Investigating Stress-relaxation and Failure Responses in the Trachea
Summary
The present protocol determines the tensile stress-relaxation and failure properties of porcine tracheae. Results from such methods can help improve the understanding of the viscoelastic and failure thresholds of the trachea and help advance the capabilities of computational models of the pulmonary system.
Abstract
The biomechanical properties of the trachea directly affect the airflow and contribute to the biological function of the respiratory system. Understanding these properties is critical to understanding the injury mechanism in this tissue. This protocol describes an experimental approach to study the stress-relaxation behavior of porcine trachea that were pre-stretched to 0% or 10% strain for 300 s, followed by mechanical tensile loading until failure. This study provides details of the experimental design, data acquisition, analyses, and preliminary results from the porcine tracheae biomechanical testing. Using the detailed steps provided in this protocol and the data analysis MATLAB code, future studies can investigate the time-dependent viscoelastic behavior of trachea tissue, which is critical to understanding its biomechanical responses during physiological, pathological, and traumatic conditions. Furthermore, in-depth studies of the biomechanical behavior of the trachea will critically aid in improving the design of related medical devices such as endotracheal implants that are widely used during surgeries.
Introduction
Despite its critical role in pulmonary disease, the largest airway structure, the trachea, has limited studies detailing its viscoelastic properties1. An in-depth understanding of the time-dependent, viscoelastic behavior of the trachea is critical to pulmonary mechanics research since understanding the airway-specific material properties can help advance the science of injury prevention, diagnosis, and clinical intervention for pulmonary diseases, which are the third leading cause of death in the United States2,3,4.
Available tissue characterization studies have reported the stiffness properties of the trachea5,6,7,8. The time-dependent mechanical responses have been minimally investigated despite their importance in tissue remodeling, which is also altered by pathology9,10. Moreover, the lack of time-dependent response data also limits the predictive capabilities of the pulmonary mechanics computational models that currently resort to using the generic constitutive laws. There is a need to address this gap by performing stress-relaxation studies that can provide the required material characteristics to inform biophysical studies of the trachea. The current study offers details of testing methods, data acquisition, and data analyses to investigate the stress-relaxation behavior of the porcine trachea.
Protocol
All methods described were approved by the Institutional Animal Care and Use Committee (IACUC) at Drexel University. All cadaveric animals were acquired from a United States Department of Agriculture (USDA)-approved farm located in Pennsylvania, USA. A cadaver of a male Yorkshire pig (3 weeks old) was used for the present study.
1. Tissue harvest
- Acquire a cadaver of a pig from an approved farm and perform the experiments within 2 h from euthanasia. Keep the cadaver on ice until tissue harvest is completed to ensure that the fresh tissue biomechanical properties are conserved.
NOTE: In the published literature, fresh tissue testing in animals is commonly performed within 2 h post-euthanasia. For details, see references11,12,13,14,15,16,17,18,19. - Place the cadaver in the supine position, make a vertical midline incision along the neck, and expose the thyroid cartilage, cricoid cartilage, and trachea from the hyoid bone to the suprasternal notch.
- Harvest the larynx and the full-length trachea using a #10 blade (Figure 1A).
- Separate the trachea sample from the larynx and then cut the tracheal tube longitudinally along the entire length on one side using the #10 blade (Figure 1B).
- Measure the thickness of the trachea using the acquired cross-sectional image (obtained using ImageJ20, see Table of Materials) (Figure 1C). Use the measured tissue thickness to calculate the cross-sectional area during data analysis.
- Cut the trachea into two circumferential strips approximately 5 mm wide (proximally) and two longitudinal strips approximately 5 mm wide (distally), with the minimum length of these strips being 25 mm (Figure 1D).
- Obtain images of the four samples (i.e., cut trachea strips) alongside a ruler. Use these images to provide digitized measurements of the sample width using Image J (Figure 1E). Then, use this measured width to calculate the sample's cross-sectional area during data analysis.
- Ensure that all tissue samples are kept hydrated using sterile phosphate-buffered saline (PBS) solution throughout the study. Keep the samples hydrated in PBS-soaked gauze until ready to test. Immerse the tissue in PBS just before testing for proper hydration.
2. Biomechanical testing
- Attach each sample to a custom-designed clamp (see the previous reports11,12,13,14,15,16,17,18,19) so that the sample is held longitudinally between the clamps (Figure 1F).
- Carefully fix the clamps (without inducing any stretch) to a material testing machine (see Table of Materials), which has a 50 N load cell attached to the top actuator (Figure 1G).
