Fluorescent In Situ Hybridization and 5-Ethynyl-2′-Deoxyuridine Labeling for Stem-Like Cells in the Hydrozoan Jellyfish Cladonema pacificum
Summary
Here, we describe a protocol for visualizing stem-like proliferating cells in the jellyfish Cladonema. Whole-mount fluorescent in situ hybridization with a stem cell marker allows for the detection of stem-like cells, and 5-ethynyl-2'-deoxyuridine labeling enables the identification of proliferating cells. Together, actively proliferating stem-like cells can be detected.
Abstract
Cnidarians, including sea anemones, corals, and jellyfish, exhibit diverse morphology and lifestyles that are manifested in sessile polyps and free-swimming medusae. As exemplified in established models such as Hydra and Nematostella, stem cells and/or proliferative cells contribute to the development and regeneration of cnidarian polyps. However, the underlying cellular mechanisms in most jellyfish, particularly at the medusa stage, are largely unclear, and, thus, developing a robust method for identifying specific cell types is critical. This paper describes a protocol for visualizing stem-like proliferating cells in the hydrozoan jellyfish Cladonema pacificum. Cladonema medusae possess branched tentacles that continuously grow and maintain regenerative capacity throughout their adult stage, providing a unique platform with which to study the cellular mechanisms orchestrated by proliferating and/or stem-like cells. Whole-mount fluorescent in situ hybridization (FISH) using a stem cell marker allows for the detection of stem-like cells, while pulse labeling with 5-ethynyl-2'-deoxyuridine (EdU), an S phase marker, enables the identification of proliferating cells. Combining both FISH and EdU labeling, we can detect actively proliferating stem-like cells on fixed animals, and this technique can be broadly applied to other animals, including non-model jellyfish species.
Introduction
Cnidaria is considered a basally branching metazoan phylum containing animals with nerves and muscles, placing them in a unique position for understanding the evolution of animal development and physiology1,2. Cnidarians are categorized into two main groups: Anthozoa (e.g., sea anemones and corals) possess only planula larvae and sessile polyp stages, while Medusozoa (members of Hydrozoa, Staurozoa, Scyphozoa, and Cubozoa) typically take the form of free-swimming medusae, or jellyfish, as well as planula larvae and polyps. Cnidarians commonly exhibit high regenerative capacity, and their underlying cellular mechanisms, particularly their possession of adult stem cells and proliferative cells, have attracted much attention3,4. Initially identified in Hydra, hydrozoan stem cells are located in the interstitial spaces between ectodermal epithelial cells and are commonly referred to as interstitial cells or i-cells3.
Hydrozoan i-cells share common characteristics that include multipotency, the expression of widely conserved stem cell markers (e.g., Nanos, Piwi, Vasa), and migration potential3,5,6,7,8. As functional stem cells, i-cells are extensively involved in the development, physiology, and environmental responses of hydrozoan animals, which attests to their high regenerative capacity and plasticity3. While stem cells, similar to i-cells, have not been identified outside of hydrozoans, even in the established model species Nematostella, proliferative cells are still involved in the maintenance and regeneration of somatic tissue, as well as the germ line9. As studies in cnidarian development and regeneration have been predominantly conducted on polyp-type animals such as Hydra, Hydractinia, and Nematostella, the cellular dynamics and functions of stem cells in jellyfish species remain largely unaddressed.
The hydrozoan jellyfish Clytia hemisphaerica, a cosmopolitan jellyfish species with different habitats around the world, including the Mediterranean Sea and North America, has been utilized as an experimental model animal in several developmental and evolutionary studies10. With its small size, easy handling, and large eggs, Clytia is suitable for lab maintenance, as well as for the introduction of genetic tools such as the recently established transgenesis and knockout methods11, opening up the opportunity for detailed analysis of the cellular and molecular mechanisms underlying jellyfish biology. In the Clytia medusa tentacle, i-cells are localized in the proximal region, called the bulb, and progenitors such as nematoblasts migrate to the distal tip while differentiating into distinct cell types, including nematocytes12.
During regeneration of the Clytia manubrium, the oral organ of jellyfish, Nanos1+ i-cells that are present in the gonads migrate to the region where the manubrium is lost in response to damage and participate in the regeneration of the manubrium7. These findings support the idea that i-cells in Clytia also behave as functional stem cells that are involved in morphogenesis and regeneration. However, given that the properties of i-cells differ among representative polyp-type animals such as Hydra and Hydractinia3, it is possible that the characteristics and functions of stem cells are diversified among jellyfish species. Furthermore, with the exception of Clytia, experimental techniques have been limited for other jellyfish, and the detailed dynamics of proliferative cells and stem cells are unknown13.
The hydrozoan jellyfish Cladonema pacificum is an emerging model organism that can be kept in a laboratory environment without a water pump or filtration system. The Cladonema medusa has branched tentacles, a common characteristic in the Cladonematidae family, and a photoreceptor organ called the ocellus on the ectodermal layer near the bulb14. The tentacle branching process occurs at a new branching site that appears along the adaxial side of the tentacle. Over time, the tentacles continue to elongate and branch, with the older branches being pushed out toward the tip15. In addition, Cladonema tentacles can regenerate within a few days upon amputation. Recent studies have suggested the role of proliferating cells and stem-like cells in tentacle branching and regeneration in Cladonema16,17. However, while conventional in situ hybridization (ISH) has been utilized to visualize gene expression in Cladonema, due to its low resolution, it is currently difficult to observe stem cell dynamics at the cellular level in detail.
This paper describes a method for visualizing stem-like cells in Cladonema by FISH and co-staining with EdU, a marker of cell proliferation18. We visualize the expression pattern of Nanos1, a stem cell marker5,17, by FISH, which allows for the identification of stem-like cell distribution at the single-cell level. In addition, the co-staining of Nanos1 expression with EdU labeling makes it possible to distinguish actively proliferating stem-like cells. This method for monitoring both stem-like cells and proliferative cells can be applied to a wide range of investigative areas, including tentacle branching, tissue homeostasis, and organ regeneration in Cladonema, and a similar approach can be applied to other jellyfish species.
Protocol
NOTE: See the Table of Materials for details related to all materials, reagents, and equipment used in this protocol.
