Synthesis and spectroscopic characterization of rhenium complexes ReH5(triphos)] and [ReH6(triphos)]+ [triphos = PPh(CH2CH2PPh2)2]. Journal of the Chemical Society. 1991 (5), 587-590 (1991).
- Kim, Y., Deng, H., Gallucci, J. C., Wojcicki, A. Rhenium polyhydride complexes containing PhP(CH2CH2CH2PCy2)2 (Cyttp): protonation, insertion, and ligand substitution reactions of ReH5(Cyttp) and structural characterization of ReH5(Cyttp) and [ReH4(η2-H2)(Cyttp)]SbF6. Inorganic Chemistry. 35 (24), 7166-7173 (1996).
- Bolano, S., et al. Synthesis, characterization, protonation studies and X-ray crystal structure of ReH5(PPh3)2(PTA) (PTA = 1,3,5-triaza-7-phosphaadamantane). Journal of Organometallic Chemistry. 691 (4), 629-637 (2006).
- Ginsberg, A. P., Abrahams, S. C., Jamieson, P. B. Nonrigid stereochemistry in eight-coordinate pentahydridorhenium complexes. Journal of the American Chemical Society. 95 (14), 4751-4752 (1973).
- Bolano, S., Bravo, J., Garcia-Fontan, S. Mono- and dinuclear rhenium polyhydride complexes bearing the chelating ligand 1,2-bis(dicyclohexylphosphinanyloxy)ethane. European Journal of Inorganic Chemistry. 2004 (24), 4812-4819 (2004).
- Leeaphon, M., Rohl, K., Thomas, R. J., Fanwick, P. E., Walton, R. A. Reactions of the polyhydride complex ReH7(PPh3)2 with quinoline, 2-hydroxyquinoline, and 2-mercaptoquinoline. The preparation and characterization of hydrido complexes of rhenium(V) and chloro complexes of rhenium(III). Inorganic Chemistry. 32 (24), 5562-5568 (1993).
- Mejia, E., Togni, A. Rhenium complexes containing the chiral tridentate ferrocenyl ligand pigiphos. Organometallics. 30 (17), 4765-4770 (2011).
- Moehring, G. A., Walton, R. A. Reactions of heptahydrobis(triphenylphosphine)rhenium with bidentate aromatic heterocycles. Inorganic Chemistry. 26 (17), 2910-2912 (1987).
- Kosanovich, A. J., Reibenspies, J. H., Ozerov, A. V. Complexes of high-valent rhenium supported by the PCP pincer. Organometallics. 35 (4), 513-519 (2016).
- Emge, T. J., Koetzle, T. F., Bruno, J. W., Caulton, K. G. Pentahydridorhenium: crystal and molecular structure of ReH5(PMePh2)3. Inorganic Chemistry. 23 (24), 4012-4017 (1984).
- Costello, M. T., Fanwick, P. E., Green, M. A., Walton, R. A. Reactions of Heptahydridobis(triphenylphosphine)rhenium with 1-(diphenylphosphino)-2-(diphenylarsino)ethane (arphos) and 1,2-bis(diphenylarsino)ethane (dpae). Structural characterization of ReH5(PPh3)2(arphos-As) and ReH5(PPh3)2(dpae-As). Inorganic Chemistry. 30 (4), 861-864 (1991).
- Alvarez, D., Lundquist, E. G., Ziller, J. W., Evans, W. J., Caulton, K. G. Synthesis, structure and applications of transition-metal polyhydride anions. Journal of the American Chemical Society. 111 (22), 8392-8398 (1989).
- Albinati, A., et al. Synthesis, characterization, and interconversion of the rhenium polyhydrides ReH3(η4-NP3)] and [ReH4(η4-NP3)]+ {NP3 = tris[2-(diphenylphosphanyl)ethyl]amine}. European Journal of Inorganic Chemistry. 2002 (6), 1530-1539 (2002).
- Bosque, R., et al. Site preference energetics, fluxionality, and intramolecular M−H···H−N hydrogen bonding in a dodecahedral transition metal polyhydride. Inorganic Chemistry. 36 (24), 5505-5511 (1997).
- Tao, Y., Sou, W., Luo, G. -. G., Kraka, E. Describing polytopal rearrangement processes of octacoordinate structures. I. renewed insights into fluxionality of the rhenium polyhydride complex ReH5(PPh3)2(Pyridine). Inorganic Chemistry. 60 (4), 2492-2502 (2021).
- Beringhelli, T., D’Alfonso, G., Minoja, A. P. Rhenium-platinum mixed metal clusters. Characterization in solution and dynamic behavior of the isomers of [Re3Pt(µ-H3)(CO)14]. An example of a labile metal fragment that undergoes intermolecular exchange. Organometallics. 13 (2), 663-668 (1994).