- Measure the grip-to-grip (i.e., clamp) distance using a ruler. Use this distance as the initial tissue length for strain calculations.
- Perform preconditioning by tensile loading each sample five times at a 1%/s strain rate to 1% strain.
- Hold each sample at peak elongation of 0% or 10% for 300 s to investigate the viscoelastic stress-relaxation response of the tissue.
- Post the stress-relaxation test, stretch the tissue immediately at 1%/s until mechanical failure occurs.
- Document the failure site and confirm that no slip has occurred by ensuring the presence of samples within the clamps post testing (Figure 1H).
3. Data acquisition
- Do not acquire any data during preconditioning.
- Record the stress-relaxation and failure testing videos using any digital camera at a minimum of 30 frames/s.
- Acquire time (s), load (N), and displacement (mm) data using a data acquisition software (see Table of Materials) at a sampling rate of 250 samples/s during both stress-relaxation and failure testing.
- Save the acquired data as a .csv file and use it for data analysis as detailed in step 4.
- Acquire still images of the clamped tissue before stress-relaxation, after stress-relaxation, and after failure (Figure 2).
4. Data analysis
- Data input
- Download and install MATLAB data analysis software (see Table of Materials), including the "Optimization" and "Image Processing" toolboxes.
- Download the zipped folder (Supplementary Coding File 1), which includes the MATLAB codes and a sample dataset that will be used to explain the data analysis steps.
- Navigate to the downloaded zipped folder and extract its contents.
- Open MATLAB and set the unzipped folder as the working directory. Ensure that the working directory has the following folders and files labeled as mentioned in the note below. Ensure that no additional folders or files are present in this working directory since they may interfere with the code and result in an error.
NOTE: (1) Failure (Post-Relaxation), (2) Failure Only, (3) Relaxation, (4) calc_relax_failure.m, (5) main_relax_failure.m, (6) testingDates.xlsx. - Navigate to the Failure Only folder.
NOTE: Data contained within this folder are derived from the control group in this study, i.e., biomechanical data from tracheal samples that were subjected to mechanical failure following a 0% elongation. - Store data from samples tested on a particular date in one Microsoft Excel file using the following file naming convention: mmddyy. For example, data from all control group tracheal samples tested on April 30th, 2022, must be stored in Failure Only | 043022.xlsx.
NOTE: Please note that, in the current study, all biomechanical testing was conducted on a single day; however, if data were derived from multiple testing dates, then create a new Microsoft Excel file, named in the convention described, for each of those testing dates. - Open Failure Only | 043022.xlsx and recognize that there are multiple worksheet tabs, each containing the raw data from each sample subjected to mechanical failure on this particular date, i.e., April 30th, 2022.
- Ensure that the samples are labeled using the following convention: [sample type]_[sample number]_[insert pre-stretch strain level]%.
NOTE: For example, in the current study, the control group tracheal samples were subjected to mechanical failure testing under axial or circumferential loading without any prior stress relaxation. Therefore, these samples are named in the following format: TA_1_0% and TC_1_0%, respectively. The 0% denotes no pre-stretch. Tracheal samples from our experimental group were first held at fixed elongation under axial or circumferential loading of 10% to evaluate the viscoelastic stress relaxation response and then subjected to mechanical failure. Therefore, these samples are named in the following format: TA_1_10% and TC_1_10% (please refer to step 4.1.16 and step 4.1.23, representing the axial and circumferential loading conditions, respectively). - Select the worksheet tab TA_1_0%. Ensure that the raw data header columns are labeled exactly as typed in bold in the note below.
NOTE: (1) Time (sec), (2) Load (N), (3) Position (mm), (4) Diameters (mm) (step 1.7), (5) Average Cross-sectional Area (Thickness x Width, mm2) (obtained in step 1.5 and step 1.7), (6) Initial Length (mm) (step 2.3). - Close the current Microsoft Excel file, Failure Only | 043022.xlsx.
- Return to the working directory of the data analysis software.
- Navigate to the Relaxation folder.
NOTE: Data contained within this folder are derived from the experimental group in this study, i.e., biomechanical data from tracheal samples that were subjected to stress relaxation testing at a fixed elongation of 10% for 300 s. - Store data from the experimental group samples tested on a particular date in one Microsoft Excel file using the following labeling convention: mmddyy.
NOTE: For example, data from all experimental group tracheal samples tested on April 30th, 2022, must be stored in Relaxation | 043022.xlsx. Refer to the note in step 4.1.6 for additional details. - Open Relaxation | 043022.xlsx and recognize that there are multiple worksheet tabs, each containing the raw load relaxation data from each sample in the experimental group that was tested on this particular date, i.e., April 30th, 2022.