1. Probe synthesis
- RNA extraction
- Place three live Cladonema medusae that are cultured in artificial seawater (ASW) in a 1.5 mL tube using a 3.1 mL transfer pipette with the tip cut off, and remove as much ASW as possible.
NOTE: ASW is prepared by dissolving a mixture of mineral salts in tap water with chlorine neutralizer; the final specific gravity (S.G.) is 1.018 or the parts per thousand (ppt) is ~27. - Freeze the 1.5 mL tubes in liquid nitrogen to inhibit RNase activity. Add 30 µL of lysis buffer from the total RNA isolation kit in which 2-mercaptoethanol (1 µL/100 µL of lysis buffer) has been added and homogenize the samples in the lysis buffer using a homogenizer.
NOTE: To avoid overflow of the buffer and sample from the tubes, homogenization with a small amount of lysis buffer is recommended. - Add 570 µL of lysis buffer and extract the total RNA following the protocol of the total RNA isolation kit (Figure 1).
- Measure the concentration of the extracted RNA using a spectrophotometer and store at −80 °C until use.
- Place three live Cladonema medusae that are cultured in artificial seawater (ASW) in a 1.5 mL tube using a 3.1 mL transfer pipette with the tip cut off, and remove as much ASW as possible.
- cDNA synthesis
- Using a kit, synthesize cDNA using the total RNA extracted from the medusae as a template (Figure 1 and Table 1).
- Incubate at 65 °C for 5 min.
- Cool rapidly on ice.
- Perform cDNA synthesis with the mixture from step 1.2.1 (Table 1). Mix thoroughly by pipetting and incubate at 42 °C for 60 min.
- Incubate at 95 °C for 5 min.
- Cool rapidly on ice.
- Measure the concentration of the synthesized cDNA using a spectrophotometer and store at or below −20 °C until use.
- PCR product synthesis
- To create a PCR template, design the specific primer using Primer-BLAST. Source the reference sequence from NCBI data or RNA-seq data.
NOTE: See the Table of Materials for the primers used in this protocol. - To amplify the target sequence, perform TA cloning, which does not require the use of restriction enzymes. To obtain a PCR product in which an adenine is added at the 3' end, use the following settings: 98 °C for 2 min; 35 cycles of 98 °C for 10 s, 55-60 °C for 30 s, 72 °C for 1 min. Use Taq DNA polymerase for the reactions (Table 1).
NOTE: To determine the PCR conditions, follow the protocol that accompanies the DNA polymerase to be used, which generally provides a recommended annealing temperature and extension time. - Run the PCR product through a 1% agarose gel and cut out the band of interest. Extract the PCR products from the cut gels using a gel extraction kit.
- To create a PCR template, design the specific primer using Primer-BLAST. Source the reference sequence from NCBI data or RNA-seq data.
- Ligation
- Ligate the PCR product to the vector with 3' thymine overhangs by mixing the reagents (Table 1) and incubating at 37 °C for 30 min (Figure 1).
NOTE: The molecular ratio of vector:PCR should be 1:>3. The pTA2 vector size is approximately 3 kb. If the PCR product (insert) is A (kb) and B (ng/µL), the volume of the insert (X in Table 1) to be taken can be calculated by ([50 ng of vector × A kb of insert])/(3 kb vector × [1/3]) = 50·A ng of insert. If the concentration of insert is B ng/µL, then volume taken is 50·A/B µL of insert.
- Ligate the PCR product to the vector with 3' thymine overhangs by mixing the reagents (Table 1) and incubating at 37 °C for 30 min (Figure 1).
- Transformation and plating
- For transformation, thaw the competent cells on ice and dispense them into 20 µL aliquots each. Add 1 µL of the plasmids containing the PCR product (less than 5% of competent cell volume) and vortex for 1 s. Incubate on ice for 5 min, and then incubate at 42 °C for 45 s, and vortex for 1 s.
- Add 180 µL of Super Optimal broth with Catabolite repression (SOC) medium (a nutritionally rich bacterial culture medium) to the competent cells and incubate at 37 °C for 30 min. After 30 min, spread the competent cells onto an agar plate containing ampicillin, 5-bromo-4-chloro-3-indolyl-beta-D-galacto-pyranoside (X-gal), and isopropyl-β-d-1-thiogalactopyranoside (IPTG), and incubate at 37 °C for 12-16 h (Figure 1).
NOTE: X-gal and IPTG are used for selecting blue and white colonies.
- Liquid culture
- Pick a white colony out of the white and blue colonies on the plate. Add it to 3-5 mL of LB medium with ampicillin and incubate on a shaker for 12-16 h at 37 °C (Figure 1).
- Miniprep
- Extract the plasmid from the LB medium using a plasmid miniprep (Figure 1).
- Quantify the plasmid concentration using a spectrophotometer.
- Read the sequence.
- To read the DNA sequence of the plasmid, send the plasmid for Sanger sequencing and then use software to align it with the genome/transcriptome sequence to confirm whether the target sequence is properly synthesized and assess in which direction the target sequence is inserted into the plasmid (5' to 3' or 3' to 5').
NOTE: If the target sequence is inserted into the plasmid in the 5' to 3' direction from the RNA polymerase binding site near the 3' end, an antisense probe can be generated by in vitro transcription. If the target sequence is inserted in the reverse 3' to 5' direction, a sense probe (negative control) can be generated. If using vectors such as pTA2, two transcription binding sites can be used depending on the purpose.
- To read the DNA sequence of the plasmid, send the plasmid for Sanger sequencing and then use software to align it with the genome/transcriptome sequence to confirm whether the target sequence is properly synthesized and assess in which direction the target sequence is inserted into the plasmid (5' to 3' or 3' to 5').
- In vitro transcription
- Prepare the DNA template from the created plasmid by performing PCR using primers outside the RNA polymerase binding site (e.g., T7/T3 binding sites) in the plasmid (Figure 1).
NOTE: Universal primers such as the M13-20 forward primer and the M13 reverse primer can be used to prepare a DNA template that includes RNA polymerase binding sites. - Purify the template after PCR amplification using a gel/PCR extraction kit.
- Mix the reagents shown below, and perform the transcription reaction at 37 °C for 3 h (Figure 1 and Table 1).
- Add 1.5 µL of DNase and incubate at 37 °C for 30 min.
- Add 10 µL of RNase-free water and purify the probe from the transcription reaction solution using a clean-up column.