- Grieco, G., Blacque, O. Solution and solid-state structure of the first NHC-substituted rhenium heptahydrides. European Journal of Inorganic Chemistry. 2019 (34), 3810-3819 (2019).
- Wazio, J. A., Jimenez, V., Soparawalla, S., John, S., Moehring, G. A. Hydrogen exchange of rhenium(VII) heptahydridobis(triphenylphosphine) with water, aniline, methanol, and itself. Inorganica Chimica Acta. 362 (1), 159-165 (2009).
- Chatt, J., Coffey, R. S. Hydrido-complexes of rhenium-containing tertiary phosphines. Journal of the Chemical Society, A. 1969, 1963-1972 (1969).
- Tao, Y., Wang, X., Zou, W., Luo, G. -. G., Kraka, E. Unusual intramolecular motion of ReH92- in K2ReH9 crystal: circle dance and three-arm turnstile mechanisms revealed by computational study. Inorganic Chemistry. 61 (2), 1041-1050 (2022).
- Berger, X., Braun, S. . 200 and More NMR Experiments a Practical Course. , (2004).
- He, G., Chen, J., Sung, H. H. -. Y., Williams, I. D., Jia, G. Substituent effect on reactions of ReH5(PMe2Ph)3 with propargyl alcohols. Inorganica Chimica Acta. 518 (1), 120239 (2021).
- Donnelly, L. J., Parsons, S., Morrison, C. A., Thomas, S. P., Love, J. B. Synthesis and structures of anionic rhenium polyhydride complexes of boron-hydride ligands and their application in catalysis. Chemical Science. 11 (9), 9994-9999 (2020).
- Donnelly, L. J., et al. C-H borylation catalysis of heteroaromatics by a rhenium boryl polyhydride. ACS Catalysis. 11 (12), 7394-7400 (2021).
- Jin, H., et al. CO-enabled rhenium hydride catalyst for directed C(sp2)-H bond alkylation with olefins. Organic Chemistry Frontiers. 2 (4), 378-382 (2015).
- Takaya, H., Ito, M., Murahashi, S. -. I. Rhenium-catalyzed addition of carbonyl compounds to the carbon−nitrogen triple bonds of nitriles: α-C−H activation of carbonyl compounds. Journal of the American Chemical Society. 131 (31), 10824-10825 (2009).
- Carr, S. W., Fowles, E. H., Fontaine, X. L. R., Shaw, B. L. Multihydride complexes of rhenium, osmium or iridium containing monodentate ditertiary phosphine ligands: selective hydrogen-deuterium exchanges of the rhenium multihydrides. Journal of the Chemical Society, Dalton Transactions. 1990 (2), 573-579 (1990).
- Jin, H., et al. Rhenium-catalyzed acceptorless dehydrogenative coupling via dual activation of alcohols and carbonyl compounds. ACS Catalysis. 3 (10), 2195-2198 (2013).
- Loza, M. L., de Gala, S., Crabtree, R. H. Steric crowding in a rhenium polyhydride induced by a chelating disilyl ligand: synthesis, characterization, and reactivity of ReH5(disil)(PPh3)2 (disil = 1,2-Bis(dimethylsilyl)benzene and 1,2-Bis(dimethylsilyl)ethane). Inorganic Chemistry. 33 (22), 5073-5078 (1994).
- Lin, Y., Zhu, X., Xiang, M. Transition metal polyhydrides-catalyzed addition of activated nitriles to aldehydes and ketones via Knoevenagel condensation. Journal of Organometallic Chemistry. 448 (1-2), 215-218 (1993).
- Abdukader, A., Jin, H., Cheng, Y., Zhu, C. Rhenium-catalyzed amination of alcohols by hydrogen transfer process. Tetrahedron Letters. 55 (30), 4172-4174 (2014).
- Lin, Y., Zhou, Y. Selective transfer hydrogenation catalyzed by transition metal polyhydrides. Fenzi Cuihua. 5 (2), 119-124 (1991).
- Green, M. A., Huffman, J. C., Caulton, K. G., Rybak, W. K., Ziolkowski, J. J. Ligand scavenging and catalytic utilization of the phototransient ReH5(PMe2Ph)2. Journal of Organometallic Chemistry. 218 (2), 39-43 (1981).
- Komiya, S., Chigira, T., Suzuki, T., Hirano, M. Polymerization of alkyl methacrylate catalyzed by hydridorhenium complexes. Chemistry Letters. 4 (4), 347-348 (1999).
- Michos, D., Luo, X. L., Faller, J. W., Crabtree, R. H. Tungsten(VI) hexahydride complexes supported by chelating triphosphine ligands: protonation to give η2-dihydrogen complexes and catalytic dehydrogenation of cyclooctane to cyclooctene. Inorganic Chemistry. 32 (8), 1370-1375 (1993).