- Pause and note that each of the samples, as indicated by the worksheet tabs included in this Microsoft Excel file, was subsequently subjected to mechanical failure under tensile mechanical loading.
NOTE: The corresponding failure data for each of the samples present must be stored in the Failure (Post-Relaxation) folder, further described in step 4.1.20. - Ensure that the samples are labeled using the convention described in step 4.1.8.
- Toggle through each worksheet tab found in the current Microsoft Excel file and refer to step 4.1.9 to ensure that raw load relaxation data for each sample, indicated by any given worksheet tab, are formatted correctly.
- Save and close the current Microsoft Excel file, Relaxation | 043022.xlsx.
- Return to the working directory of the data analysis software.
- Navigate to the folder Failure (Post-Relaxation).
- Ensure that there is a Microsoft Excel file(s) with the same date(s) (refer to step 4.1.6 for details about naming the Microsoft Excel files corresponding to each testing date) as that present in the folder Relaxation.
NOTE: Data contained within the current folder, Failure (Post-Relaxation), are the corresponding raw mechanical failure data from tracheal samples subjected to stress relaxation testing at a fixed elongation of 10% for 300 s. - Open Failure (Post-Relaxation) | 043022.xlsx and recognize multiple worksheet tabs, each of which contains raw mechanical failure data from the same samples present in Relaxation | 043022.xlsx.
- Ensure that the samples are labeled using the convention mentioned in step 4.1.8 and that the labels match those in Relaxation | 043022.xlsx.
NOTE: For example, the data in the current Microsoft Excel file for TA_1_10% represent raw mechanical failure data for tracheal specimen #1 under axial loading that previously underwent stress relaxation testing at 10% fixed elongation for 300 s. - Toggle through each worksheet tab and refer to step 4.1.9 to ensure that the header column for the raw mechanical failure data for each sample is formatted correctly.
- Close the current Microsoft Excel file, Failure (Post Relaxation) | 043022.xlsx.
- Return to the working directory of the data analysis software.
- Repeat steps 4.1.5-4.1.26 for additional testing dates, as applicable.
- Open the Microsoft Excel file, testingDates.xlsx, which will direct the code to analyze user-specified testing dates.
- List testing dates in the first column in the following format: mm/dd/yy.
- In the second column, indicate using a Y (for yes) or N (for no) whether any samples on this particular testing date were from the experimental (stress relaxation followed by mechanical failure) group.
- In the third column, indicate using a Y (for yes) or N (for no) whether any samples on this particular testing date were from the control (direct mechanical failure) group.
- Repeat steps 4.1.29-4.1.31 for additional testing dates.
- Save and close the current Microsoft Excel file, testingDates.xlsx.
- Return to the working directory of the data analysis software.
- Open the main script file, main_relax_failure.m.
- Select the large, green arrow on the software interface to run the code. Alternatively, type run main_calc_relax in the command window.
- Upon being prompted, input comma-separated fixed elongation levels (in %) for the various experimental groups and press OK.
NOTE: In the current study, only one stress relaxation elongation was used, i.e., enter 10. Do not include 0% for the control group. However, if data were derived from multiple elongations, for example 10% and 20%, then enter 10,20. - Upon being prompted, input comma-separated stress-relaxation testing durations (in seconds) for the various experimental groups and press OK.
NOTE: In the current study, tracheal samples were held at fixed elongation for 300 s and hence, input 300. However, if data were derived from multiple stress relaxation durations, for example 90 s and 300 s, then enter 90,300.
- Viscoelastic stress relaxation response
- Using the code (main_relax_failure.m), convert load-time data (Code Line 144) to nominal stress-time data using the following equation19:
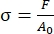 , where σ represents stress (mega Pascals [MPa]), F represents circumferential or axial load (Newtons [N]), and A0 represents the initial cross-sectional area (millimeters squared [mm2]).
, where σ represents stress (mega Pascals [MPa]), F represents circumferential or axial load (Newtons [N]), and A0 represents the initial cross-sectional area (millimeters squared [mm2]). - Using the code (main_relax_failure.m), determine the peak load and stress magnitudes (Code Lines 138 and 146) in response to the application of the 10% fixed elongation on the sample at the beginning of the 300 s relaxation test.
NOTE: These values are hereafter termed initial peak load and initial peak stress, respectively. - Using the code (main_relax_failure.m), calculate the percentage reduction in stress (or load) at 300 s (Code Lines 141 and 149) using the following equation:
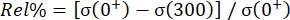 , where Rel% represents the relaxation percentage, σ(0+) represents the initial peak stress (or initial peak load), and σ(300) represents the recorded stress (or load) level after relaxation over 300 s.