- Check the size of the synthesized RNA by loading 1 µL onto a 1% agarose gel and determine the concentration using a spectrophotometer.
NOTE: Synthesized RNA probes should have a concentration of at least 100 ng/µL. - Add 30 µL of formamide for storage at or below −20 °C.
- Prepare the DNA template from the created plasmid by performing PCR using primers outside the RNA polymerase binding site (e.g., T7/T3 binding sites) in the plasmid (Figure 1).
2. EdU incorporation and fixation
- Place Cladonema medusae (5-10 animals per tube) into 1.5 mL tubes using a 3.5 mL transfer pipette and add ASW up to a total volume of 500 µL. Add 7.5 µL of 10 mM EdU stock solution and incubate the samples for 1 h at 22 °C (Figure 2). The EdU final concentration is 150 µM.
NOTE: Use 5-7-day-old medusae to monitor third branch formation (Figure 3A). The day that the medusae detach from the polyp is counted as day 1, and on day 5, medusae typically start to exhibit a third branch on their main tentacles. - After 1 h, remove as much ASW containing EdU as possible.
- To anesthetize the medusae, add 7% MgCl2 in H2O and incubate for 5 min.
- Remove the 7% MgCl2 in H2O and fix the medusae overnight (O.N.) at 4 °C with 4% paraformaldehyde (PFA) in ASW (Figure 2).
NOTE: When performing FISH without EdU incorporation, samples can be fixed similarly to those treated with EdU following anesthetization (steps 2.3-2.4).
3. Fluorescent in situ hybridization
- Proteinase treatment and post fixation
- Remove the PFA and wash the samples with PBS containing 0.1% Tween-20 (PBST) for 3 x 10 min. Use 300-500 µL of PBST per wash.
NOTE: From this step to the end of hybridization, the experiment should be performed in an RNase-free environment, wearing gloves and a mask. The 1x PBS in this step must be made with DEPC-treated water. After hybridization, 1x PBS made with water without DEPC treatment can be used. Some ISH and FISH protocols dehydrate samples using methanol steps, which makes it possible to save the fixed samples at −20 °C until use. To avoid superfluous work, the dehydration process is omitted from this protocol, particularly since the samples can be kept in PBST for up to a few days. - After fixation, place the sample in 1.5 mL tubes on a shaker, except for the anti-DIG-POD antibody O.N. incubation step (step 3.4.2). After washing, add 10 mg/mL Proteinase K stock solution in PBST and incubate the medusae with Proteinase K (final concentration: 10 µg/mL) at 37 °C for 10 min.
- Remove the Proteinase K solution and wash the samples for 2 x 1 min with PBST.
- To postfix the medusae, add 4% PFA in 1x PBS and incubate at 37 °C for 15 min.
- Remove the PFA solution and wash the samples for 2 x 10 min with PBST.
NOTE: If the target gene is highly expressed with low background signal, steps 3.1.2-3.1.5 can be skipped.
- Remove the PFA and wash the samples with PBS containing 0.1% Tween-20 (PBST) for 3 x 10 min. Use 300-500 µL of PBST per wash.
- Hybridization
- Remove the PBST and add Hybridization Buffer (HB Buffer, Table 1). Incubate the samples in HB Buffer for 15 min at room temperature (RT). Use 300-400 µL of HB Buffer, which provides enough volume for successful hybridization.
NOTE: HB Buffer, Wash Buffer 1, and Wash Buffer 2 are prepared with 20x SSC stock. HB Buffer is stored at or below −20 °C and must be brought to RT before use. - Remove the HB Buffer and add new HB Buffer. Prehybridize at 55 °C for at least 2 h in a hybridization incubator.
- Remove the HB Buffer and incubate with HB Buffer containing the probes that were stored in step 1.9.7 (final probe concentration: 0.5-1 ng/µL in HB Buffer). Hybridize at 55 °C for 18-24 h (Figure 2) in a hybridization incubator.
NOTE: FISH signal intensity and specificity can vary due to target gene expression, as well as probe length and specificity. To increase the intensity, parameters such as hybridization duration (18-72 h) and temperature (50-65 °C) can be adjusted, and different probes can be tested. To avoid non-specific signals, Proteinase K treatment (concentration and duration) can be modified19.
- Remove the PBST and add Hybridization Buffer (HB Buffer, Table 1). Incubate the samples in HB Buffer for 15 min at room temperature (RT). Use 300-400 µL of HB Buffer, which provides enough volume for successful hybridization.
- Probe removal
- Remove the HB Buffer containing probes, and then add Wash Buffer 1 (Table 1). Wash the samples with Wash Buffer 1 for 2 x 15 min at 55 °C. Use 300-400 µL of wash buffer.
NOTE: HB Buffer containing probes can be used repeatedly, up to about ten times. Instead of discarding, store the used HB Buffer containing probes at or below −20 °C. Preheat Wash Buffer 1, Wash Buffer 2, 2x SSC, and PBST at 55 °C before use. - Remove Wash Buffer 1, and then add Wash Buffer 2 (Table 1). Wash the samples with Wash Buffer 2 for 2 x 15 min at 55 °C.
- Remove Wash Buffer 2, and then add 2x SSC. Wash the samples with 2x SSC for 2 x 15 min at 55 °C.
- Remove 2x SSC, and then add preheated PBST. Wash the samples for 1 x 15 min with PBST at RT.
- Remove the HB Buffer containing probes, and then add Wash Buffer 1 (Table 1). Wash the samples with Wash Buffer 1 for 2 x 15 min at 55 °C. Use 300-400 µL of wash buffer.
- Anti-DIG antibody incubation
- Remove PBST, and then add 1% blocking buffer. Incubate the samples for at least 1 h at RT while shaking slowly on a rocker.
NOTE: Prepare 1% blocking buffer freshly by diluting 5% blocking buffer stock (Table 1). Check the samples before the next antibody reaction because the sample tends to stick to the back of the lid or the wall as a result of shaking. - After blocking, remove the 1% blocking buffer, add anti-DIG-POD solution (1:500, in 1% blocking buffer), and incubate the samples O.N. at 4 °C (Figure 2).
NOTE: Be careful to keep the incubation time within 12-16 h to prevent the detection of non-specific signals.
- Remove PBST, and then add 1% blocking buffer. Incubate the samples for at least 1 h at RT while shaking slowly on a rocker.
- Detection of DIG-labeled probe
- Remove the anti-DIG-POD solution, and then add Tris-NaCl-Tween Buffer (TNT, Table 1). Wash the samples with TNT for 3 x 10 min at RT.
NOTE: The use of the tyramide signal amplification (TSA) technique provides a better resolution image against horseradish peroxidase-labeled reagents (e.g., anti-DIG-POD). - Dilute fluorescent dye-conjugated tyramide (Cy5-tyramide) stock solution (1:50) in the amplification diluent buffer to make the active Cy5-tyramide solution (Figure 2).
- Remove as much TNT as possible, and then add the active Cy5-tyramide solution. Incubate the samples for 10 min in the dark.
- Wash the samples with PBST for 3 x 10 min in the dark.
- Remove the anti-DIG-POD solution, and then add Tris-NaCl-Tween Buffer (TNT, Table 1). Wash the samples with TNT for 3 x 10 min at RT.
- Detection of EdU
NOTE: The EdU kit detects incorporated EdU as fluorescent signals (Figure 2).- To prepare the EdU detection cocktail, mix the components as shown in Table 1. Use 100 µL of EdU detection cocktail for each sample.
NOTE: Prepare 1x Reaction Buffer additive by diluting 10x Reaction Buffer additive with ultrapure water. - Remove PBST, and then add the EdU detection cocktail. Incubate in the dark for 30 min.
- Wash with PBST for 3 x 10 min in the dark.
- To prepare the EdU detection cocktail, mix the components as shown in Table 1. Use 100 µL of EdU detection cocktail for each sample.
- DNA staining
- Dilute Hoechst 33342 (1:500) in PBST to prepare the Hoechst solution. Remove the PBST, and then add the Hoechst solution. Incubate the samples for 30 min in the dark (Figure 2).
- Wash the samples with PBST for 3-4 x 10 min in the dark.
- Mounting
- Make a bank on the glass slide to prevent the sample from being crushed. Apply vinyl tape to the glass slide and hollow out the center.
- Transfer the medusae to the bank on the slide glass using a 3.1 mL transfer pipette with the tip cut off.
NOTE: Be careful not to let the tentacles overlap. - Remove any PBST with a P200 pipette, and then slowly add 70% glycerol as the mounting medium (Figure 2).
NOTE: Instead of 70% glycerol, anti-fading mounting medium can be used to prevent the fluorescence from fading. - Gently place a coverslip on the medusae with forceps, and seal the side of the coverslip with clear nail polish.
- Keep the slides at 4 °C in the dark if not immediately performing microscopy observation.
- Imaging
- Use a laser scanning confocal microscope to acquire images. For single-cell resolution, use a 40x oil lens or a lens for higher magnification.
- After image acquisition, open the images using ImageJ/FIJI software and count cells that are positive for EdU+ and/or Nanos1+ by the multipoint tool20.
Representative Results
Cladonema tentacles have been used as a model to study the cellular processes of morphogenesis and regeneration15,16,17. The tentacle structure is composed of an epithelial tube where stem-like cells, or i-cells, are located in the proximal region, called the tentacle bulb, and new branches are sequentially added to the rear of the distal region of the bulb along the adaxial side (Figure 3A)15. Previous reports have indicated that cell proliferation is active both in the tentacle bulb and at the new branching sites using either EdU or BrdU labeling16,17. However, due to the resolution of in situ hybridization, it is unclear whether stem-like cells are truly proliferative or not. To visualize both stem-like and proliferative cells simultaneously at the cellular level, we performed FISH for stem cell markers (Nanos1 or Piwi) and EdU labeling for S phase cells in the same samples.
At cellular resolution by FISH, the expression of Nanos1 was localized at the tentacle bulb and the new branching site (Figure 3B). Piwi was also expressed in the tentacle bulb and at the new branching site in a pattern similar to that of Nanos1 (Figure 3C). These results were consistent with the observations from whole-mount in situ hybridization in a previous report17, where the budding branch of a 7-day-old medusa was almost uniformly labeled by Nanos1 and Piwi. To visualize the beginning of the accumulation of stem-like cells, we monitored the new branching site of a 5-day-old medusa. The co-labeling of Nanos1 expression and EdU-positive cells revealed the spatial pattern of stem-like cells and proliferative cells in the tentacle (Figure 4A). Although the gross distributions of EdU+ and Nanos1+ cells were consistent with previous reports16,17, EdU+ cells were more widely distributed throughout the tentacle bulb, at least at the beginning of branching, while Nanos1+ cells accumulated more locally at the tentacle bulb and the new branching site (Figure 4A and Figure 4E). These observations suggest that distinct distributions of stem-like cells and proliferating cells are detected depending on the developmental timing and different stages.
A more detailed view of the bulb and the new branching site revealed that EdU signals merge with nuclear staining, whereas Nanos1 expression is constrained to the cytoplasm surrounding the nucleus, consistent with a previous report5 (Figure 4B,C). A fraction (19.79%) of the cells exhibited co-labeling of EdU and Nanos1 (EdU+ Nanos1+; Figure 4B,C, yellow arrowheads, and Figure 4D), suggesting that these cells are an actively-proliferating stem cell population. Intriguingly, 14.46% of the cells were found to be EdU+ Nanos1− in the middle of the bulb and at the new branching site, suggesting the presence of non-stem-like proliferative cells (Figure 4B,C, white arrows, and Figure 4D). In contrast, 26.32% of cells were observed to be EdU− Nanos1+ at the base of the bulb and at the new branching site, indicating the presence of a stem cell population that is either slow-cycling or quiescent, neither of which is detected by EdU pulse labeling (Figure 4B,C, yellow arrows, and Figure 4D).