, where Rel% represents the relaxation percentage, σ(0+) represents the initial peak stress (or initial peak load), and σ(300) represents the recorded stress (or load) level after relaxation over 300 s. - Refer to the code (main_relax_failure.m) for modeling the viscoelastic stress relaxation response (Code Lines 152-161) using a two-term Prony series exponential decay model. This model is commonly used to describe the viscoelastic behavior of various biological tissues, including various cartilaginous airway levels (trachea, large bronchi, and small bronchi)21,22.
NOTE: Calculated stress values [σ(t)] are normalized to produce the following reduced relaxation function: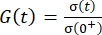 and G(0) = 1. To compare viscoelastic stress-relaxation responses, G(t) is curve-fitted using nonlinear least-squares regression as follows:
and G(0) = 1. To compare viscoelastic stress-relaxation responses, G(t) is curve-fitted using nonlinear least-squares regression as follows: 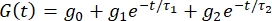 , where t is the time during stress relaxation hold, g is the relaxation coefficient, τ1 and τ2 and indicate the relaxation times (in seconds) that describe the short- (initial) and long-term (equilibrium) behavior of the tissue, respectively.
, where t is the time during stress relaxation hold, g is the relaxation coefficient, τ1 and τ2 and indicate the relaxation times (in seconds) that describe the short- (initial) and long-term (equilibrium) behavior of the tissue, respectively.
- Using the code (main_relax_failure.m), convert load-time data (Code Line 144) to nominal stress-time data using the following equation19:
- Mechanical failure response
- Use code (main_relax_failure.m) to convert the load-displacement data (Code Lines 143-144) recorded by the tensile testing machine to nominal stress-strain data using the equations mentioned in the note below.
NOTE: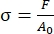 , where σ represents nominal stress (MPa), F represents circumferential or axial load (N), and A0 represents the initial cross-sectional area (mm2);
, where σ represents nominal stress (MPa), F represents circumferential or axial load (N), and A0 represents the initial cross-sectional area (mm2); 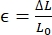 , where
, where  represents resultant strain, ΔL represents displacement, and L0 represents the initial length of the sample. For samples subjected to failure tensile testing following stress relaxation hold, L0 represents the pre-stretched length of the tissue. For example, sample 3 (initial length of 8 mm) was pre-stretched to 10%, and therefore, L0 was considered 8.8 mm for computing the resultant strain values19.
represents resultant strain, ΔL represents displacement, and L0 represents the initial length of the sample. For samples subjected to failure tensile testing following stress relaxation hold, L0 represents the pre-stretched length of the tissue. For example, sample 3 (initial length of 8 mm) was pre-stretched to 10%, and therefore, L0 was considered 8.8 mm for computing the resultant strain values19. - Use the function (calc_relax_failure.m) to identify the maximum load (i.e., failure load) and corresponding failure displacement, as well as the maximum stress (i.e., failure stress) and corresponding failure strain (Code Lines 33 and 61-63).
- Use the function (calc_relax_failure.m) to discard the load-displacement data following the failure load (Line 34).
- Use the function (calc_relax_failure.m) to plot the load-displacement curve and, when prompted, manually select two points in the linear region of the curve to approximate the tissue stiffness (N/mm) (Code Lines 37-58).
NOTE: Since the load-displacement curve is normalized by the cross-sectional area and initial length of the sample to yield the stress-strain curve, the code utilizes the user-selected x- and y- coordinates from the load-displacement curve to compute the modulus of elasticity (MPa) using the following equation19: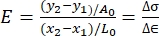 , where E represents the modulus of elasticity, x and y represent the selected coordinates on the load-displacement curve, A0 represents the cross-sectional area, L0 represents the length of the sample at the beginning of mechanical failure, and Δσ and Δ
, where E represents the modulus of elasticity, x and y represent the selected coordinates on the load-displacement curve, A0 represents the cross-sectional area, L0 represents the length of the sample at the beginning of mechanical failure, and Δσ and Δ represent the change in stress and strain over the linear region of the failure response, respectively.
represent the change in stress and strain over the linear region of the failure response, respectively. - Repeat step 4.3.4 for every sample.
- Use code (main_relax_failure.m) to convert the load-displacement data (Code Lines 143-144) recorded by the tensile testing machine to nominal stress-strain data using the equations mentioned in the note below.