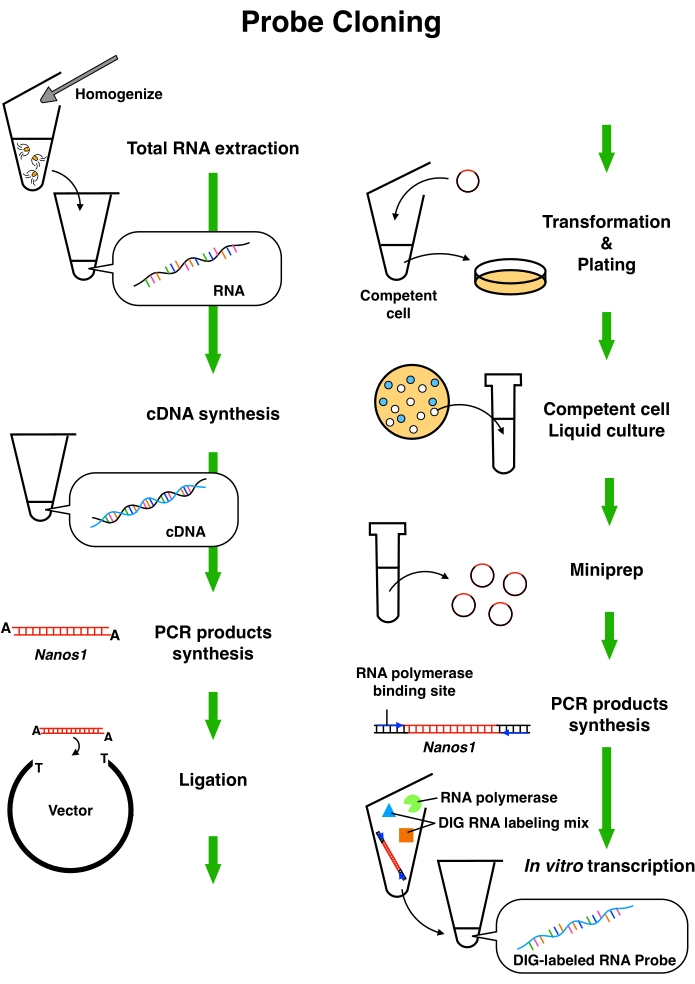
Figure 1: Scheme of the probe synthesis for in situ hybridization. Extraction of total RNA from medusae and cDNA synthesis from total RNA. Nanos1-specific PCR products were synthesized from the cDNA. The PCR products were ligated into the vectors, and amplified vectors were collected through competent cell culture. The PCR products with RNA polymerase binding sites were synthesized using the plasmids as templates. DIG-labeled RNA probes were synthesized by in vitro transcription. Abbreviations: DIG = digoxigenin. Please click here to view a larger version of this figure.
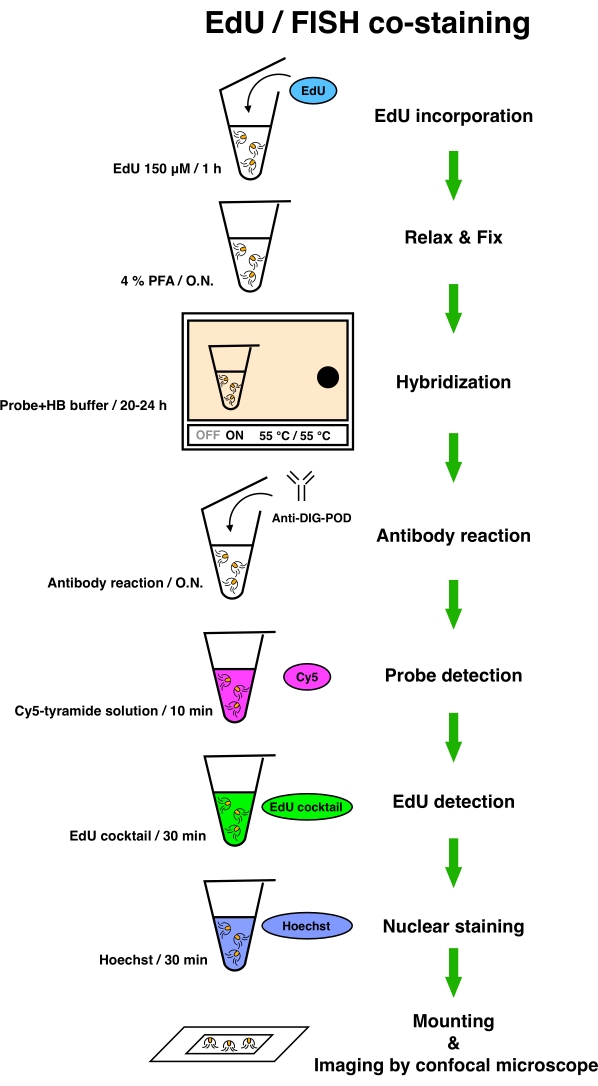
Figure 2: Scheme of EdU and fluorescent in situ hybridization co-staining. Medusae were incubated with 150 µM EdU for 1 h. Subsequently, the medusae were anesthetized (to relax the tissue) with 7% MgCl2 in H2O and fixed with 4% PFA O.N. at 4 °C. After fixation, the samples were hybridized with HB Buffer with a probe for 20-24 h at 55 °C. After the hybridization reaction, the samples were washed and incubated with anti-DIG-POD solution O.N. at 4 °C. The medusae were stained with Cy5-tyramide solution for 10 min, followed by the detection of EdU for 30 min and staining with Hoechst 33342 for 30 min. After completing all the staining processes, the medusae were mounted on the glass slide, and images were obtained with a confocal microscope. Abbreviations: EdU = 5-ethynyl-2'-deoxyuridine; FISH = fluorescent in situ hybridization; PFA = paraformaldehyde; O.N. = overnight; HB = hybridization. Please click here to view a larger version of this figure.
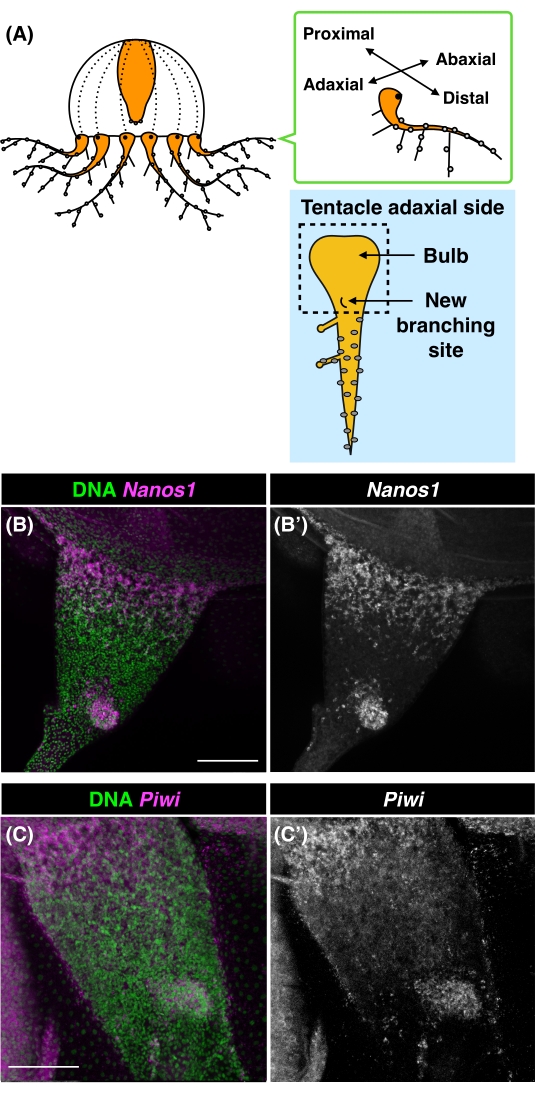
Figure 3: Nanos1 and Piwi expression patterns on the proximal adaxial side of the Cladonema medusa tentacle. (A) Schematic of a Cladonema medusa and tentacle. The adaxial side of the tentacle: the tentacle bulb (the most proximal region) with new branches sequentially formed on the new branching site. The inset (dashed square) indicates the area captured by the confocal image. (B) FISH images of Nanos1 gene expression from the proximal, adaxial side of the tentacle of a 7-day-old Cladonema medusa. (C) FISH images of Piwi gene expression from the proximal, adaxial side of the tentacle of a 7-day-old Cladonema medusa. DNA: green, Nanos1: magenta. B'–C' for Nanos1 FISH only images. Scale bars = 100 µm (B,C). Abbreviation: FISH = fluorescent in situ hybridization. Please click here to view a larger version of this figure.
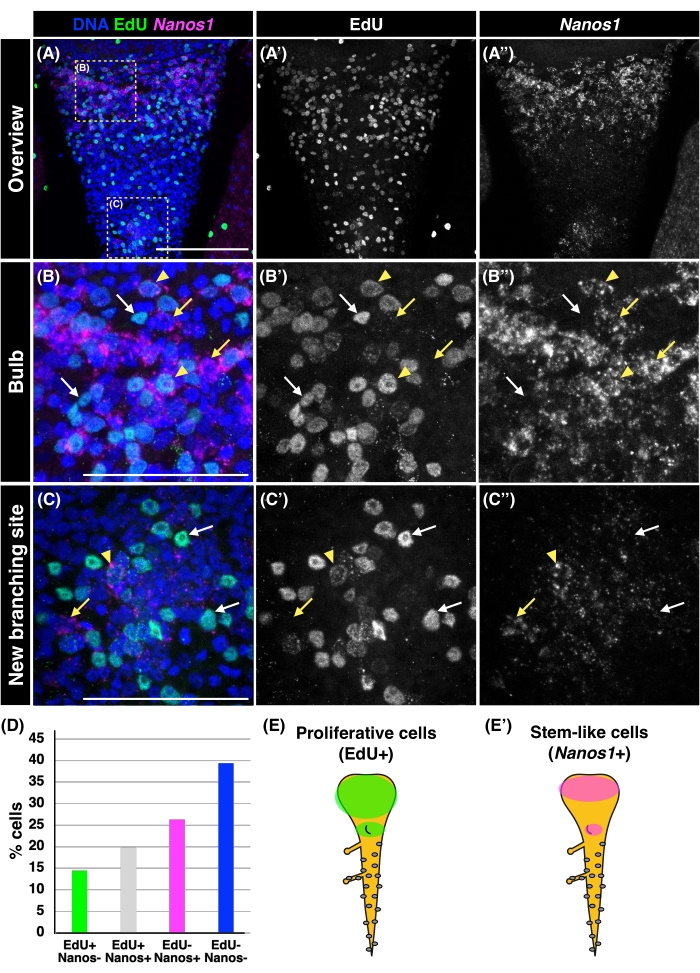
Figure 4: EdU and Nanos1 expression patterns on the proximal, adaxial side of Cladonema medusa tentacle. (A–C) Images of the proximal adaxial side of the Cladonema medusa tentacle co-labeled with Nanos1 expression and EdU; 5-day-old medusae were used. (A) An overview of the tentacle. Yellow dashed squares indicate the areas of B and C. (B) Magnification of the tentacle bulb. (C) Magnification of a new branching site. Yellow arrowheads indicate the cells that are positive for both EdU and Nanos1. Yellow arrows indicate the cells that are only positive for Nanos1. White arrows indicate cells only positive for EdU. A–C panels are merged images for DNA (blue), EdU (green), and Nanos1 (magenta). A'–C' are panels for EdU only images; A''–C'' are for Nanos1 FISH only images. Scale bars = 100 µm (A), 50 µm (B, C). (D) The quantification of EdU- and/or Nanos1-positive cells in the basal side of the tentacle (quantification area = 30.10 µm2 square, n = 6, a total of 249 cells). EdU+ Nanos1− cells, 14.46%; EdU+ Nanos1+ cells, 19.79%; EdU− Nanos1+ cells, 26.32%; EdU− Nanos1− cells, 39.44%. (E) Schematic of a Cladonema medusa tentacle from the adaxial side.The overall distribution of EdU+ cells and Nanos1+ cells are shown in E and E', respectively. Please click here to view a larger version of this figure.
Table 1: Composition of different PCR reactions and buffers in this protocol. To calculate the volume of the PCR product (X µL) in the ligation reaction, see the note after protocol step 1.4. Please click here to download this Table.
Discussion
Proliferating cells and stem cells are important cellular sources in various processes such as morphogenesis, growth, and regeneration21,22. This paper describes a method for co-staining the stem cell marker Nanos1 by FISH and EdU labeling in Cladonema medusae. Previous work using EdU or BrdU labeling has suggested that proliferative cells localize to the tentacle bulbs16,17, but their molecular characteristics were unclear. The present study shows the simultaneous determination of the distribution of proliferative cells and the localization of Nanos1+ stem-like cells (Figure 4). The results showed that some proliferative cells expressed Nanos1, but other cells were marked only with EdU and did not express Nanos1, suggesting the existence of stem cell heterogeneity or, potentially, of other proliferative cells. It will be interesting to dissect the detailed stem cell distribution during different processes in Cladonema, including tentacle branching, tissue homeostasis, organ regeneration, and germ cell maintenance.