- Data output
- Once the code is successfully run, ensure that the computed results are available in the working directory of the data analysis software as a Microsoft Excel file in the following naming convention: relax_failure_results_mmddyy.xlsx, where mmddyy will be replaced by the date on which the code was run.
Representative Results
Figure 1 shows the failed tissue near the clamping site and the presence of tissue within the clamp, confirming no slip during tensile testing. Figure 2 indicates various failure sites, including the top or bottom clamping sites or along the length of the tissue, that were observed during tensile testing among the tested samples. Data analysis results are summarized in Figures 3–4 and Tables 1–2. Stress relaxation responses for tracheal samples following axial or circumferential pre-stretch to 10% strain are shown in Figure 3. The initial peak load and stress, the percentage reduction in stress over the 300 s hold, and time constants, t1 and t2, in a 2-term Prony series relaxation function were calculated from these relaxation curves. These viscoelastic parameters are included in Table 1. The stress-strain responses of the tracheal sample subjected to failure testing under axial or circumferential loads following no pre-stretch or 10% pre-stretch are shown in Figure 4. From these curves, failure stress and the corresponding failure strain, as well as the modulus of elasticity, were determined and are listed in Table 2.
The preliminary tests successfully characterized the stress-relaxation responses of the tracheal tissue. In these initial experiments, the 10% pre-stretch stress-relaxation response reported the initial peak stress to be higher in axial loading directions, while the percentage reduction in stress was higher in the circumferential loading direction when compared to the axial loading direction (Table 1). The relaxation times (τ1 and τ2 that describe the short- [initial] and long-term [equilibrium] behavior of the tissue) were also higher in the axial loading direction when compared to the circumferential loading direction for the same 10% pre-stretch group. When comparing the failure data, the failure stress and E values were higher in circumferential loading directions in both the 0% and 10% pre-stretch groups, whereas the failure strain reported in the axial loading directions was higher (Table 2). These preliminary findings warrant additional experiments to further characterize the stress-relaxation and failure responses in tracheal tissue to better understand its stress-relaxation responses in tensile loading conditions, both axially or circumferentially. The steps outlined in this protocol can help accomplish this goal.
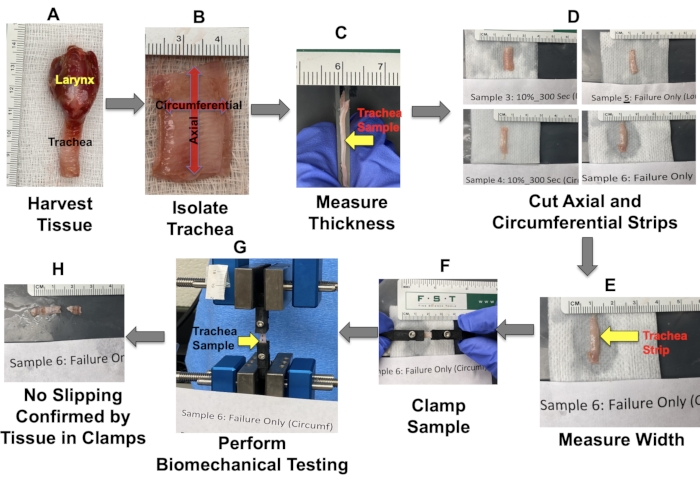
Figure 1: Tissue harvesting and mechanical testing details. Please click here to view a larger version of this figure.
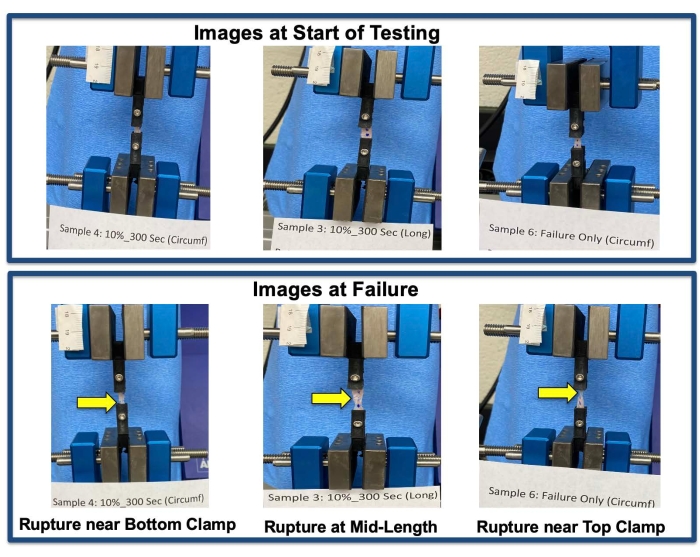
Figure 2: Failure sites. Sample failure sites as indicated by yellow arrows. Please click here to view a larger version of this figure.