The major bottleneck for visualizing stem cells in animals is the initial identification of stem cell markers. In cnidarians, stem cell markers have not been identified in non-hydrozoans3, and thus, at this stage, the direct application of FISH for stem cell markers remains limited to hydrozoans. Nevertheless, FISH allows for the detection of specific gene expression at the cellular level, and thus, by changing probes, this method can be extended to observe the spatial expression patterns of any genes of interest in detail. For example, using markers of progenitor cells and differentiated cells, we can verify the distribution of specific cell types in the Cladonema tentacles. As a caveat, the present protocol may have to be modified depending on the genes of interest due to differences in expression levels and mRNA stability. In particular, non-specific signals and weak signals are common problems associated with FISH. Changing the hybridization time and temperature, using bleaching reagents (formamide or methanol), and adjusting other parameters (TSA reaction time, Proteinase K treatment, washing after hybridization) may yield clearer signals and fewer nonspecific signals23,24. It is also important to select a FISH protocol appropriate to the animal model being used, since one FISH protocol may not be applicable to other species, even within the same taxon7,25,26.
EdU labeling is generally used to detect proliferative cells but could be used for different experimental purposes by varying the concentration and incubation time27. To detect proliferating cells, it is critical to determine a successful duration and concentration for EdU incorporation. In the pulse labeling used in this work, only proliferative cells that passed through S phase for a short period of time were marked, and similar short incubation methods have been utilized for detecting proliferating cells in other cnidarians6,28,29. By contrast, the combination of prolonged EdU incorporation and Nanos1 FISH may reveal the presence of slow-cycling or quiescent stem cells27. It is also possible to mark not only proliferative cells but also endocycling cells that have undergone DNA synthesis without division. Furthermore, by tracking EdU-labeled cells for a longer duration, we can determine the cellular capacity for migration and differentiation associated with proliferative cells and their cell lineage6,30.
While the combination of FISH and EdU or BrdU staining has been used7,31, the method established here can easily be applied to other marine invertebrates and non-model animals, including different jellyfish species. EdU staining is simpler and more sensitive than BrdU staining18, and with its short time incubation, it enables the detection of proliferating cells, unlike the previous study that used EdU for long-term cell labeling7. In recent years, with the advancement of next-generation sequencing technologies, genome and gene expression information has become available for many species. Identifying stem cells and proliferative cells will continue to be an effective approach for understanding stem cell heterogeneity and diversity and providing insights into the cellular dynamics underlying different biological phenomena.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by AMED under Grant Number JP22gm6110025 (to Y.N.) and by the JSPS KAKENHI Grant Number 22H02762 (to Y.N.).
Materials
| 2-Mercaptoethanol | Wako | 137-06862 | |
| 3.1 mL transfer pipette | Thermo Scientific | 233-20S | |
| 5-Bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-Gal) | Wako | 029-15043 | |
| anti-DIG-POD | Roche | 11207733910 | |
| Cladonema pacificum Nanos1 forward primer | 5’-AAGAGACACAGTCATTATCAAGC GA-3’ |
||
| Cladonema pacificum Nanos1 reverse primer | 5’-CGACGTGTCCAATTTTACGTGCT -3’ | ||
| Cladonema pacificum Piwi forward primer | 5’- AAAAGAGCAGCGGCCAGAAAGA AGGC -3’ |
||
| Cladonema pacificum Piwi reverse primer | 5’- GCGGGTCGCATACTTGTTGGTA CTGGC -3’ |
||
| Click-iT EdU Cell Proliferation Kit for Imaging, Alexa Fluor 488 dye | Invitrogen | C10337 | EdU kit |
| Coroline off | GEX Co. ltd | N/A | chlorine neutralizer |
| DIG Nucleic Acid Detection Kit Blocking Reagent | Roche | 11175041910 | blocking buffer |
| DIG RNA labeling mix | Roche | 11277073910 | |
| DTT | Promega | P117B | |
| ECOS competent cell DH5α | NIPPON GENE | 316-06233 | competent cell |
| Fast gene Gel/PCR Extraction kit | Fast gene | FG-91302 | gel extraction kit |
| Fast gene plasmid mini kit | Fast gene | FG-90502 | plasmid miniprep |
| Formamide | Wako | 068-00426 | |
| Heparin sodium salt from porcine | SIGMA-ALDRICH | H3393-10KU | |
| Isopropyl-β-D(-)-thiogalactopyranoside (IPTG) | Wako | 096-05143 | |
| LB Agar | Invitrogen | 22700-025 | agar plate |
| LB Broth Base | Invitrogen | 12780-052 | LB medium |
| Maleic acid | Wako | 134-00495 | |
| mini Quick spin RNA columns | Roche | 11814427001 | clean-up column |
| NaCl | Wako | 191-01665 | |
| NanoDrop OneC Microvolume UV-Vis Spectrophotometer with Wi-Fi | Thermo Scientific | ND-ONEC-W | spectrophotometer |
| Polyoxyethlene (20) Sorbitan Monolaurate (Tween-20) | Wako | 166-21115 | |
| PowerMasher 2 | nippi | 891300 | homogenizer |
| Proteinase K | Nacarai Tesque | 29442-14 | |
| RNase Inhibitor | TaKaRa | 2313A | |
| RNeasy Mini kit | Qiagen | 74004 | total RNA isolation kit |
| RQ1 RNase-Free Dnase | Promega | M6101 | |
| Saline Sodium Citrate Buffer 20x powder (20x SSC) | TaKaRa | T9172 | |
| SEA LIFE | Marin Tech | N/A | mixture of mineral salts |
| T3 RNA polymerase | Roche | 11031163001 | |
| T7 RNA polymerase | Roche | 10881767001 | |
| TAITEC HB-100 | TAITEC | 0040534-000 | Hybridization incuvator |
| TaKaRa Ex Taq | TaKaRa | RR001A | Taq DNA polymerase |
| TaKaRa PrimeScript 2 1st strand cDNA Synthesis Kit | TaKaRa | 6210A | cDNA synthesis kit |
| Target Clone | TOYOBO | TAK101 | pTA2 Vector |
| tRNA | Roche | 10109541001 | |
| TSA Plus Cyanine 5 | AKOYA Biosciences | NEL745001KT | tyramide signal amplification (TSA) technique |
| Zeiss LSM 880 | ZEISS | N/A | laser scanning confocal microscope |
References
- Leclère, L., Röttinger, E. Diversity of cnidarian muscles: Function, anatomy, development and regeneration. Frontiers in Cell and Developmental Biology. 4, 157 (2017).
- Bosch, T. C. G., et al. Back to the basics: Cnidarians start to fire. Trends in Neurosciences. 40 (2), 92-105 (2017).
- Gold, D. A., Jacobs, D. K. Stem cell dynamics in Cnidaria: Are there unifying principles. Development Genes and Evolution. 223 (1-2), 53-66 (2013).
- Technau, U., Steele, R. E. Evolutionary crossroads in developmental biology: Cnidaria. Development. 138 (8), 1447-1458 (2011).
- Leclère, L., et al. Maternally localized germ plasm mRNAs and germ cell/stem cell formation in the cnidarian Clytia. Biologie du développement. 364 (2), 236-248 (2012).
- Bradshaw, B., Thompson, K., Frank, U. Distinct mechanisms underlie oral vs aboral regeneration in the cnidarian Hydractinia echinata. eLife. 4, 05506 (2015).
- Sinigaglia, C., et al. Pattern regulation in a regenerating jellyfish. eLife. 9, 54868 (2020).
- David, C. N. Interstitial stem cells in Hydra: Multipotency and decision-making. The International Journal of Developmental Biology. 56 (6-7-8), 489-497 (2012).
- Röttinger, E. Nematostella vectensis, an emerging model for deciphering the molecular and cellular mechanisms underlying whole-body regeneration. Cells. 10 (10), 2692 (2021).
- Houliston, E., Momose, T., Manuel, M. Clytia hemisphaerica: A jellyfish cousin joins the laboratory. Trends in Genetics. 26 (4), 159-167 (2010).
- Peron, S., Houliston, E., Leclère, L., Boutet, A., Shierwater, B. The Marine Jellyfish Model, Clytia hemisphaerica. Handbook of Marine Model Organisms in Experimental Biology. , 129-147 (2021).
- Denker, E., Manuel, M., Leclère, L., Le Guyader, H., Rabet, N. Ordered progression of nematogenesis from stem cells through differentiation stages in the tentacle bulb of Clytia hemisphaerica (Hydrozoa, Cnidaria). Biologie du développement. 315 (1), 99-113 (2008).
- Fujita, S., Kuranaga, E., Nakajima, Y. Regeneration potential of jellyfish: Cellular mechanisms and molecular insights. Genes. 12 (5), 758 (2021).
- Suga, H., et al. Flexibly deployed Pax genes in eye development at the early evolution of animals demonstrated by studies on a hydrozoan jellyfish. Proceedings of the National Academy of Sciences. 107 (32), 14263-14268 (2010).
- Fujiki, A. Branching pattern and morphogenesis of medusa tentacles in the jellyfish Cladonema pacificum (Hydrozoa, Cnidaria). Zoological Letters. 5 (12), 13 (2019).
- Fujita, S., Kuranaga, E., Nakajima, Y. Cell proliferation controls body size growth, tentacle morphogenesis, and regeneration in hydrozoan jellyfish Cladonema pacificum. PeerJ. 7, 7579 (2019).
- Hou, S., Zhu, J., Shibata, S., Nakamoto, A., Kumano, G. Repetitive accumulation of interstitial cells generates the branched structure of Cladonema medusa tentacles. Development. 148 (23), (2021).
- Salic, A., Mitchison, T. J. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proceedings of the National Academy of Sciences of the United States of America. 105 (7), 2415-2420 (2008).
- Angerer, L. M., Angerer, R. C. Detection of poly A + RNA in sea urchin eggs and embryos by quantitative in situ hybridization. Nucleic Acids Research. 9 (12), 2819-2840 (1981).
- Rakotomamonjy, J., Guemez-Gamboa, A. Purkinje cell survival in organotypic cerebellar slice cultures. Journal of Visualized Experiments. (154), e60353 (2019).
- Tanaka, E. M., Reddien, P. W. The cellular basis for animal regeneration. Developmental Cell. 21 (1), 172-185 (2011).
- Penzo-Méndez, A. I., Stanger, B. Z. Organ-size regulation in mammals. Cold Spring Harbor Perspectives in Biology. 7 (9), 019240 (2015).
- Sinigaglia, C., Thiel, D., Hejnol, A., Houliston, E., Leclère, L. A safer, urea-based in situ hybridization method improves detection of gene expression in diverse animal species. Biologie du développement. 434 (1), 15-23 (2018).
- King, R. S., Newmark, P. A. In situ hybridization protocol for enhanced detection of gene expression in the planarian Schmidtea mediterranea. BMC Developmental Biology. 13 (1), 8 (2013).
- Flici, H., et al. An evolutionarily conserved SoxB-Hdac2 crosstalk regulates neurogenesis in a cnidarian. Cell Reports. 18 (6), 1395-1409 (2017).
- He, S., et al. An axial Hox code controls tissue segmentation and body patterning in Nematostella vectensis. Science. 361 (6409), 1377-1380 (2018).
- Govindasamy, N., Murthy, S., Ghanekar, Y. Slow-cycling stem cells in hydra contribute to head regeneration. Biology Open. 3 (12), 1236-1244 (2014).
- Passamaneck, Y. J., Martindale, M. Q. Cell proliferation is necessary for the regeneration of oral structures in the anthozoan cnidarian Nematostella vectensis. BMC Developmental Biology. 12 (1), 34 (2012).
- Gold, D. A., et al. Structural and developmental disparity in the tentacles of the moon jellyfish Aurelia sp.1. PLoS One. 10 (8), 0134741 (2015).
- Gold, D. A., Nakanishi, N., Hensley, N. M., Hartenstein, V., Jacobs, D. K. Cell tracking supports secondary gastrulation in the moon jellyfish Aurelia. Development Genes and Evolution. 226 (6), 383-387 (2016).
- Cheng, L. -. C., Alvarado, A. S. Whole-mount BrdU staining with fluorescence in situ hybridization in planarians. Planarian Regeneration. 1774, 423-434 (2018).