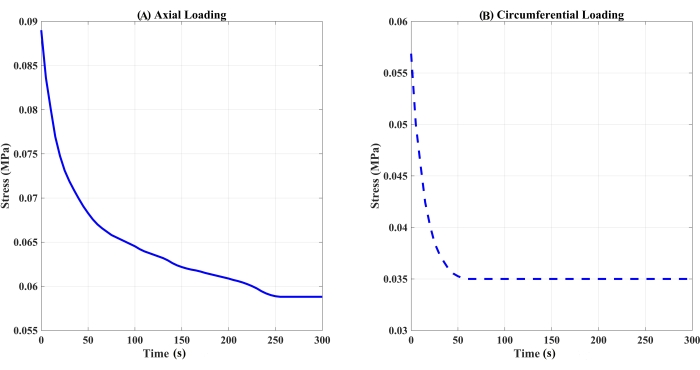
Figure 3: Stress relaxation response over a 300 s hold of trachea samples pre-stretched to 10% strain. (A) Axial or (B) circumferential loading (n = 1 per loading condition). Please click here to view a larger version of this figure.
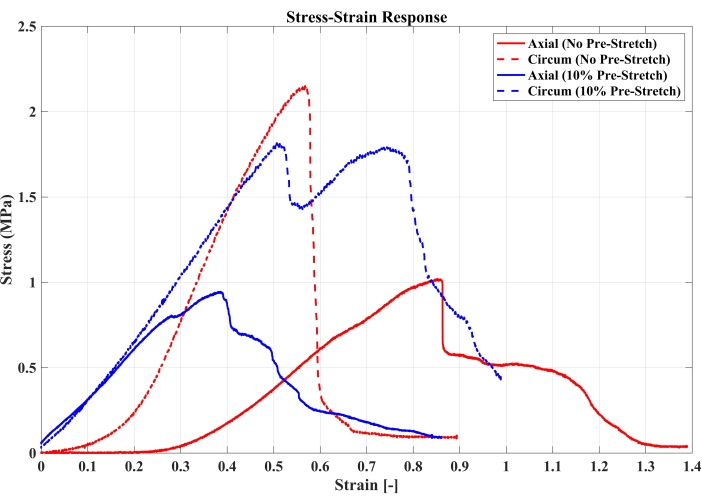
Figure 4: Stress-strain responses for failure testing of trachea samples under axial or circumferential loading following no pre-stretch or 10% pre-stretch (n = 1 per loading condition). Please click here to view a larger version of this figure.
| Sample | Pre-stretch strain | Loading Orientation | Initial Peak Load (N) | Initial Peak Stress (MPa) | % Reduction in Stress | τ1 (s) | τ2 (s) | Adjusted R2 (%) | |
| 3 | 10% | Axial | 0.56 | 0.089 | 33.93 | 11.59 | 152.44 | 98.79 | |
| 4 | Circumferential | 0.26 | 0.057 | 42.31 | 1.58 | 14.86 | 99.08 | ||
Table 1: Measured and calculated stress relaxation parameter values for trachea samples subjected to a pre-stretch of 10% strain to undergo stress relaxation for 300 s.
| Sample | Pre-stretch strain | Loading Orientation | Failure Stress (MPa) | Failure Strain | Modulus of Elasticity (MPa) |
| 3 | 10% | Axial | 0.89 | 0.38 | 2.9 |
| 4 | Circumferential | 1.78 | 0.51 | 3.74 | |
| 5 | 0% (Failure Only) | Axial | 1.02 | 0.86 | 2.3 |
| 6 | Circumferential | 2.15 | 0.57 | 6.3 |
Table 2: Failure responses of trachea samples under various experimental groups.
Supplementary Coding File 1: The custom codes to study the stress-relaxation behavior of the trachea. Please click here to download this File.
Discussion
Very few studies have reported the stress-relaxation properties of the trachea21,23. Studies are needed to further strengthen our understanding of the time-dependent responses of the tracheal tissue. This study offers detailed steps to perform such investigations; however, the following critical steps within the protocol must be ensured for reliable testing: (1) proper tissue hydration, (2) similar tissue-type (number of cartilaginous rings and muscle) distribution in circumferential and longitudinal samples, (3) clamping of the sample without pre-stretch, (4) using sample thickness and width to estimate the cross-sectional area that is used to calculate the tissue stress during biomechanical tensile testing, (5) proper clamping of the tissue sample, 6) using the gauge length of the clamped sample to input the strain rate of 1%/s for tensile testing, and (7) confirming no slippage with the presence of tissue in the clamp after testing. Additionally, troubleshooting may require restarting the data acquisition software to re-establish communication with the testing device controller.
The current study also provides detailed descriptions of the test methods, data analyses, and the custom MATLAB codes (Supplementary Coding File 1) created to study the stress-relaxation behavior of the trachea. No prior studies provide such comprehensive information. Furthermore, on the educational front, the methods described in the current study can be easily integrated as a teaching module for stress-relaxation labs in engineering courses in both traditional as well as virtual reality formats24,25,26,27.
Currently available stress-relaxation studies on the trachea and other soft tissue fit the relaxation function of a two-term Prony series28,29,30. The current study also uses this function; however, future studies could extend their investigation by utilizing quasi-linear viscoelastic modeling techniques to characterize viscoelastic behavior. Such studies will not only help create a robust predictive computational model of airway biomechanics but also help design implants such as airway stents that require tissue material properties for performance testing.
Finally, the methods described in this study can not only be used to assess the effects of age and species on the stress-relaxation behavior of the trachea but can also be applied to other soft and hard tissue such as ligaments, intervertebral discs, and bones. Such viscoelastic data can be integrated to improve existing high-fidelity finite element computational models31,32,33.
Divulgations
The authors have nothing to disclose.
Acknowledgements
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number R15HD093024 and the National Science Foundation CAREER Award Number 1752513.
Materials
| Disposable safety scalpels | Fine Science Tools Inc | 10000-10 | |
| eXpert 7600 | ADMET Inc. | N/A | Norwood, MA |
| Forceps | Fine Science Tools Inc | 11006-12 and 11027-12 or 11506-12 | |
| Gauge Safe | ADMET Inc. | N/A | Free Download |
| Image J | NIH | N/A | Open Source |
| Proramming Software – MATLAB | Mathworks | N/A | version 2018A |
| Scissors | Fine Science Tools Inc | 14094-11 or 14060-09 | |
| Sterile phosphate buffer solution | Millipore, Thomas Scientific | MFCD00131855 |
References
- Brand-Saberi, B. E. M., Schäfer, T. Trachea: Anatomy and physiology. Thoracic Surgery Clinics. 24, 1-5 (2014).
- Barnett, S. B., Nurmagambetov, T. A. Costs of asthma in the United States: 2002-2007. The Journal of Allergy and Clinical Immunology. 127 (1), 145-152 (2011).
- Chronic Obstructive Pulmonary Disease (COPD). Centers for Disease Control and Prevention Available from: https://www.cdc.gov/copd/index.html (2022)
- Wilson, L., Devine, E. B., So, K. Direct medical costs of chronic obstructive pulmonary disease: chronic bronchitis and emphysema). Respiratory Medicine. 94 (3), 204-213 (2000).
- Codd, S. L., Lambert, R. K., Alley, M. R., Pack, R. J. Tensile stiffness of ovine tracheal wall. Journal of Applied Physiology. 76 (6), 2627-2635 (1994).
- Noble, P. B., Sharma, A., McFawn, P. K., Mitchell, H. W. Elastic properties of the bronchial mucosa: epithelial unfolding and stretch in response to airway inflation. Journal of Applied Physiology. 99 (6), 2061-2066 (2005).
- Teng, Z., et al. Anisotropic material behaviours of soft tissues in human trachea: An experimental study. Journal of Biomechanics. 45 (9), 1717-1723 (2012).
- Wang, L., et al. Mechanical properties of the tracheal mucosal membrane in the rabbit. I. steady-state stiffness as a function of age. Journal of Applied Physiology. 88 (3), 1014-1021 (2000).
- Ambrosi, D., et al. Perspectives on biological growth and remodeling. Journal of the Mechanics and Physics of Solids. 59 (4), 863-883 (2011).
- Bai, T. R., Knight, D. A. Structural changes in the airways in asthma: Observations and consequences. Clinical Science. 108 (6), 463-477 (2005).
- Singh, A. Extent of impaired axoplasmic transport and neurofilament compaction in traumatically injured axon at various strains and strain rates. Brain Injury. 31 (10), 1387-1395 (2017).
- Singh, A., Kallakuri, S., Chen, C., Cavanaugh, J. M. Structural and functional changes in nerve roots due to tension at various strains and strain rates: An in-vivo study. Journal of Neurotrauma. 26 (4), 627-640 (2009).
- Singh, A., Lu, Y., Chen, C., Cavanaugh, J. M. Mechanical properties of spinal nerve roots subjected to tension at different strain rates. Journal of Biomechanics. 39 (9), 1669-1676 (2006).
- Singh, A., Lu, Y., Chen, C., Kallakuri, S., Cavanaugh, J. M. A new model of traumatic axonal injury to determine the effects of strain and displacement rates. Stapp Car Crash Journal. 50, 601-623 (2006).
- Singh, A., Magee, R., Balasubramanian, S. Methods for in vivo biomechanical testing on brachial plexus in neonatal piglets. Journal of Visualized Experiments. (154), e59860 (2019).
- Singh, A., Magee, R., Balasubramanian, S. Mechanical properties of cervical spinal cord in neonatal piglet: in vitro. Neurology and Neurobiology. 3 (2), (2020).
- Singh, A., Magee, R., Balasubramanian, S. An in vitro study to investigate biomechanical responses of peripheral nerves in hypoxic neonatal piglets. Journal of Biomechanical Engineering. 143 (11), 114501 (2021).
- Singh, A., Shaji, S., Delivoria-Papadopoulos, M., Balasubramanian, S. Biomechanical responses of neonatal brachial plexus to mechanical stretch. Journal of Brachial Plexus and Peripheral Nerve Injury. 13, 8-14 (2018).
- Singh, A. . The effects of tensile loading on mechanical, neurophysiological and morphological changes in spinal nerve roots. , (2006).
- Schneider, C. A., Rasband, W. S., Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 9 (7), 671-675 (2012).
- Eskandari, M., Arvayo, A. L., Levenston, M. E. Mechanical properties of the airway tree: Heterogeneous and anisotropic pseudoelastic and viscoelastic tissue responses. Journal of Applied Physiology. 125 (3), 878-888 (2018).
- Toby, E. B., Rotramel, J., Jayaraman, G., Struthers, A. Changes in the stress relaxation properties of peripheral nerves after transection. Journal of Hand Surgery. 24 (4), 694-699 (1999).
- Safshekan, F., Tafazzoli-Shadpour, M., Abdouss, M., Shadmehr, M. B. Viscoelastic properties of human tracheal tissues. Journal of Biomechanical Engineering. 139 (1), (2017).
- Singh, A. A new approach to teaching biomechanics through active, adaptive, and experiential learning. Journal of Biomechanical Engineering. 139 (7), 0710011-0710017 (2017).
- Singh, A., Ferry, D., Balasubramanian, S. Efficacy of clinical simulation based training in biomedical engineering education. Journal of Biomechanical Engineering. 141 (12), 121011-121017 (2019).
- Singh, A., Ferry, D., Mills, S. Improving biomedical engineering education through continuity in adaptive, experiential, and interdisciplinary learning environments. Journal of Biomechanical Engineering. 140 (8), 0810091-0810098 (2018).
- Singh, A., Ferry, D., Ramakrishnan, A., Balasubramanian, S. Using virtual reality in biomedical engineering education. Journal of Biomechanical Engineering. 142 (11), 111013 (2020).
- Majmudar, T., Balasubramanian, S., Magee, R., Gonik, B., Singh, A. In-vitro stress relaxation response of neonatal peripheral nerves. Journal of Biomechanical Engineering. 128, 110702 (2021).
- Orozco, V., Magee, R., Balasubramanian, S., Singh, A. A systematic review of the tensile biomechanical properties of the neonatal brachial plexus. Journal of Biomechanical Engineering. 143 (11), 110802 (2021).
- Singh, A. M. T., Magee, R., Gonik, B., Balasubramanian, S. Effects of pre-stretch on neonatal peripheral nerve: An in-vitro study. Journal of Brachial Plexus and Peripheral Nerve Injury. 17 (1), 1-9 (2022).
- Balasubramanian, S., D’Andrea, C., Viraraghavan, G., Cahill, P. J. Development of a finite element model of the pediatric thoracic and lumbar spine, ribcage, and pelvis with orthotropic region-specific vertebral growth. Journal of Biomechanical Engineering. 144 (10), 101007 (2022).
- Hadagali, P., Peters, J. R., Balasubramanian, S. Morphing the feature-based multi-blocks of normative/healthy vertebral geometries to scoliosis vertebral geometries: Development of personalized finite element models. Computer Methods in Biomechanics and Biomedical Engineering. 21 (4), 297-324 (2018).
- Singh, A. Available computational and physical models to understand the mechanisms of neonatal brachial plexus injury during shoulder dystocia. Open Access Journal of Neurology and Neurosurgery. 9 (4), 555768 (2019).

